Abstract
Objective
The objective of the study was to report mature outcomes in patients treated on a prospective feasibility study of helical tomotherapy-based craniospinal irradiation.
Methods
Patients needing craniospinal irradiation were accrued, treated, and followed up longitudinally for survival and toxicity on an institutional review board-approved study.
Results
Twenty patients (median age of 15 years) constituted the study cohort. Tomotherapy-based craniospinal irradiation was well tolerated with self-limiting and reversible acute toxicity. Four (20 %) patients needed growth factor or platelet support during craniospinal irradiation. Significant late neuro-toxicity was seen in only one (5 %) patient. None of the patients developed symptomatic radiation pneumonitis or second new malignancy. At a median follow-up of 62 months (inter-quartile range 24–71 months), the 5-year progression-free survival and overall survival for the entire cohort was 50 and 55 %, respectively. Outcomes within the cohort varied significantly; patients with favorable biology disease had good outcomes while patients with high-risk, metastatic, or recurrent disease fared poorly reflecting inherently aggressive biology.
Conclusions
Helical tomotherapy is an ideal platform for planning, verification, and delivery of supine craniospinal irradiation in clinical practice resulting in moderate, self-limiting, reversible acute toxicity and modest delayed toxicity. Patterns of failure and survival outcomes are largely dependent upon disease biology and are not any different compared to conventional techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Craniospinal irradiation (CSI) is an integral component [1, 2] in the multi-modality management of central nervous system (CNS) tumors with a propensity of neuraxis dissemination via the cerebrospinal fluid pathways such as medulloblastoma, primitive neuro-ectodermal tumor (PNET), atypical teratoid/rhabdoid tumor (AT/RT), pineoblastoma, and germinoma. It is also useful as a salvage treatment for patients with disseminated neuraxial disease [3] such as high-grade ependymoma with drop metastases and primary CNS lymphoma (PCNSL) with leptomeningeal dissemination. CSI remains one of the most technically challenging processes in radiotherapy planning, verification, and delivery due to the need of uniform and homogeneous irradiation of a long and complex shaped target volume. It is associated with several acute toxicities [4] such as anorexia, nausea, vomiting, dysphagia, alopecia, dermatitis, somnolence, and fatigue that are generally mild and self-limiting. Irradiation of a substantial proportion of hematopoetically active red bone marrow during CSI results in moderate acute hematologic toxicity, which at times may lead to unwarranted treatment interruptions and need for supportive interventions [5], particularly if combined with chemotherapy. Late effects of CSI are the major cause of concern [4, 6], more so in growing children, and include neuro-cognitive impairment, hearing loss, growth retardation, hormonal dysfunction, cataract formation, impaired fertility, cardio-pulmonary insufficiency, cerebrovascular accidents, and second malignancies.
Traditionally, CSI had been delivered in the prone position under fluoroscopic guidance with multi-leaf collimator (MLC)-shaped bilateral cranial fields geometrically matched to direct posterior spinal field using couch and collimator rotation [7]. Older children and adults necessitate the use of two adjacent spinal fields with an appropriate gap [7] on the surface to encompass the entire spinal target volume. These junctions are shifted periodically throughout the course of irradiation to feather the dose across the junction. Difficulty in administering anesthesia to young children and relative lack of comfort in the prone position [8, 9] coupled with widespread availability of computed tomographic (CT) simulation heralded the shift to CSI in the supine position [10, 11] in contemporary neuro-oncologic practice. Target volume coverage is more easily assured and delivery more reproducible with supine CSI [9]. Emphasis on calculation of dose-volume parameters of planning target volumes (PTVs) as well as organs at risk (OARs) has led to progressive improvements in supine CSI techniques from simple beam geometry [10, 11] to more conformal approaches such as forward planned segmented technique [12] and inversely planned intensity modulated radiation therapy (IMRT) [13, 14] either using multiple static/dynamic fields or rotational IMRT [15–17]. These modern high-precision techniques, particularly IMRT for CSI, have shown potential for reduction in several late toxicities consequent to reduced doses to normal tissues compared to conventional radiotherapy. There is unequivocal data demonstrating dosimetric superiority of IMRT for CSI [18–21] over conventional techniques both for PTV (in terms of coverage, homogeneity, and conformality) as well as OARs (in terms of mean and maximum doses). Although the moderate (50–80 %) to intermediate (20–50 %) dose envelopes are substantially lesser with IMRT, concerns have been raised regarding increased low-dose (<20 %) spillage [19, 22] and consequentially high integral doses with potential long-term implications.
