Abstract
Bamboos are versatile multipurpose forest products that are so important economically that they are often referred to as “green gold”. With the development of modern gardens, bamboos have become one of the main garden plants because of their unique aesthetic beauty. However, flowering usually causes bamboo to die. To explore the genetic and molecular mechanisms that underlie the transition from the vegetative to reproductive stage in bamboos, two FT-like genes, PvFT1 and PvFT2, were identified in a bamboo species (Phyllostachys violascens). The cDNA sequences of PvFT1 and PvFT2 consisted of 962 bp and 890 bp, respectively, and each encodes 178 amino acids. These two proteins belonged to the PEBP family and contained a Tyr residue that is critical to differentiate FT and TFL1. Like other florigen, PvFT1 is expressed only in the leaves and reached its highest level 20 to 30 days before flowering. It’s transcript is not detected in those plants that never flowered, while PvFT2 mRNA is observed only in spikelet. PvFT1 transcript accumulation was diurnally expressed with a peak at dusk. Constitutive expression of full-length PvFT1 in rice cause early flowering relative to wild-type and in vitro, 50 % of shooting plants displayed structures that resembled floral organs. Our results suggested that PvFT1 might be a candidate gene for florigen and played a role in the induction of bamboo flowering, while PvFT2 might be involved in the development of floral organs. This information could lead to a better understanding of the mechanisms involved in bamboo flowering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In plants, the transition from the vegetative to reproductive stage is a critical developmental switch regulated by both environmental and endogenous factors (Baurle and Dean 2006). A complicated network of signaling pathways controlling the transition was identified in Arabidopsis thaliana. The gibberllin (GA) and autonomous pathways in this species respond to endogenous factors, such as plant age and leaf number, while its photoperiod and vernalization pathways respond to environmental factors, such as day length and temperature (Mouradov et al. 2002).
The FLOWERING LOCUS T (FT) is a floral promoter gene in Arabidopsis, which can integrate signaling pathways, including photoperiod, temperature, vernalization, and light quality. It is expressed in leaves under flowering-inductive conditions, and therefore, it controls flowering (Kardailsky et al. 1999). The Hd3a gene, a FT-homolog, which is identified as a quantitative trait locus for flowering time, is a key activator of flowering in rice (Kojima et al. 2002). RNAi suppression of the FT or Hd3a genes cause late flowering (Corbesier et al. 2007; Komiya et al. 2008; Tamaki et al. 2007). FT protein (florigen) activate transcription of the floral meristem identity gene APETALA1 (AP1) and possibly other related MADS-box genes to promote flowering (Abe et al. 2005; Corbesier et al. 2007; Wigge et al. 2005). FT-like proteins from several different species act in a manner similar to FT with respect to the induction of flowering (Lee et al. 2013; Li and Dubcovsky 2008; Lifschitz et al. 2006; Lin et al. 2007; Navarro et al. 2011; Oda et al. 2012; Tamaki et al. 2007). This general mechanism is likely to be widely conserved in many plants, including Arabidopsis, rice, citrus, tomato, poplar, sunflower, and pea (Blackman et al. 2010; Endo et al.2005; Hecht et al. 2011; Hsu et al. 2006; Kardailsky et al. 1999; Lifschitz et al. 2006). Like Hd3a, RFT1 is another florigen gene. Double RFT1-Hd3a RNAi plants do not flower for up to 300 days after seeding (DAS) under SD or LD conditions (Komiya et al. 2008).
Various bamboo species (Phyllostachys spp.) are very important as economic crops for fibers, bioenergy, ornamentals, and ecosystem uses (Chen et al. 2010; Kumiko et al. 2001; Nath et al. 2009). The production and trade of bamboos in horticulture is increasing rapidly, and sales are rising year after year. P. violascens is one of the economically important bamboo species (Lin et al. 2009). This species is perennial and has the unique attribute of flowering even though it is a member of the grass family (Poaceace). Compared with other plant species, the time of its flowering is unpredictable (Lin et al. 2009; Peng et al. 2013). Different plants in this species may flower at different times during the year but most plants flower between April and May. The duration of flowering could last 2 to 3 months. Based on our observations, the flowering buds began to develop and differentiate 1 to 2 months before its flowering. Some plants died after they flowered, resulting in bamboo forestland degradation, but others did not die after flowering. Interestingly, some flowering plants in this species could flower again the following year but some plants never flowered. Furthermore, some new bamboo plants without leaves did not grow well, but still flowered and died.
