Abstract
Objective
Intensity modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT) techniques were compared in terms of their dosimetric quality, treatment efficiency, and delivery accuracy for hippocampal sparing prophylactic whole brain radiotherapy.
Methods
Ten previously treated patients were selected for this study. All plans were prescribed to deliver 30 Gy in 10 fractions to 90% of the target volume. RTOG 0933 recommendations were applied for treatment planning. Plans were compared based on the organ at risk (OAR) sparing, homogeneity and conformity indexes, monitor unit (MU), and beam on time (BOT). Delivery accuracy of the plans was also compared.
Results
VMAT plans had better homogeneity index and conformity index than IMRT plans. In terms of hippocampus sparing, VMAT plans were superior to other plans. Since brainstem, optic nerves, and chiasm were in the PTV, their doses were nearly equal to each other for both techniques. So, there were no statistical differences between techniques. Although both eyes were not in the PTV, there was no significant dose difference between techniques. However, due to the posterior gantry angles of IMRT plans, lens doses were lower in IMRT plans than those in VMAT plans. The VMAT technique had lower MU and BOT values than the IMRT technique. In terms of delivery accuracy, VMAT plans were superior than IMRT plans.
Conclusion
VMAT plans provide better target volume coverage, homogeneity, conformity, and hippocampus sparing when compared with IMRT plans. VMAT plans are also the best in terms of treatment efficiency since they require a much smaller number of MUs and thus a shorter treatment time than IMRT plans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In small cell lung cancer (SCLC), the rate of brain metastasis at the time of diagnosis is approximately 10–15% [1]. This rate reaches up to 80% in the last period of the disease [2]. Development of brain metastasis disrupts the patient’s quality of life and shortens survival. Primary treatment of the SCLC is chemotherapy, but chemotherapy alone does not reduce the incidence of brain metastases [3]. Therefore, patients who do not have brain metastasis in cranial MRI are treated with prophylactic cranial irradiation (PCI) after chemotherapy. PCI is used in order to reduce the incidence of brain metastasis. This prophylactic technique increases the probability of disease-free survival and overall survival in patients [4,5,6,7,8].
However, whole brain radiotherapy (WBRT) may cause neurocognitive decline and significantly affect the quality of life. Radiation-induced damage to the hippocampus plays a significant role in this decline following WBRT. The hippocampus plays a vital role in regulating learning, memory encoding, memory consolidation, spatial location perception (orientation), and mood regulation, and it is a rare site for brain metastasis. Radiation Therapy Oncology Group (RTOG) 0933 [9] is a phase II clinical trial that aims to explore the hypothesis that sparing the hippocampus during cranial irradiation may mitigate radiation-induced neurocognitive toxicity.
The purpose of this study was to compare treatment plan quality and delivery accuracy assessments of different treatment techniques for hippocampal sparing prophylactic whole brain radiotherapy (HS WBRT).
Methods
Ten patients previously treated for HS WBRT using the VMAT technique with a 10 × 250-cGy prescription dose at our institution were selected for this study.
CT simulation
All patients had a planning computed tomography (CT) scan for treatment planning. With regard to patient immobilization, the thermoplastic mask was used to eliminate potential movement of the head during the scan. Also, Portrait Type-S™ was mounted to a couch to prevent movement during the procedure. Initial alignment was conducted by manually aligning the built-in planar positioning lasers, to intersect at the patients’ brain center. Once positioning was satisfactory, a full helical CT scan of the head at 120 kVp and 500 mA at 1.25-mm increments was performed in the supine position by using Philips Brilliance Big Bore CT machine (Philips Medical Systems, Cleveland, OH, USA). A total of 250 images were collected in a time of 46 s through a 17.5-mm cone beam, and the thickness of the slices was 1 mm. 3D image was created from the reconstruction of X-ray images acquired during the simulation. The imaging DICOM data from the CT scan was sent to the treatment planning system (TPS). All CT images of the patients were fused with their recent T1-weighted magnetic resonance images.