Helical tomotherapy [23] has emerged as a revolutionary and novel platform of radiation therapy whereby a 6-MV linear accelerator (LA) mounted on a ring gantry continuously rotates around the patient to deliver IMRT treatment in helical mode as the patient translates through the ring, allowing irradiation of large complex volumes without the need for any junctions or abutment dosimetry. An integrated megavoltage CT image-guidance system for verification allows rapid online assessment and correction of set-up errors [24, 25]. These unique features of tomotherapy have been explored by several groups for CSI with promising dosimetric results [18–21]. However, long-term outcome data of tomotherapy-based CSI is relatively sparse and lacking in the indexed medical literature. The purpose of this analysis was to report mature outcome data (disease outcomes as well as toxicity) in a cohort of patients treated on a prospective feasibility study of tomotherapy-based CSI at a major academic center.
Materials and methods
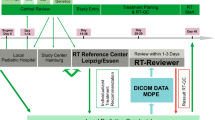
After an appropriate neuro-surgical procedure (biopsy or resection), patients referred for postoperative neuraxial irradiation were accrued on an institutional review board (IRB)-approved prospective feasibility study of tomotherapy-based CSI. Written informed consent was obtained from adult patients (≥18 years), while parents provided consent for children (<18 years). The study was partially funded by a competitive institutional intramural research grant.
CSI planning, verification, and delivery
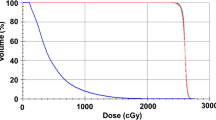
The technique of tomotherapy-based CSI planning, verification, and delivery [19, 25] has been previously described. Briefly, patients were immobilized in the supine position using customized four-point fixation thermoplastic head-neck mask and a rigid knee rest to prevent pelvic rotation. The use of whole-body vacuum cradle was at the discretion of the treating physician. Patients were aligned in the mid-sagittal plane using fluoroscopy guidance before placing external fiducial markers for set-up reference. Planning CT images were acquired using 5-mm slice thickness from vertex till upper thigh and transferred via a network to a contouring workstation for delineation of target volumes and OARs. The clinical target volume (CTV) for the brain included the entire brain and its covering meninges, while CTV for the spine included the entire spinal canal and the exiting nerve roots till the inferior end of the thecal sac (generally S1–S2 junction). PTVs were generated differentially for the brain and spine considering better immobilization of the brain compared to the body. An isotropic margin of 5 mm was applied to the CTV brain to generate the PTV brain, while the CTV spine was uniformly expanded by 8–10 mm to create the PTV spine. OARs included the eyes, lens, parotid glands, thyroid, heart, lungs, esophagus, liver, bowel, kidneys, gonads, rectum, and bladder. In addition, the skull bones, vertebral bodies, and pelvis including bilateral proximal femur were contoured as surrogate for active or red bone marrow. CSI planning was done on a dedicated planning workstation (TomoPlan version 4.2, Tomotherapy Inc, WI, USA). Planning parameters included a fan-beam thickness of 5 cm with a pitch of 0.287 and a modulation factor of 3. Directional blocks were applied for the eyes and kidneys to prevent any beamlets from entering through them. The typical prescription dose for CSI was 35 Gy in 21 fractions for medulloblastoma and 25 Gy in 15 fractions for intracranial germinoma. A typical dose-wash and dose-volume histogram (Fig. 1) of tomotherapy-based CSI shows excellent target volume coverage and sparing of OARs. Treatment was delivered using 6-MV photons on Tomotherapy Hi-ART-II (Tomotherapy Inc, WI, USA). Set-up verification was done daily through megavoltage CT images acquired at three levels, viz the brain (skull-base), thoracic spine (carina), and lumbar spine (pelvis), and co-registered with the planning CT dataset using mutual information exchange algorithm. The auto-fusion was manually fine tuned by clinicians, and translational shifts were recorded at all three levels for all three cardinal directions (lateral, longitudinal, and vertical). Final shifts were applied using a shift calculator program developed in-house taking into account differential CTV to PTV margins from the brain towards spine. Rotational errors though noted during image guidance were disregarded and not corrected.
Mid-sagittal section (a) showing good high-dose (95 % isodose wash-green) and moderate-dose (50 % isodose wash-blue) conformality but increased low-dose spillage (25 % isodose wash-pink) of tomotherapy-based craniospinal irradiation. Directional block achieves good sparing of the eyes (b) while treating the cribriform region adequately. Kidneys are also spared from moderate to high doses (c) using directional block. Typical dose-volume histogram (d) showing excellent coverage of planning target volumes (PTVs) of the brain and spine with sparing of most organs at risk
Other therapy
Ondansetron (4–8 mg per hour orally prior to CSI) was given as anti-emetic prophylaxis during CSI. Rescue dosing was reserved for breakthrough vomiting. Steroids were not prescribed routinely but only for symptoms of raised intracranial pressure. Blood and blood component transfusion and growth factor support were given on physician discretion. After completion of CSI, patients received boost irradiation based upon histology and extent of disease. None of the patients received any chemotherapy concurrently during the course of radiotherapy. However, patients with high-risk disease received sequential adjuvant systemic chemotherapy after radiotherapy. The most common regimen used was six cycles of institutional CNS embryonal tumor (CET) multi-agent systemic chemotherapy protocol comprising of cyclophosphamide (1000 mg/m2 in divided doses on days 1–2) plus vincristine (1.5 mg/m2 on days 1 and 8) alternating with high-dose cisplatin (75 mg/m2 on day 1), cyclophosphamide (1000 mg/m2 in divided doses on days 2–3), and vincristine (1.5 mg/m2 on days 1 and 8) along with appropriate hydration, forced saline dieresis, mesna, and anti-emetic prophylaxis. Therapy at relapse was at the discretion of the treating physician and ranged from best supportive care to salvage chemotherapy (cytotoxic and/or metronomic) and occasionally reirradiation.