In bamboos, several flowering-related genes, such as DlMADS8 and DlMADS18 from the species Dendrocalamus latiflorus, are cloned using homologous cloning (Lin et al. 2009; Tian et al. 2005, 2006; Xu et al. 2010). Both DlMADS8 and DlMADS18 are typical plant MADS-box genes and their individual overexpression in Arabidopsis leads to curled leaves and early flowering. This suggests that the two genes probably play a role in floral meristem determinacy and are involved in controlling the flowering time of D. latiflorus (Tian et al. 2005, 2006). However, the genes that induce flowering in bamboo are not identified. Even though the draft genome of the fast-growing non-timber forest species moso bamboo (P. heterocycla) had been completed (http://202.127.18.221/bamboo/), the FT orthologous gene was not found in 31,987 protein-coding genes (Peng et al. 2013).
In this study, we isolated two FT-like genes, PvFT1 and PvFT2, from a bamboo species (P. violascens), and measured their expression levels at different developmental stages using RT-PCR and real time PCR analysis. Our results demonstrated that PvFT1 might play a role in the induction of flowering and PvFT2 might be involved in the development of floral organs in P. violascens.
Materials and methods
Plant materials
The P. violascens plants for detecting the PvFT expression before flowering and during flowering were transplanted from a village located nearly our campus to the University’s greenhouse. According to our observation, some of the plants transplanted would flower in spring next year. So, the leaves of twenty plants were labeled and sampled at 4:00 pm before flowering and during flowering to determine the expression levels of PvFT1 and PvFT2 until five plants appeared the first spikelet, respectively. The leaves of three naturally blossom growing plants of the same species were also collected to determine the diurnal expression of PvFT1 and PvFT2 genes on 20th April, on that day the day length was about 13hs from five twenty seven to half past eighteen. Samples were taken every 3 h for consecutive eight times daily during the spring. In addition, leaves and spikelet from flowering plants and leaves and vegetative shoots (4–5 cm in length) from non-flowering plants were collected to determine the expression levels of PvFT1 and PvFT2.
Transgenic plants and wild-type (WT) rice (Oryza sativa L.ssp. Japonica (CV. Nipponbare) were cultivated in a paddy field at China National Rice Research Institute (119°57′E, 30 o 03′N) by conventional management.
DNA/RNA isolation
Total genomic DNA was isolated from the collected leaves using the CTAB method (Doyle and Doyle 1987), and total RNA was isolated from the collected leaves, vegetative shoots, and spikelet using the Trizol kit (Invitrogen, USA) based on the manufacturer’s instructions.
Cloning and sequencing of FT-like genes
Two pairs of degenerate primers, PpFTF/PpFTR and HLTOPFTF2/HLTOPFTR1 (Table 1), were created based on the conserved region of FT homologous genes in Gramineae using Primer Premier 5.0 software (Premier, Canada). 5′ RACE and 3′ RACE primers (Table 1) were then designed according to the obtained sequence information. Polymerase chain reaction (PCR) amplification was used to clone PvFT1 and PvFT2. PCR was performed in 20 μl reaction mixture with 100 ng DNA or cDNA, 0.5 μM of each of the forward and reverse primers, 0.2 mM dNTPs, 1 U of TaqDNA polymerase and 1× reaction buffer that included 3 mM MgCl2. The cycling conditions consisted of an initial denaturing step at 94 °C for 5 min, followed by 38 cycles at 94 °C for 30 s, 52–57 °C for 30 s, and 72 °C for 1 min, and a final elongation step at 72 °C for 10 min. The PCR was conducted in a PTC-200TM thermal controller with 96 wells (MJ Research, USA). PCR products were separated by electrophoresis on 1.5 % agarose gels in 1 × TAE buffer and visualized under ultraviolet light after staining with ethidium bromide. The PCR products were purified and recovered using an agarose gel DNA purification and recovery kit (Takara, Japan). The recovered fragments were cloned into the pMD19-T vector (Takara) and finally transformed into Escherichia coli strain DH5α competent cells. The positive colonies were validated by PCR, and then sequenced (Sangon Biological Engineering and Technology and Service, Shanghai, China).
Phylogenetic analysis of FT-like genes
A combination of the FT-like genes from other plant species including rice, maize, barley, and sorghum was used for phylogenetic analysis. A protein sequence alignment was generated using DNAMAN software (Lynnon Biosoft, USA). Phylogenetic analyses were conducted using protein sequences. Maximum likelihood (ML) and neighbor-joining (NJ) methods were used to construct the phylogenetic trees. The NJ tree was built using MEGA4 (Kumar et al. 2004) and the ML phylogenetic tree was built using PHYML version 2.4.3 (Guindon and Gascuel 2003). Bootstraps with 100 replicates were performed to assess node support.