Target volume and OAR delineation
The delineations of the brain and OARs were performed for all 10 cases as described in RTOG 0933 guidelines. The clinical target volume (CTV) is defined as the whole brain parenchyma to C1. The planning target volume (PTV) is defined as the whole brain volume excluding the hippocampal avoidance region. Hippocampal avoidance regions will be generated by three-dimensionally expanding the hippocampal contours by 5 mm.
Planning objectives
RTOG 0933 recommendations were applied for treatment planning. The prescription was for a total of 30 Gy with 10 fractions. The protocol stipulated that 98% of the PTV had to receive at least 25 Gy and the volume of PTV receiving over 37.5 Gy was limited to 2%. Additionally, the prescription dose of 30 Gy had to cover at least 90% of the PTV. Hippocampus constraints are as follows: (1) The maximum dose to the hippocampus was limited to 17 Gy. (2) The dose to the entire hippocampal volume could not exceed 10 Gy.
In this study, the same target volume dose constraints and OAR dose constraints were used for each technique.
Treatment planning
Intensity modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) treatment plans were created for HS WBRT.
IMRT plans were generated using commercial inverse planning software Eclipse, version 13.0 (Varian Medical Systems, Palo Alto, CA). Six-megavolt photon beams with 600 MU/min dose rate of Varian Triology (Varian Medical Systems, Palo Alto, CA) were used. Beam shaping with the Trilogy is achieved with the 120-leaf Millennium multi leaf collimator (MLC). Beam geometry consisted of seven coplanar fields with the posterior gantry angles of 105°, 130°, 155°, 175°, 200°, 225°, and 250°. Default smoothing values were used during optimization. The normal tissue objective to deliver the highly steep gradient dose to target volume was values of 350 with a 0.5 fall-off between the start dose of the 100% and the end dose of the 50%. The maximum iteration of calculations was limited to 500 times. The MLC motion was optimized using the sliding window technique, resulting in a slightly higher number of monitor units (MUs) and a significantly lower beam on time (BOT) was selected. The dose-volume optimizer (DVO) for IMRT plans and progressive resolution optimizer (PRO) for VMAT plans were used. The anisotropic analytical algorithm (AAA) was used for dose calculations in the TPS. Inhomogeneity correction was on, and 1-mm grid size was used. The calculation time for IMRT was 3 min.
VMAT plans were created with 2 full arcs and noncoplanar 2 half arcs combination using 6-MV photon beams. The dose rate was variable with a maximum value of 600 MU/min (averaging around 300 MU/min). Two full arcs were delivered with one arc moving counterclockwise from 179.9° to 180.1° and the second arc moving in the opposite direction so as to minimize the off-treatment between the two beams about 25 s. For the first noncoplanar arc, gantry rotated from 0° to 90°, and for the second, gantry rotated from 90° to 0°. Four arc VMAT plans shared the same isocenter and optimized independently and simultaneously.
For the first full arc, field size and collimator rotation were determined by the automatic tool from Eclipse to encompass the PTV. The second full arc was similar to the first arc except for the rotation of the collimator, which was 360-X for the second arc (X corresponded to the rotation of the collimator of the first arc). We controlled that the collimator was always rotated to a value different from zero in order to avoid tongue and groove effect. For noncoplanar arcs, couch angle was 90° and collimator rotation was determined with the same method used for full arcs. AAA with a grid size of 1 mm was selected for dose calculations. The calculation time for VMAT was 9 min. Dosimetric parameters were evaluated using dosimetric tool dose-volume histograms (DVH).
Plan evaluation
Plan quality
Treatment planning efficiency was evaluated based on the comparison of the conformity index (CI) and homogeneity index (HI), the average dose delivered to 90% (D90%) and 2% (D2%) of PTV, mean and maximum dose to the hippocampus and maximum dose to optic nerves, chiasm, brainstem, eyes, and lenses.
HI defined as
used to quantify dose homogeneity in the PTV.