Assessments and outcome measures
Patients were reviewed at least once weekly during CSI for documentation of acute toxicity. Acute radiation morbidity including acute hematological toxicity during tomotherapy-based CSI was graded and scored according to Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer (EORTC) toxicity criteria [26]. Initial response assessment magnetic resonance imaging (MRI) of the neuraxis was done 4–6 weeks after completion of all radiotherapy treatment. For patients receiving adjuvant systemic chemotherapy, final response assessment MRI was done 4–6 weeks after completion of the chemotherapy regimen. Patients were required to follow-up at 3-month intervals for the first 2 years, 6-month intervals till 5 years, and annually thereafter. Patients underwent periodic assessment of cognitive function, hearing, and hormonal status using appropriate tests, in addition to annual surveillance MRI on follow-up. Delayed radiation toxicity was graded as per the RTOG/EORTC late morbidity criteria [26]. Disease outcomes were evaluated as progression-free survival (PFS) and overall survival (OS) and reported at 5 years with respective standard errors (±SE). PFS was calculated from date of starting of tomotherapy-based CSI till documented clinico-radiological progression or death. Overall survival was calculated from date of starting tomotherapy-based CSI till death from any cause. Patients without the event of progression or death were censored on date of last follow-up. All time-to-event analyses were done using the product-limit method of Kaplan-Meier. The cutoff date for analysis was specified as 31st March 2015.
Results
From August 2008 till December 2010, 20 patients referred for neuraxial irradiation were accrued on this prospective feasibility study of tomotherapy-based CSI. The study cohort comprised a heterogeneous patient population with significant differences in terms of histology, risk stratification, prior therapy, and prognosis. Table 1 describes the disease and treatment characteristics of the study cohort. The median age of the study cohort was 15 years (range 7–51 years), with males comprising the majority (n = 15). Medulloblastoma (n = 11) was the most common histology followed by intracranial germinoma (n = 5). Sixteen patients had newly diagnosed malignancies (referred for adjuvant therapy after recent neuro-surgery), while four patients had either received prior chemotherapy and/or radiotherapy or developed recurrent disease after first surgery. One of these was a PCNSL with leptomeningeal dissemination treated with high-dose methotrexate and referred for CSI as consolidation therapy. The second patient was a survivor of childhood acute lymphoblastic leukemia (ALL) treated earlier with chemotherapy and whole brain radiotherapy for CNS prophylaxis who 6 years later developed supratentorial PNET and was referred for further adjuvant therapy. The third patient was a case of recurrent medulloblastoma (leptomeningeal dissemination) who had been treated with CSI plus posterior fossa boost at initial diagnosis (4 years ago) and had documented progressive disease after three cycles of multi-agent systemic chemotherapy at first relapse just prior to being considered for craniospinal reirradiation. The fourth patient was a young girl with medulloblastoma who defaulted after first surgery and came back within 4 months with local recurrence and suspected leptomeningeal disease for which she underwent reexcision before being referred for CSI.
Acute toxicity
All 20 patients completed the planned radiotherapy regimen (CSI plus boost if any). Tomotherapy-based CSI was very well tolerated with only 1 (5 %) patient requiring 2 days of interruption due to intractable vomiting that necessitated hospitalization for intravenous fluids and rescue anti-emetics (grade III nausea/vomiting). Vast majority of patients had only grade I–II nausea/vomiting with no need of additional rescue anti-emetics during CSI. Acute radiation dermatitis was generally mild (grade I–II), with no patient developing moist desquamation. No patient developed any significant lower gastro-intestinal toxicity during the course of therapy. Seven of 20 (35 %) patients developed grade III–IV leukopenia/neutropenia while 3 (15 %) patients experienced grade III thrombocytopenia during tomotherapy-based CSI that was generally self-limiting and reversible. Growth factor support was given once during CSI to only 3 (15 %) patients while 1 (5 %) patient needed platelet transfusion. Blood counts started to drop from 2nd week itself and reached their nadir in the 3rd or 4th week, after which myelorecovery started (which generally coincided with completion of CSI or growth factor support/platelet transfusion). Vast majority of patients had achieved complete myelorecovery by the end of boost radiotherapy and prior to initiation of sequential chemotherapy.