Expression level of FT-like genes
Total RNA (2 μg) was primed with the dT18 primer in a First-Strand cDNA Synthesis Kit (MBI, USA) based on the manufacturer’s instructions. Reverse transcription (RT)-PCR was used to determine the expression levels of PvFT1 and PvFT2. The cDNA was diluted to 20 μl with water, and 2 μl was used for amplification. The amplification conditions were as follows: 5 min at 94 °C, 30 cycles of 30 s at 94 °C, 30 s at 60 °C, and 1 min at 72 °C; followed by 5 min at 72 °C. PCR products were analyzed by 1.5 % agarose gel electrophoresis stained with ethidium bromide. A bamboo actin gene was used as the internal standard. Its PCR amplification conditions were as follows: 94 °C for 4 min, 25 cycles of 94 °C for 50 s, 55 °C for 50 s, and 72 °C for 2 min, and a final extension at 72 °C for 5 min.
Real-time PCR was carried out with respective primers PvFT1-F and PvFT1-R which was designed based on non-conserved region of the PvFT1 using ABI Primer express 3.0. PCR amplification and analysis were carried out using CFX96™ Real-Time PCR Detection System (Bio-Rad) with SYBR Premix Ex Taq™ GC (Perfect Real Time) (Takara). The final volume was 20 μl containing 2 × SYBR Premix Ex Taq GC 10 μl, 10 μM each primer 0.4 μl, cDNA 1.0 μl and ddH2O 8 μl. The relative value of the gene expression was done with 2-ΔΔCt method with Actin as control gene. Three independent replicates were performed for each experiment.
Copy number of transgene
For the copy number estimated, the single copy gene Os8 in rice was used as control gene. The most robust method for copy number determination by real-time PCR is the comparative Ct (2-ΔΔCt) method. The procedure was based on the reference (Ingham et al. 2001)
Vector construction and transformation of rice
The full-length ORF of the PvFT1 cDNA was amplified with forward primer PPFTEF and reverse primer PPFTER and the partial-length ORF with forward primer PpFTE3 containing the restriction enzyme sites KpnI and reverse primer PpFTE4 containing the restriction enzyme sites XbaI. Both PCR products were separately recombined into the pCAMBIA1301 vector containing the cauliflower mosaic virus (CaMV) 35S promoter. The two recombined vectors were confirmed by restriction enzyme digestion and sequencing, and they were separately transformed into the Agrobacterium tumefaciens strain GV3101 by electroporation. The rice (Oryza sativa L.ssp. Japonica (CV. Nipponbare)) transformation was performed according to the Agrobacterium-mediated method described by Hiei et al. (1994). The transgenic plants were confirmed by hygromycin resistant test and PCR.
Results
PvFT1 and PvFT2 are FT homologues
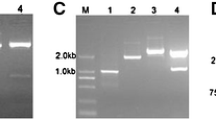
In order to identify the potential FT orthlogues in P. violascens, two pairs of primers, HLTOPFTF2/HLTOPFTR1 and PpFTF/PpFTR (Table 1) were used for PCR amplification. A 345 bp DNA fragment (referred to as PvFT1) was amplified using the primers HLTOPFTF2/HLTOPFTR1, while a 537 bp DNA fragment (referred to as PvFT2) was created using the primers PpFTF/PpFTR. As shown in Fig. 1, their complete coding sequences and 5′ and 3′ UTRs were obtained by 5′ and 3′ RACE with single primers (Table 1). PvFT1 had a 24 bp 5′ UTR and at least two 3′ UTRs with different lengths (Fig. 1 and S1) while PvFT2 had a 36 bp 5′ UTR and a 320 bp 3′ UTR (Fig. S2). The full-length PvFT1 and PvFT2 cDNAs were 962 bp and 893 bp, respectively. A comparison of the genomic sequence to the cDNA sequence showed that both PvFT genes contained four exons and three introns. Like FT homologous genes, their second and third exons were 62 bp and 41 bp in size, respectively (Fig. 1). Both encoded putative proteins of 178 amino acid residues (Fig. 2). Blastp showed that PvFT1 and PvFT2 contained PEBP domain with 91.6 % identity. An amino acid sequence alignment demonstrated that both PvFT1 and PvFT2 shared high homology to Gramineae FT homologues, particularly they shared 85.6 and 87.8 % identities, respectively, to Hd3a of rice (Figs. 2 and 3) and contained the critical amino acid residue Tyr at position 86 in PvFT1 (Fig. 2) and position 85 in PvFT2 (Fig. 2 and S2).