CI was used to assess the quality of target coverage. Paddick conformity index was defined as [10]
where target volume ∩ V100% is the target volume covered by the reference isodose, V100% = volume of the reference isodose, and target volume (PTV) = CTV − hippocampus avoidance region (in this study, reference isodose used for 90%).
Plan efficiency
Treatment efficiency was evaluated based on the comparison of the:
-
MU, defined as the average number of monitor units required to deliver the prescribed dose
-
BOT, defined as the time the treatment planning system predicts the beam will be “on” to deliver the prescribed monitor units. BOT did not account for the time required to reach each gantry position, as in the case of IMRT.
For each one of the evaluated dosimetric parameters, the average value and the standard deviation (SD) were calculated. The treatment plans satisfying RTOG 0933 objectives were considered acceptable for treatment.
Data and statistical analysis
For statistical analysis, the student paired t tests were performed to compare the results for parametric data. Wilcoxon signed-rank test was performed for the nonparametric data. The data were tested by the Statistical Package of Social Sciences (SPSS v16.0) with statistical significance level set at p < 0.05.
Treatment plan verification
The planning parameters of all plans including beam angles, MLC leaf settings, jaws sizes, and MUs were transferred to the CT images of the ArcCHECK phantom and then recalculated. Finally, the planar dose distributions of the calculated plans were exported from the Eclipse TPS. SNC patient software, version 5 (Sun Nuclear, Melbourne, USA), was used to perform the absolute dose comparisons. Gamma index analysis with the criteria of 3% dose difference (DD) within a distance to agreement (DTA) of 3 mm and 2% ± 2 mm was performed. Gamma index evaluation was done by the global evaluation method.
Results
The ten patients in this study were aged between 49 and 67 years. Table 1 shows the volume range and mean volume of CTV, PTV, hippocampus, and hippocampus avoidance region volume.
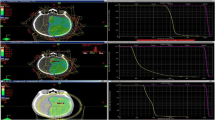
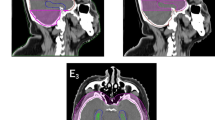
The typical isodose distribution of the 4 arc VMAT and IMRT techniques in the transverse, sagittal, and coronal planes were shown in Figs. 1 and 2 respectively. In comparing the discrepancy between the 90% isodose lines, dose distribution in the VMAT plan was more conformal to the target volume especially in the sagittal plane. The typical plan comparison DVH was shown in Fig. 3.
Plan evaluation parameters
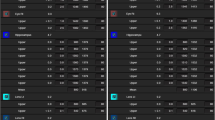
Table 2 shows the summary of the dosimetric results of the target volume. The mean doses and SD for CTV and PTV (= CTV − hippocampus avoidance region) were given over 10 patient data. In RTOG 0933 protocol, dose inhomogeneity was allowed in the target volume as long as ≥ 90% of the target volume receives the prescribed dose. But, as the protocol recommends, the dose received by 2% of PTV was kept below 37.5 Gy. The maximum doses of IMRT plans were higher than those of VMAT plans, and the difference between the two techniques was statistically meaningful. Since, the normalization of plans was made regarding that 90% of PTV receives 30 Gy. There was no statistically meaningful difference between the two techniques in terms of PTV90% doses.
The mean and SD of HI and CI values of ten patients and the statistical analysis between techniques were also shown in Table 2 for IMRT and VMAT plans. The average HI and CI were better for VMAT plans than those for IMRT plans. There was statistically significant difference between the plans in terms of HI and CI (p > 0.05).
Target volume
Organ at risk
All the planning dose constraints were achieved with all the plans. The dosimetric findings for the OAR and hippocampus were reported in Table 3.
The dose constraint suggested by RTOG 0933 for the hippocampus max dose is below 17 Gy and D100% of it should be less than 10 Gy. Although all of the plans met these criteria, the VMAT technique lowers hippocampus doses more than the IMRT technique. And the difference between techniques for both constraints of the hippocampus was statistically meaningful.