Patterns of failure and disease outcomes
The median time to clinico-radiological progression was 20 months (range 2–48 months). The predominant pattern of failure was leptomeningeal dissemination (five of nine relapses), two of which had local recurrence in the tumor bed as well. Isolated local failure at the primary site was seen in three patients (one each of PCNSL, anaplastic medulloblastoma, and supratentorial PNET), while one patient had isolated systemic disease (diffuse bone marrow involvement) with no evidence of disease in the neuraxis confirmed on imaging. The patient with PCNSL had been in complete remission for 4 years following consolidation CSI after which she had developed an intracranial relapse that was salvaged with high-dose methotrexate and cytosine arabinoside followed by whole brain reirradiation. This patient continues to be in sustained remission 2 years after salvage therapy. The child with anaplastic medulloblastoma underwent reexcision of the isolated local relapse followed by further chemotherapy and focal conformal irradiation of the tumor bed. She eventually succumbed to progressive leptomeningeal disease 18 months after first relapse. The child with supratentorial PNET (ALL survivor) developed local recurrence almost a year after tomotherapy-based CSI that could not be salvaged. Nine of 20 (45 %) patients included in the study cohort have died by the time of this analysis, seven due to disease progression and two of unrelated causes (Table 1). Five of nine patients with high-risk, metastatic, or recurrent embryonal tumors (including the patient treated with craniospinal reirradiation) succumbed to progressive disease, while the girl child with recurrent high-risk medulloblastoma died of suspected sepsis due to community-acquired pneumonia nearly 18 months after the last cycle of adjuvant chemotherapy. One child with germinoma died of herpetic encephalitis (unrelated cause). The last patient on the study was initially labeled as average-risk medulloblastoma but presented with leptomeningeal dissemination within 1 month of completion of radiotherapy (CSI plus boost). Histopathology review at that time revealed it to be high-grade primary Burkitt-like lymphoma of the cerebellum. Although he was started on salvage chemotherapy for the same with high-dose methotrexate-based regimen, he succumbed to progressive disease within 6 months of initial diagnosis. At a median follow-up of 62 months (inter-quartile range 24–71 months), the 5-year PFS and OS (with their respective SEs) for the entire cohort was 50 % (±11.2 %) and 55 % (±11.1 %), respectively (Fig. 2). Outcomes within the cohort varied significantly depending upon disease biology. Long-term outcomes were excellent for favorable biology disease with 5-year estimates of PFS and OS of 100 % each for average-risk medulloblastoma and 80 % (±17.9 %) each for localized germinoma (Fig. 3). Five-year PFS and OS was 33.3 % (±15.7 %) each for the high-risk, metastatic, or recurrent medulloblastoma cohort (Fig. 3) reflecting their inherently aggressive and poor biology. The other or miscellaneous group of tumors also fared poorly with a 5-year PFS and OS of 25 % (±21.7 %) and 50 % (±25 %), respectively (Fig. 3).
Late toxicity
Long-term survivors (median follow-up of 70 months) in the study cohort had their share of late toxicities, as expected. However, in the context of multi-modality treatment (given either at initial diagnosis or for salvage), it may be inappropriate to ascribe late toxicity exclusively to any particular component of multi-modality therapy including tomotherapy-based CSI. All surviving children and adolescents (excepting one) had returned to their school or college and were either pursuing or completed higher education with fair scholastic performance. All adults who were working prior to treatment returned to gainful work and were fully socially integrated. New-onset endocrinopathy in pituitary-hypothalamic axis (thyroid, cortisol, growth hormone) was seen in six patients on follow-up that was corrected with appropriate replacement therapy with thyroxine and prednisone. Three long-term survivors (aged <10 years as CSI) had significant growth retardation due to growth hormone deficiency that could not be corrected due to the high cost of growth hormone replacement. Clinically and developmentally significant sensorineural hearing loss (grade II or worse ototoxicity) was documented in five children on serial pure-tone audiometry, which could also partially be ascribed to platinum-based adjuvant chemotherapy. A small asymptomatic intracranial bleed (possibly related to radiation-induced cavernoma) was detected in one long-term survivor on surveillance imaging. Only one long-term survivor (relapsed PCNSL salvaged with further chemotherapy and whole brain reirradiation) developed significant dementia due to therapy-induced leukoencephalopathy (delayed grade IV neuro-toxicity). Although objective testing of cardio-pulmonary insufficiency was not a part of the study, none of the patients (including long-term survivors) developed symptomatic radiation pneumonitis or cardiac dysfunction. Second malignant neoplasm was not detected in any of the patients in the study cohort on follow-up.