Full-length DNA sequence of the FT-like gene, PvFT1, and its putative amino acid sequence. The initiation codon (ATG) and termination codon (TAGTGA) are boxed. The putative amino acid sequence is shown below the DNA sequence. Sequence code with capital letter indicates exon regions (coding regions). The exon-intron boundaries follow the /GT-AG/ rule. The deletion sequences in other 3′ UTR regions are underlined. An arrowhead marks the key amino acid residue distinguishing FT- and TFL1-type functions
ClustalW multiple alignment of the complete protein sequences of FT family proteins in rice, maize and other grasses(including cymbidium faberi,Lolium perenne, Lolium temulentum, Triticum aestivum, Hordeum vulgare). The PEBP domain boundaries are marked with a broad overline. An arrowhead marks the key amino acid residue distinguishing FT- and TFL1-type functions. All the FT-like proteins have a tyrosine at this position. The 14-amino-acid stretch and the Leu-Tyr-Asn (LYN) triad are boxed
Phylogenetic relationships between FT-like proteins, TFL1-like and MFT-like proteins from rice, barley and other grasses. Numbers such as 51, 60, and 100 represent the percentage bootstrap values (re-sampling). Accession numbers corresponding to the FT proteins are as follows: OsFTL1/FTL (AP002745), OsFTL2/Hd3a (AB052941), OsFTL3/RFT1 (AP007223), OsFTL4 (AC108760), OsFTL5 (AP004124), OsFTL6 (AL662946), OsFTL7 (AL831806), OsFTL8 (AP003105), OsFTL9 (AP003076), OsFTL10 (AC130603), OsFTL11 (AC136448), OsFTL12 (AP003682), OsFTL13 (AP004070), HvFT1 (DQ100327), HvFT2 (DQ297407), hypothetical protein (Sorghum bicolor) (XP_002453364), HvFT3(DQ411319), ZCN8(EU241924), OsMFT1(AK107056), OsMFT2(AP002882), RCN2(AK243046), RCN4(AL662947), RCN1(AAD42895), and RCN3(AAD42896)
In addition, we constructed a phylogenetic tree based on aligned amino acid sequences among FT homologues using the DNAMAN software program. These included 18 rice sequences, three barley sequences, two maize sequences, a sorghum sequence, and our two bamboo sequences (Fig. 3). The phylogenetic analysis showed that these 26 PEBP genes appeared to be grouped into three clusters. The first cluster, named the FT-LIKE subfamily, consisted of 13 rice genes (OsFTL1 to OsFTL13), three barley genes (HvFTL1, HvFTL2 and HvFTL13), one maize gene (ZCN8) and our two bamboo genes (PvFT1 and PvFT2). The second cluster, termed the TFL1-LIKE subfamily, is composed of three rice genes (RCN1 to RCN3) and one maize gene (RCN4). The third cluster, referred to as the MFT-LIKE subfamily, contained two rice genes (OsMFT1 and OsMFT2).
Taken together, our results suggest that PvFT1 and PvFT2 are indeed FT homologues.
PvFT1 and PvFT2 are expressed in different parts of bamboo plants
To determine whether PvFT1 and PvFT2 were involved in the induction of flowering, we measured their expression levels in leaves before and after flowering using RT-PCR analysis and real-time PCR. We selected leaves sampled from three out of five plants that appeared the first spikelet between February 24th and March 6th and tested the expression level of these two genes, respectively. The analysis was repeated twice with similar results. The PvFT1 transcript was detected in the leaves before flowering but it was not detectable after flowering. It was only detectable approximately 20 to 30 days before flowering although its expression level was very low (Fig. 4a and e). We also measured its level in the leaves of plants that were going to flower and plants that never flowered. We found that PvFT1 expressed only in the plants that would flower (Fig. 4b). That PvFT1 were expressed higher in Fig. 4b than Fig. 4a was because the sample used in Fig. 4a was from the plant which had little spikelet, and the sample used in Fig. 4b was from another plant that was in blossom and had lots of spikelet. Unlike PvFT1, PvFT2 could not be detectable in leaves before or after flowering even when the expression of PvFT1 reached its highest level (Fig. 4c). Our results suggest that PvFT1 may be a candidate gene for florigen and plays a role in the induction of bamboo flowering.
RT-PCR analysis of expression levels of the two FT-like PvFT genes. The analysis was repeated twice with similar results. a and e Expression of PvFT-1 before and after flowering. a The arrow indicates the target band. A bamboo Actin gene was used as an internal control. The flowering time of the plant was between 24 February and 6 March. e Real-time PCR was performed using PvFT-1 or Actin-specific primers. Error bars represent SD. The number of biological replicates was three. b Expression of PvFT-1 and PvFT-2 genes in different tissue. UL and US: leaves and vegetative shoots from a plant that did not flower until 60 days after sampling; FL and FS: leaves and spikelets from a plant that is flowering. c Expression of PvFT-1 and PvF-2 genes in leaves at 5:30 pm d Diurnal expression of PvFT-1 under natural conditions. PvFT-1 shows diurnal expression patterns with a peak at dusk. The sun set arise at 5:27. The open and filled bars at the bottom represent the light and dark periods, respectively
To test if PvFT1’s expression pattern was also diurnal, we measured its level at different time points during the day. As shown in Fig. 4d, its maximum expression occurred near 5:30 pm, and its expression exhibited the diurnal pattern. Therefore, our data supported PvFT1 may act as a candidate for florigen.