Since brainstem, optic nerves, and chiasm were in the PTV, their doses were nearly equal to each other for both techniques. So, there was no statistical difference between techniques for these OARs.
Although both eyes were not in the PTV, there was no significant dose difference between techniques. However, lens doses were lower in IMRT plans than those in VMAT plans. The differences were statistically meaningful for both lenses. The reason could be that although VMAT delivers the doses dynamically during rotation of the gantry around the head, IMRT delivers doses with fixed posterior gantry angles.
Plan delivery efficiency
The mean and SD of MU and BOT values of treatment plans were given in Table 4. VMAT technique had a lower MU and BOT values than the IMRT technique, and the differences were statistically meaningful.
Patient-specific QA results
The mean and SD of patient-specific QA results of treatment plans which were analyzed using two different gamma evaluation criteria were given in Table 5. For 3% ± 3-mm evaluation criteria, there is no statistically meaningful difference between passing rates of patient-specific QA plans. However, for 2% ± 2-mm evaluation criteria, passing rates of IMRT plans were worse than those of VMAT plans and the difference was statistically meaningful.
Discussion
Radiation-induced cognitive impairment is reported to occur in up to 50–90% of adult brain tumor patients [11]. This damage can be reduced by protecting the hippocampus, an important part of the limbic system, during radiotherapy. Redmond et al. [12] prospectively evaluate cognitive function and intracranial failure patterns after hippocampal sparing PCI for limited-stage SCLC. There was no significant decline in performance between baseline and 6 or 12 months for any of the tests. The association between baseline intelligence quotient and change in performance on testing was not significant, but two patients (10%) developed metastasis in the under-dosed region.
A phase II RTOG 0933 study [9] compared 113 patients treated by HS WBRT for brain metastases with previous studies in which patients received WBRT with no avoidance of hippocampus. Forty-two patients were evaluated at 4 months. There was significant reduction (7.0%) in the mean Hopkins Verbal Learning Test–Revised Delayed Recall from baseline to 4 months, compared to the historical control (p < 0.001), with no reduction in quality of life scores.
In this study, IMRT and VMAT techniques were compared in terms of their dosimetric quality, treatment efficiency, and delivery accuracy for HS WBRT. RTOG 0933 recommendations were applied for treatment planning. We found that the VMAT technique was superior in terms of plan quality and hippocampus VMAT plans provide better target volume coverage, homogeneity, conformity, and better hippocampus sparing when compared with IMRT plans. VMAT plans are also the best in terms of treatment efficiency since they require a much smaller number of MUs and thus a shorter treatment time than IMRT plans.
Gondi et al. [13] compared the efficacy of helical tomotherapy and IMRT in the hippocampus sparing. The target volume coverage and OAR sparing were similar between these two techniques. However, the median and maximum doses received by the hippocampus were 5.5 Gy and 12.8 Gy, respectively, for helical tomotherapy and 7.8 and 15.3 Gy, respectively, for IMRT.
Rong et al. [14] evaluated the dosimetric differences among three modalities, IMRT, helical tomotherapy, and VMAT, in delivering HS WBRT. The authors concluded that helical tomotherapy was considered to be the preferred modality for HS WBRT due to its superior dose distribution. When helical tomotherapy was not available or treatment time was a concern, VMAT could provide sufficient dose distribution meeting RTOG 0933 criteria and efficient treatment delivery.
Saad et al. [15] compared dosimetrically IMRT and VMAT techniques in sparing of the hippocampus, OARs, and PTV coverage while treating brain metastasis with WBRT incorporating a simultaneous integrated boost. They found a statistically significant advantage in WB-PTV CI and HI with VMAT, compared with IMRT. Also, they calculated lower hippocampus mean and maximum doses in VMAT than those in IMRT. They concluded that using the WBRT-SIB technique, VMAT showed better PTV coverage with less mean and maximum doses to the hippocampus than IMRT.