Discussion
Technological improvements in radiotherapy planning, verification, and delivery have led to the shift towards CSI in the supine position ensuring easier target volume coverage, enhanced patient comfort, improved reproducibility, and better repositioning accuracy [8, 9, 24]. The promise of exquisite high-dose conformality and excellent OAR sparing has fuelled the use of advanced photon-irradiation techniques such as static multi-field IMRT, dynamic arc IMRT, and rotational IMRT in contemporary radiotherapy practice. Neuraxial irradiation with its large, complex volume and several adjacent normal critical structures that need sparing lends itself automatically to the IMRT paradigm. Although it is feasible to do IMRT for CSI in the supine position on a conventional LA [19, 21], the entire length of the neuraxis cannot be covered even by the maximum field size (40 cm) generally available on standard machines mandating field junctions and inherent uncertainty of abutment dosimetry both for static/dynamic IMRT as well as rotational IMRT. Proton beam therapy with its physical characteristics that includes lack of exit doses was always hailed as the ultimate platform for CSI planning and delivery [27]. The theoretical advantages of protons based on dosimetry and predictive toxicity models are undeniable. Yoon et al. [28] reported the dosimetric benefit of proton-based CSI in comparison to photon-based IMRT techniques (both LA-based and tomotherapy-based CSI). Of note, tomotherapy-based CSI, similar to LA-based IMRT for CSI, showed intermediate doses to several normal tissues compared to proton-based CSI, but significantly increased low-dose bath. Tomotherapy, however, had favorable dose-volume indices for the parotid glands, lenses, and thyroid gland compared to proton-based CSI as well as LA-based techniques. Outcome data of proton-based CSI also demonstrates clinical benefit (although modest at best) compared to photon-based CSI (including tomotherapy). Several authors [29–31] have reported favorable acute toxicity profile (nausea/vomiting and myelotoxicity) as well as reduced late radiation morbidity (mainly ototoxicity and endocrinopathy) with proton-based CSI across all ages (young children, adolescents, and adults). Some concerns have been raised regarding the technical aspects of proton beam therapy including its relative biologic effectiveness (RBE). However, it is reassuring to note that there was no demonstrable relationship between treatment failure (n = 16) and proton therapy technique, proton end of range, linear energy transfer, and RBE in a large cohort of medulloblastoma patients (n = 109) treated with proton-based CSI [32], confirming the safety of such an approach. Recent innovations in proton beam therapy such as spot scanning, intensity modulation, and image guidance have further enhanced its capability of substantially reducing toxicity while preserving quality of life during neuraxial irradiation; however, accessibility and affordability of protons [33] remain a major challenge even in most developed countries today.
Amongst advanced photon-irradiation techniques, helical tomotherapy is ideally suited for supine CSI [15, 19], as it can encompass a length of up to 160 cm in a single plan obviating the need for any junctions. In addition, an integrated megavoltage CT allows daily image guidance for easy set-up verification [24, 25]. The dosimetric superiority of tomotherapy-based CSI over conventional techniques as well as LA-based IMRT has been reported by several groups [18–21]; however, clinical outcome data has been sparse. Table 2 summarizes all studies [34–38] reporting clinical outcomes with tomotherapy-based CSI. The present study reinforces the feasibility of using tomotherapy-based CSI in clinical practice and provides the most mature outcome data of all. All previously published studies of tomotherapy-based CSI have reported only short-term (2–3 years) survival outcomes [34, 37, 38] at a much shorter median follow-up. Compared to them, the survival outcomes of the present study may seem somewhat inferior, but it is pertinent to note that the median follow-up (62 months) is longest for this study cohort that also included a large number of patients with biologically unfavorable and aggressive disease (high risk, metastatic, or recurrent) with inherently poor prognosis. Leptomeningeal dissemination as the predominant pattern of relapse in this study cohort is also a reflection of poor biology and is in accordance with previously published patterns of failure in high-risk medulloblastoma [39, 40]. Patients with good biology disease (average-risk medulloblastoma and germinoma) did very well with excellent long-term survival outcomes. The treatment was well tolerated with no significant interruptions. Despite significant low-dose (<20 %) spillage inherent to tomotherapy-based CSI [19, 22], acute hematologic toxicity was moderate, self-limiting, and reversible with only 20 % patients needing any intervention in the form of blood components or growth factor support. This is in contrast to 50–90 % incidence of grade III or worse acute hematologic toxicity with nearly a third to half requiring supportive care (blood, blood components, growth factors, and/or antibiotics) in most other reported series of tomotherapy-based CSI. The main reason for such high rates of acute hematologic toxicity in other reports was the use of chemotherapy either before tomotherapy-based CSI or sometimes even concurrently. It is well known that pre-irradiation as well as concurrent chemotherapy increases the risk of severe hematologic toxicity [5]. Late radiation morbidity was modest, acceptable, and comparable to previously published data. It was reassuring to note that no one developed a second new malignancy on follow-up, although this could change with longer follow-up.