While PvFT2 did not express in leaves, it might still be involved in the development of floral organs. To determine this hypothesis, we measured its level in spikelet using RT-PCR analysis and found that PvFT2 was expressed in the spikelet (Fig. 4b). In addition, we measured the level of PvFT1 in the spikelet to determine if it had an additional role in the development of floral organs. Unlike PvFT2, PvFT1 did not express in the spikelet (Fig. 4b).
Taken together, our results demonstrate that PvFT1 may be a candidate for florigen to induce the flowering of bamboo while PvFT2 is involved in the development of floral organs. This provides information that will lead to a better understanding of the mechanisms of bamboo flowering.
Constitutive expression of PvFT1 causes early flowering
To explore the function of PvFT1 in the timing of flowering, a CaMV 35S∷PvFT1 overexpression vector containing the full-length PvFT1 gene ORF (Fig. 5a) and a CaMV 35S∷PvFT1Δ5′ 102 bp overexpression vector containing a partial-length PvFT1 gene ORF with a 102 bp deletion at the 5′ terminal (Fig. 5b) were separately transformed into the calli of rice through Agrobacterium tumefaciens-mediated transformation and were introduced into the genome of the rice, Oryza sative L. japonica.cv. Nipponbare, separately (Fig. 5c and d). When transformed calli (containing the full-length PvFT1 gene) were transferred onto shoot differentiation media, less than 12 % developed into plants. The rate of differentiation of PvFT1Δ5-transgenics was 98 %. Transgene expression examinations by RT-PCR showed that compared with WT, transcript level was increased in all tested leaves of transgenic plants (Fig. 5e and f). We transferred 43 independent lines containing the full-length PvFT1 gene from the test tubes into the greenhouse and observed that 50 % of these plants displayed structures that resembled floral organs (Fig. 5g). Unfortunately, all but five of these plants eventually died. Then, five independent transgenic plants (T0) containing the full-length PvFT1 construct were planted in a paddy field and only three lines survived. Sixty-eight T1 plants of the line R5 were used for genetic analysis. Fifty transgenic plants and 18 wild type individuals fitted to 3:1 segregation ratio(X 2 = 0.02 < X2 0.05 = 3.84) and thus a predicted single locus insertion of 35S::PvFT1. Then, line R5 copy number was estimated by real-time PCR (Table 2). Those 50 transgenic plants were further examined with regard to flowering time. Meanwhile, we obtained 45 T0 PvFT1Δ5 independent lines. After determining the genetic characterization of transgenic plant line 78 T1 generation, which also fitted to 3:1 segregation ratio(X 2 = 0.06 < X2 0.05 = 3.84), those 59 T1 positive transgenic plants were also examined for flowering time. PvFT1-ox plants flowered early by 6-10 day relative to PvFT1Δ5′ 102 bp-ox plants and wild type plants (Figs. 5h and 6). A t-test confirmed that the flowering time was different between PvFT1-ox plants and wild type plants (p = 0.038) and between PvFT1-ox plants and PvFT1Δ5′ 102 bp-ox plants (p = 0.041)but not between PvFT1Δ5′ 102 bp-ox plants and wild type plants (p = 0.053). These results suggested that the full-length PvFT1 protein might promote early flowering, but the partial-length PvFT1 gene (deleting the 5′ 102 bp) did not function during the transition from vegetable to reproductive phase.
Phenotype of CaMV 35S::PvFT1 plants compared with wild-type (WT) plants. (a) and (b) Schematic diagrams of the full-length PvFT1 (a) and the partial-length PvFT1Δ5′102 bp (b) sense constructs. CaMV 35S: cauliflower mosaic virus 35S promoter; Tnos: nos terminator. (c) and (d) PCR analyses using specific-primers for full length PvFT1 gene (c) and partial-length PvFT1Δ5′102 bp fragment (d). (e) and (f) RT-PCR analyses of transgenes, full length PvFT1 gene (e) and partial-length PvFT1Δ5′102 bp(f). The Actin gene was used as an internal control. (g) and (h). Phenotype of transgenic plants (full-length PvFT1) compared with WT. Regenerated shootings (T0) with panicle just transplanted from test tubes. Arrows indicate panicles emerging at the heading stage (g). Plants (T1) were cultivated in a paddy field. Arrows indicate panicles emerging at the heading stage (h)
Discussion
Bamboos play important roles in economic and ecological systems. They can be used as a material for making paper and their shoots can be used as food. With the development of the modern garden, bamboos have also become one of the main urban garden plants. However, once they flower they often die, and the timing of their flowering is unpredictable. To date, no bamboo flowering mutant has been identified and the mechanism of bamboo flowering remains unknown. Thus, the genes involved in bamboo flowering were usually obtained using homologous cloning (Lin et al. 2009; Tian et al. 2005, 2006; Xu et al. 2010). In this study, we identified two FT-like homologues, PvFT1 and PvFT2, in P. violascens, which may regulate flowering. Our findings provide information that will lead to a better understanding of the mechanisms of bamboo flowering.