The above-mentioned studies are compatible with our study results which showed that VMAT statistically significantly protected the hippocampus compared with IMRT. Using the VMAT technique, D100% of the hippocampus ranged between 975 and 747 cGy, with a mean value for all patients reaching 859 cGy. While using the IMRT technique, the mean dose of the hippocampus ranged between 1074 and 862 cGy, with a mean value for all patients reaching 957 cGy.
Our results also demonstrated statistically significant superiority of the VMAT approach over the IMRT approach, regarding the reduction in the hippocampus maximum point dose which ranged between 1682 and 1418 cGy by VMAT and with a mean value of 1540 cGy. While it ranged between 1824 and 1551 cGy and with a mean value 1685 cGy with IMRT.
Conclusion
Hippocampal sparing whole brain radiotherapy is useful for preventing neurocognitive function loss. VMAT technique allows for sparing of the hippocampus with acceptable target coverage and homogeneity.
References
Hardy J, Smith I, Cherryman G, Vincent M, Judson I, Perren T, Williams M (1990) The value of computed tomographic (CT) scan surveillance in the detection and management of brain metastases in patients with small cell lung cancer. Br J Cancer 62:684–686
Hirsch FR, Paulson OB, Hansen HH et al (1982) Intracranial metastases in small cell carcinoma of the lung. Cancer 50:2433–2437
Schiller JH, Adak S, Cella D, DeVore RF III, Johnson DH (2001) Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593 — a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol 19:2114–2122
Arriagada R, LeChevalier T, Borie F et al (1995) Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 87:183–190
Gregor A, Cull A, Stephens RJ, Kirkpatrick JA, Yarnold JR, Girling DJ, Macbeth FR, Stout R, Machin D (1997) Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. Eur J Cancer 33:1752–1758
Aupérin A, Arriagada R, Pignon JP, le Péchoux C, Gregor A, Stephens RJ, Kristjansen PEG, Johnson BE, Ueoka H, Wagner H, Aisner J (1999) Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med 341:476–484
Meert AP, Paesmans M, Berghmans T, Martin B, Mascaux C, Vallot F, Verdebout JM, Lafitte JJ, Sculier JP (2001) Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer 1:5
Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, Postmus P, Collette L, Musat E, Senan S, EORTC Radiation Oncology Group and Lung Cancer Group (2007) Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 357:664–672
Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, Konski AA, Bauman GS, Shah S, Shi W, Wendland M, Kachnic L, Mehta MP (December 2014) Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 32(34):3810–3816
Paddick I (2000) A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical Note. J Neurosurg 93(Suppl 3):219–222
Crossen JR, Garwood D, Glatstein E, Neuwelt EA (1994) Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 12(3):627–642
Redmond KJ, Hales RK, Anderson-Keightly H, Zhou XC, Kummerlowe M, Sair HI, Duhon M, Kleinberg L, Rosner GL, Vannorsdall T (2017) Prospective study of hippocampal-sparing prophylactic cranial irradiation in limited-stage small cell lung cancer. Int J Radiat Oncol Biol Phys 98(3):603–611
Gondi V, Tolakanahalli R, Mehta MP, Tewatia D, Rowley H, Kuo JS, Khuntia D, Tomé WA (2010) Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator–based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 78:1244–1252
Rong Y, Evans J, Xu-Welliver M, Pickett C, Jia G, Chen Q, Zuo L (2015) Dosimetric evaluation of intensity-modulated radiotherapy, volumetric modulated arc therapy, and helical tomotherapy for hippocampal-avoidance whole brain radiotherapy. PLoS One 10:e0126222
Saad E, Elshahat K, Metwally H (2020) Dosimetric comparison between intensity-modulated radiotherapy and volumetric-modulated arc therapy in hippocampus sparing in brain metastasis treated by whole-brain irradiation and simultaneous integrated boost. J Radiother Pract 19(1):45–51
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Acar, H., Altinok, A.Y. & Doyuran, M. Dosimetric comparison of linac-based modulated techniques for hippocampal sparing whole brain radiotherapy. J Radiat Oncol 9, 123–129 (2020). https://doi.org/10.1007/s13566-020-00430-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-020-00430-2