Caveats and limitations
The major limitation of the study is the inclusion of small number of patients (n = 20) with significant heterogeneity in terms of histopathological diagnosis, risk category, prior exposure to chemotherapy and/or radiotherapy, and expected outcomes. Risk stratification for medulloblastoma was based on traditional clinico-radiological criteria without any attempt for molecular subgrouping. The study included both children and adults, thereby comprising a relatively older cohort (median age of 15 years) of patients than is typically associated with CSI (mostly children and adolescents). Patients with average-risk disease were grossly underrepresented in the study as they were accrued and treated on another parallel study testing hyperfractionated radiation therapy without upfront chemotherapy. Dose prescription and fractionation of tomotherapy-based CSI were somewhat variable and largely depended on histology and prior therapy. Further boost irradiation and use of systemic chemotherapy were also variable and based on the expected patterns of spread and disease biology. Late toxicity though documented longitudinally was largely assessed clinically without any objective, validated assessment tools or instruments. Some patients succumbed to early progressive disease, and many long-term survivors did not comply fully with late toxicity evaluation, precluding robust estimates. Health-related quality of life was not assessed in the study that also did not consider cost-effectiveness as an outcome measure.
Conclusions
Helical tomotherapy is an ideal platform for planning, verification, and delivery of supine CSI in clinical practice obviating the need for any junctions and abutment dosimetry. Tomotherapy-based CSI results in moderate, self-limiting, reversible acute toxicity and modest late toxicity. Patterns of failure and survival outcomes are largely dependent upon disease biology rather than technique and are not any different as compared to conventional CSI. Excellent survival is achieved with tomotherapy-based CSI in patients with good biology disease, while patients with high-risk, metastatic, or recurrent disease have relatively poor outcomes reflecting inherently aggressive and unfavorable biology.
References
Samkari A, Hwang E, Packer RJ (2012) Medulloblastoma/Primitive neuroectodermal tumor and germ cell tumors: the uncommon but potentially curable primary brain tumors. Hematol Oncol Clin North Am 26(4):881–895. doi:10.1016/j.hoc.2012.04.002
McGovern SL, Grosshans D, Mahajan A (2014) Embryonal brain tumors. Cancer J 20(6):397–402. doi:10.1097/PPO.0000000000000081
Wei RL, Nguyen ST, Yang JN, Wolff J, Mahajan A (2012) Salvage craniospinal irradiation with an intensity modulated radiotherapy technique for patients with disseminated neuraxis disease. Practical radiation oncology 2(4):e69–75. doi:10.1016/j.prro.2012.01.004
Fossati P, Ricardi U, Orecchia R (2009) Pediatric medulloblastoma: toxicity of current treatment and potential role of protontherapy. Cancer Treat Rev 35(1):79–96. doi:10.1016/j.ctrv.2008.09.002
Jefferies S, Rajan B, Ashley S, Traish D, Brada M (1998) Haematological toxicity of cranio-spinal irradiation. Radiother Oncol: J Euro Soc Therapeutic Radiology Oncol 48(1):23–27
Christopherson KM, Rotondo RL, Bradley JA, Pincus DW, Wynn TT, Fort JA, Morris CG, Mendenhall NP, Marcus RB Jr, Indelicato DJ (2014) Late toxicity following craniospinal radiation for early-stage medulloblastoma. Acta Oncol 53(4):471–480. doi:10.3109/0284186X.2013.862596
Scott RL (2013) An overview of craniospinal axis fields and field matching. Med Dosim: Off J Am Assoc Med Dosimetrists 38(4):424–429. doi:10.1016/j.meddos.2013.05.005
Hideghety K, Cserhati A, Nagy Z, Varga Z, Fodor E, Vincze V, Szanto E, Maraz A, Thurzo L (2012) A prospective study of supine versus prone positioning and whole-body thermoplastic mask fixation for craniospinal radiotherapy in adult patients. Radiother Oncol: J Euro Soc Therapeutic Radiology Oncol 102(2):214–218. doi:10.1016/j.radonc.2011.07.003
Verma J, Mazloom A, Teh BS, South M, Butler EB, Paulino AC (2015) Comparison of supine and prone craniospinal irradiation in children with medulloblastoma. PRO 5(2):93–98. doi:10.1016/j.prro.2014.05.004