PvFT1 and PvFT2 are FT-like homologues
Flowering-related genes in bamboos had been identified, such as DlMADS8 and DlMADS18 from Dendrocalamus latiflorus (Tian et al. 2005, 2006), PpMADS1 and PpMADS2 from P. violascens (Lin et al. 2009), and DlEMF2 from D. latiflorus (Xu et al. 2010). Recently, we identified several flower-specific unigenes in Bambusa oldhamii using large-scale analysis of expressed sequence tags (Lin et al. 2010). However, no genes involved in the induction of flowering of bamboos were reported. In this study, we identified PvFT1, which likely acts as a candidate gene for florigen and is involved in the induction of flowering in P. violascens.
PvFT1 shared similar structure with the other FT-like genes. The FT-like genes have well-conserved structure, although some variation was found in which the first and fourth exon were large while the second and third exons were small (Chardon and Damerval 2005). Our data demonstrated that the four exons of PvFT1 shared the length-based structure of FT-like genes.
Unlike PvFT1, PvFT2 was not an inducer of flowering in P. violascens but it had a role in floral organ development. Like PvFT1, it shared the structural similarity with the other FT-like genes.
Two members of the PEBP gene family, FT and TFL1, promoted and inhibited flowering in Arabidopsis, respectively, but both shared similar gene structures and protein sequences (Hanzawa et al. 2005). Their opposite functions are due to a different amino acid residue at position 83, Tyr for FT and His for TFL1 (Ahn et al. 2006). Based on Blastp analysis, PvFT1 and PvFT2 shared the amino acid Tyr at position 86 and 85 with the FT-like proteins, respectively. In addition, PvFT1 and PvFT2 also shared with the FT-like proteins the 14-amino-acid stretch and the Leu-Tyr-Asn triad, which are used to distinguish FT-like from the TFL1 (Ahn et al. 2006). Furthermore, our data demonstrated that PvFT1 and PvFT2 were FT-like proteins based on phylogenetic analyses.
PvFT1 and PvFT2 are expressed in different parts of bamboo plants
In Arabidopsis, the expression level of FT in WT increased with plant age under both LD and SD conditions (Kardailsky et al. 1999). In rice, two florigen genes, Hd3a and RFT1, were important for flowering under flowering-induction conditions, and the transcript levels of both genes peaked 30 days before flowering (Komiya et al. 2008). A FT-like gene in poplar, PtFT1, increased in expression level with plant age until the initiation of flowering (Bo¨hlenius et al. 2006). Our data (Fig. 4a) showed that PvFT1 expressed maximally 20 to 30 days in leaves before flowering, but that the expression level was very low. Thus, it was not found in Dendrocalamus latiflorus (Zhang et al. 2012) and in Phyllostachys violascens (private communication) using transcriptome sequencing. Like other FT-like homologues, PvFT1 may play a role in inducing flowering in P. violascens.
FT and PtFT1 exhibited diurnal expression patterns with a peak in the beginning of night under LD conditions (Bo¨hlenius et al. 2006). We found that PvFT1 expressed in a similar pattern with FT and PtFT1 under natural light (Fig. 4d). FT, Hd3a and PtFT1 were observed to express only in leaves (Bo¨hlenius et al. 2006; Kardailsky et al. 1999; Kojima et al. 2002). Like those florigen, PvFT1 was also expressed only in leaves. This is consistent with the observation that florigen are produced in leaves and then act in shoot apical meristem (SAM) (Corbesier et al. 2007; Jaeger and Wigge 2007; Mathieu et al. 2007).
Unlike FT, Hd3a, PtFT1, and PvFT1, PvFT2 was expressed only in spikelet. In rice, a FT-like homolog, FTL1/FTL, was also found to express in spikelet rather than in leaves (Komiya et al. 2008). Thus, PvFT1 and PvFT2 may have different physiological functions in the initiation and development of flowering in bamboos.