Michalski JM, Klein EE, Gerber R (2002) Method to plan, administer, and verify supine craniospinal irradiation. J Appl Clin Med Phys/ Am College Med Physics 3(4):310–316. doi:10.1120/1.1508013
Parker WA, Freeman CR (2006) A simple technique for craniospinal radiotherapy in the supine position. Radiother Oncol: J Euro Soc Therapeutic Radiology Oncol 78(2):217–222. doi:10.1016/j.radonc.2005.11.009
Wilkinson JM, Lewis J, Lawrence GP, Lucraft HH, Murphy E (2007) Craniospinal irradiation using a forward planned segmented field technique. Br J Radiol 80(951):209–215. doi:10.1259/bjr/61306844
Pai Panandiker A, Ning H, Likhacheva A, Ullman K, Arora B, Ondos J, Karimpour S, Packer R, Miller R, Citrin D (2007) Craniospinal irradiation with spinal IMRT to improve target homogeneity. Int J Radiat Oncol Biol Phys 68(5):1402–1409. doi:10.1016/j.ijrobp.2007.02.037
Parker W, Filion E, Roberge D, Freeman CR (2007) Intensity-modulated radiotherapy for craniospinal irradiation: target volume considerations, dose constraints, and competing risks. Int J Radiat Oncol Biol Phys 69(1):251–257. doi:10.1016/j.ijrobp.2007.04.052
Penagaricano JA, Papanikolaou N, Yan Y, Youssef E, Ratanatharathorn V (2005) Feasibility of cranio-spinal axis radiation with the Hi-Art tomotherapy system. Radiother Oncol: J Euro Soc Therapeutic Radiology Oncol 76(1):72–78. doi:10.1016/j.radonc.2005.06.013
Bedford JL, Lee YK, Saran FH, Warrington AP (2012) Helical volumetric modulated arc therapy for treatment of craniospinal axis. Int J Radiat Oncol Biol Phys 83(3):1047–1054. doi:10.1016/j.ijrobp.2011.07.039
Chen JCC, Atwood TF et al (2012) Volumetric modulated arc therapy planning method for supine craniospinal irradiation. J Radiat Oncol 1:291–297
Kunos CA, Dobbins DC, Kulasekere R, Latimer B, Kinsella TJ (2008) Comparison of helical tomotherapy versus conventional radiation to deliver craniospinal radiation. Technol Cancer Res Treat 7(3):227–233
Sharma DS, Gupta T, Jalali R, Master Z, Phurailatpam RD, Sarin R (2009) High-precision radiotherapy for craniospinal irradiation: evaluation of three-dimensional conformal radiotherapy, intensity-modulated radiation therapy and helical TomoTherapy. Br J Radiol 82(984):1000–1009. doi:10.1259/bjr/13776022
Studenski MT, Shen X, Yu Y, Xiao Y, Shi W, Biswas T, Werner-Wasik M, Harrison AS (2013) Intensity-modulated radiation therapy and volumetric-modulated arc therapy for adult craniospinal irradiation—a comparison with traditional techniques. Med Dosim: Offi J Am Assoc Med Dosimetrists 38(1):48–54. doi:10.1016/j.meddos.2012.05.006
Myers PA, Mavroidis P, Papanikolaou N, Stathakis S (2014) Comparing conformal, arc radiotherapy and helical tomotherapy in craniospinal irradiation planning. J Appl Clin Med Phys/ Am College Med Physics 15(5):4724. doi:10.1120/jacmp.v15i5.4724
Petersson K, Gebre-Medhin M, Ceberg C, Nilsson P, Engstrom P, Knoos T, Kjellen E (2014) Haematological toxicity in adult patients receiving craniospinal irradiation—indication of a dose-bath effect. Radiother Oncol: J Euro Soc Therapeutic Radiology Oncol 111(1):47–51. doi:10.1016/j.radonc.2014.01.020
Welsh JS, Patel RR, Ritter MA, Harari PM, Mackie TR, Mehta MP (2002) Helical tomotherapy: an innovative technology and approach to radiation therapy. Technol Cancer Res Treat 1(4):311–316
Al-Wassia R, Bahig H, Poon E, Parker W, Freeman C (2013) Daily setup uncertainty analysis for craniospinal irradiation using helical tomotherapy. PRO 3(4):349–355. doi:10.1016/j.prro.2012.07.005
Gupta T, Upasani M, Master Z, Patil A, Phurailatpam R, Nojin S, Kannan S, Godasastri J, Jalali R (2015) Assessment of three-dimensional set-up errors using megavoltage computed tomography (MVCT) during image-guided intensity-modulated radiation therapy (IMRT) for craniospinal irradiation (CSI) on helical tomotherapy (HT). Technol Cancer Res Treat 14(1):29–36. doi:10.7785/tcrt.2012.500391
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31(5):1341–1346. doi:10.1016/0360-3016(95)00060-C
Wolden SL (2013) Protons for craniospinal radiation: are clinical data important? Int J Radiat Oncol Biol Phys 87(2):231–232. doi:10.1016/j.ijrobp.2013.05.036
Yoon M, Shin DH, Kim J, Kim JW, Kim DW, Park SY, Lee SB, Kim JY, Park HJ, Park BK, Shin SH (2011) Craniospinal irradiation techniques: a dosimetric comparison of proton beams with standard and advanced photon radiotherapy. Int J Radiat Oncol Biol Phys 81(3):637–646. doi:10.1016/j.ijrobp.2010.06.039
Moeller BJ, Chintagumpala M, Philip JJ, Grosshans DR, McAleer MF, Woo SY, Gidley PW, Vats TS, Mahajan A (2011) Low early ototoxicity rates for pediatric medulloblastoma patients treated with proton radiotherapy. Radiat Oncol 6:58. doi:10.1186/1748-717X-6-58
Jimenez RB, Sethi R, Depauw N, Pulsifer MB, Adams J, McBride SM, Ebb D, Fullerton BC, Tarbell NJ, Yock TI, Macdonald SM (2013) Proton radiation therapy for pediatric medulloblastoma and supratentorial primitive neuroectodermal tumors: outcomes for very young children treated with upfront chemotherapy. Int J Radiat Oncol Biol Phys 87(1):120–126. doi:10.1016/j.ijrobp.2013.05.017
Barney CL, Brown AP, Grosshans DR, McAleer MF, de Groot JF, Puduvalli V, Tucker SL, Crawford CN, Gilbert MR, Brown PD, Mahajan A (2014) Technique, outcomes, and acute toxicities in adults treated with proton beam craniospinal irradiation. Neuro-Oncology 16(2):303–309. doi:10.1093/neuonc/not155
Sethi RV, Giantsoudi D, Raiford M, Malhi I, Niemierko A, Rapalino O, Caruso P, Yock TI, Tarbell NJ, Paganetti H, MacDonald SM (2014) Patterns of failure after proton therapy in medulloblastoma; linear energy transfer distributions and relative biological effectiveness associations for relapses. Int J Radiat Oncol Biol Phys 88(3):655–663. doi:10.1016/j.ijrobp.2013.11.239
Johnstone PA, McMullen KP, Buchsbaum JC, Douglas JG, Helft P (2013) Pediatric CSI: are protons the only ethical approach? Int J Radiat Oncol Biol Phys 87(2):228–230. doi:10.1016/j.ijrobp.2013.05.037
Penagaricano J, Moros E, Corry P, Saylors R, Ratanatharathorn V (2009) Pediatric craniospinal axis irradiation with helical tomotherapy: patient outcome and lack of acute pulmonary toxicity. Int J Radiat Oncol Biol Phys 75(4):1155–1161. doi:10.1016/j.ijrobp.2008.12.083
Sugie C, Shibamoto Y, Ayakawa S, Mimura M, Komai K, Ishii M, Miyamoto A, Oda K (2011) Craniospinal irradiation using helical tomotherapy: evaluation of acute toxicity and dose distribution. Technol Cancer Res Treat 10(2):187–195
Mesbah L, Matute R, Usychkin S, Marrone I, Puebla F, Minguez C, Garcia R, Garcia G, Beltran C, Marsiglia H (2011) Helical tomotherapy in the treatment of pediatric malignancies: a preliminary report of feasibility and acute toxicity. Radiat Oncol 6:102. doi:10.1186/1748-717X-6-102
Lopez Guerra JL, Marrone I, Jaen J, Bruna M, Sole C, Sanchez-Reyes A, Rivin E, Ortiz MJ, Calvo F, Matute R (2014) Outcome and toxicity using helical tomotherapy for craniospinal irradiation in pediatric medulloblastoma. Clin Transl Oncol: Off Pub Federation Spanish Oncol Soc National Cancer Institute Mexico 16(1):96–101. doi:10.1007/s12094-013-1048-7
Qu B, Du L, Huang Y, Yu W, Cai B, Xu S, Ma L (2014) Clinical analysis of intracranial germinoma’s craniospinal irradiation using helical tomotherapy. Chin J Cancer Res 26(3):247–254. doi:10.3978/j.issn.1000-9604.2014.05.02
Skowronska-Gardas A, Chojnacka M, Morawska-Kaczynska M, Perek D, Perek-Polnik M (2007) Patterns of failure in children with medulloblastoma treated with 3D conformal radiotherapy. Radiother Oncol: J Euro Soc Therapeutic Radiology Oncol 84(1):26–33. doi:10.1016/j.radonc.2007.05.018
Lee DS, Cho J, Kim SH, Kim DS, Shim KW, Lyu CJ, Han JW, Suh CO (2014) Patterns of failure following multimodal treatment for medulloblastoma: long-term follow-up results at a single institution. Breast Cancer Res Treat : Off J Korean Cancer Asso. doi:10.4143/crt.2014.067
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was partially funded by a competitive intramural research grant (IRB Project No. 197) provided by Tata Memorial Centre, Mumbai.
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
This study was duly approved by the Institutional Ethics Committee of Tata Memorial Centre, Mumbai. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all patients included in the study.
Rights and permissions
About this article
Cite this article
Gupta, T., Zade, B., Upasani, M. et al. Helical tomotherapy-based craniospinal irradiation: mature outcomes of a prospective feasibility study. J Radiat Oncol 5, 221–230 (2016). https://doi.org/10.1007/s13566-015-0235-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-015-0235-2