Constitutive expression of PvFT1 gene strongly affects the development of rice
The constitutive expression of most FT-like genes could result in earlier flowering in plants. Like other FT-like proteins, the PvFT1 full-length protein contained a PEBP conserved domain (from the 29th aa to the 168th aa) that represented 80 % of the coding sequence. To test the function of the PvFT1 gene, we separately transformed CaMV 35S∷PvFT1 and CaMV 35S∷PvFT1Δ5′ 102 bp recombinant vector into the calli of rice. To our surprise, the constitutive expression of the full-length PvFT1 gene strongly inhibited rice calli differentiation, resulting in a long differentiation period and a low differentiation rate. Furthermore, all of plantlets grew weakly with slender leaves and none rooted. 50 % of the plantlets showed floral organs, and only four plantlets survived. Transgenic T1 plants flowered about 10 day earlier than WT plants. In rice, the overexpression of Hd3a caused extremely early heading and resulted in few spikelet because of insufficient vegetative growth (Kojima et al. 2002). However, those T1 plants that constitutively expressed the partial-length PvFT1 gene with a 102 bp 5′-terminal deletion showed similar flowering times with WT plants. These results suggested that the 34 N-terminal amino acids (encoded by the 5′ 102 bp) of the PvFT1 protein may play important roles in the proteins behavior. In Arabidopsis, to initiate floral transcription in the shoot apex, the FT protein must translocate into the nucleus and then interact with the FD protein, a bZIP transcription factor, which localizes in the nucleus to regulate meristem identity genes, such as AP1 (Abe et al.2005). In rice, the Hd3a protein interacts with the GF14c protein, which mainly localizes in the cytoplasm, and lead to a mainly cytoplasmic Hd3a- GF14c protein complex. This complex inhibits the shuttling of Hd3a from the cytoplasm into the nucleus, which delays flowering (Purwestri et al. 2009; Taoka et al. 2011). Those results hinted that the 34 N-terminal amino acids may be important for translocating the PvFT1 protein from the cytoplasm into the nucleus even though nuclear localization signal (NLS) was not found. The full-length PvFT1 protein can move into nucleus, interact with proteins downstream, and inhibit development. However, the partial-length PvFT1 protein (a 34 aa N-terminal deletion) cannot transport into the nucleus and; therefore, does not exert nuclear action. In fact, both FT protein in Arabidopsis and Hd3a in rice have no classical NLS sequence based on their amino acid sequence. How these two proteins transport from cytoplasm into nucleus remain unknown.
In maize, the FT-Like ZCN8 gene functions as a floral activator (Meng et al. 2011). Constitutive expression of the gene driven by the Ubi promoter inhibited differentiation of calli (Danilevskaya et al. 2010). However, ectopic expression of ZCN8 driven by tissue-specific promoters, Pro ZMM4 or ProZM-ADF4, resulted in earlier flowering and fewer leaves in T1 progeny than those of WT (Meng et al. 2011). Ectopic expression of both FT and Hd3a driven by phloem-specific promoters induced early flowering in transgenic Arabidopsis and rice, respectively (An et al. 2004; Tamaki et al. 2007). Our studies demonstrated that the constitutive expression of PvFT1 inhibited receptor rice development and led them to flower in test tubes. The ectopic expression of PvFT1 driven by phloem- or tissue-specific promoters remains to be studied.
Abbreviations
- FT:
-
Flowering locus T
- PEBP:
-
Phosphatidylethanolamine-binding protein
- Hd3a:
-
Heading date 3a
- AP1 :
-
APETALA1
- RFT1:
-
Rice flowering locus T1
References
Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309:1052–1056
Ahn JH, Miller D, Winter VJ, Banfield MJ, Lee JH, Yoo SY, Henz SR, Brady RL, Weigel D (2006) A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J 25:605–614
An HL, Roussot C, Suarez-Lopez P, Corbesier L, Vincent C, Pineiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, Coupland G (2004) CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131:3615–3626
Baurle I, Dean C (2006) The timing of developmental transitions in plants. Cell 125:655–664
Blackman BK, Strasburg JL, Raduski AR, Michaels SD, Rieseberg LH (2010) The role of recently derived FT paralogs in sunflower domestication. Curr Biol 20:629–635
Bo¨ hlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 19:1040–1043
Chardon F, Damerval C (2005) Phylogenomic analysis of the PEBP gene family in cereals. J Mol Evol 61:579–590
Chen CY, Hsieh MH, Yang CC, Lin CS, Wang AY (2010) Analysis of the cellulose synthase genes associated with primary cell wall synthesis in Bambusa oldhamii. Phytochemistry 71:1270–1279
Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316:1030–1033
Danilevskaya ON, Meng X, Ananiev EV (2010) Concerted modification of flowering time and inflorescence architecture by ectopic expression of TFL1-like genes in maize. Plant Physiol 153:238–251
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Endo T, Shimada T, Fujii H, Kobayashi Y, Araki T, Omura M (2005) Ectopic expression of an FT homolog from Citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L.Raf.). Transgenic Res 14:703–712
Guindon S, Guindon O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Hanzawa Y, Money T, Bradley D (2005) A single amino acid converts a repressor to an activator of flowering. Proc Natl Acad Sci U S A 102:7748–7753
Hecht VR, Laurie RE, Schoor JKV, Ridge S, Knowles CL, Liew LC, Sussmilch FC, Murfet IC, Macknight RC, Weller LJ (2011) The pea GIGAS gene is a FLOWERING LOCUS T homolog necessary for graft-transmissible specification of flowering but not for responsiveness to photoperiod. Plant Cell 23:147–161
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hsu CY, Liu Y, Luthe DS, Yuceer C (2006) Poplar FT2 shortens the juvenile phase and promotes seasonal flowering in poplar trees. Plant Cell 18:1846–1861
Ingham DJ, Beer S, Money S, Hansen G (2001) Quantitative real time PCR assay for determining transgene copy number in transformed plants. Biotechniques 31:132–140
Jaeger KE, Wigge PA (2007) FT protein acts as a long-range signal in Arabidopsis. Curr Biol 17:1050–1054
Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D (1999) Activation tagging of the floral inducer FT. Science 286:1962–1965
Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M (2002) Hd3a, a rice ortholog of the arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43:1096–1105
Komiya R, Ikegami A, Tamaki S, Yokoi S, Shimamoto K (2008) Hd3a and RFT1 are essential for flowering in rice. Development 135:767–774
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Kumiko M, Hidefumi Y, Masaaki Y, Kinji T, Hiroaki Y (2001) Manufacture and properties of fiberboard made from moso bamboo. J Jpn Wood Res Soc 47:111–119
Lee R, Baldwin S, Kenel F, McCallum J, Macknight R (2013) FLOWERING LOCUS T genes control onion bulb formation and flowering. Nat Commun 4:2884. doi:10.1038/ncomms3884
Li C, Dubcovsky J (2008) Wheat FT protein regulates VRN1 transcription through interactions with FDL2. Plant J 55:543–554
Lifschitz E, Eviatar T, Rozman A, Shalit A, Goldshmidt A, Amsellem Z, Alvarez JP, Eshed Y (2006) The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc Natl Acad Sci U S A 103:6398–6403
Lin M, Belanger H, Lee Y, Varkonyi-Gasic E, Taoka K, Miura E, Xoconostle-Cázares B, Gendler K, Jorgensen RA, Phinney B (2007) FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the cucurbits. Plant Cell 19:1488–1506
Lin EP, Peng HZ, Jin QY, Deng MJ, Li T, Xiao XC, Hua XQ, Wang KH, Bian HW, Han N, Zhu MY (2009) Identification and characterization of two Bamboo (Phyllostachys praecox) AP1/SQUA-like MADS-box genes during floral transition. Planta 231:109–120
Lin XC, Chow TY, Chen HH, Liu CC, Chou SJ, Huang BL, Kuo CI, Wen CK, Huang LC, Fang W (2010) Understanding bamboo flowering based on large-scale analysis of expressed sequence tags. Genet Mol Res 9:1085–1093
Mathieu J, Warthmann N, Küttner F, Schmid M (2007) Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol 17:1055–1060
Meng X, Muszynski MG, Danilevskaya ON (2011) The FT-Like ZCN8 gene functions as a floral activator and is involved in photoperiod sensitivity in maize. Plant Cell 23:942–960
Mouradov A, Cremer F, Coupland G (2002) Control of flowering time: interacting pathways as a basis for diversity. Plant Cell 14:S111–S130
Nath AJ, Das G, Das AK (2009) Above ground standing biomass and carbon storage in village bamboos in North East India. Biomass Bioenergy 33:1188–1196
Navarro C, Abelenda JA, Cruz-Oro’ E, Cue’llar CA, Tamaki S, Silva J, Shimamoto K, Prat S (2011) Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 48:119–123
Oda A, Narumi T, Li T, Kando T, Higuchi Y, Sumitomo K, Fukai S, Hisamatsu T (2012) CsFTL3, a chrysanthemum FLOWERING LOCUS T-like gene, is a key regulator of photoperiodic lowering in chrysanthemums. J Exp Bot 63:1461–1477
Peng ZH, Lu Y, Li LB, ZhaoQ FQ, Gao ZM, Lu HY, HuT YN, Liu KY, Li Y, Fan DL, Guo YL, Li WJ, Lu YQ, Weng QJ, Zhou CC, Zhang L, Huang T, Zhao Y, Zhu CR, Liu XG, Yang XW, Wang T, Miao K, Zhuang CY, Cao XL, Tang WL, Liu GS, Liu YL, Chen J, Liu ZJ, Yuan LC, Liu ZH, Huang XH, Lu TT, Fei BH, Ning ZM, Han B, Jiang ZH (2013) The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat Genet 45:456–461
Purwestri YA, Ogaki Y, Tamaki S, Tsuji H, Shimamoto K (2009) the 14-3-3 protein GF14c acts as a negative regulator of flowering in rice by interacting with the florigen Hd3a. Plant Cell Physiol 50:429–438
Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K (2007) Hd3a protein is mobile flowering signal in rice. Science 316:1033–1036
Taoka K, Ohki I, Tsuji H, Furuita K, Hayashi K, Yanase T, Yamaguchi M, Nakashima C, Purwestri YA, Tamaki S, Ogaki Y, Shimada C, Nakagawa A, Kojima C, Shimamoto K (2011) 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 476:332–337
Tian B, Chen Y, Yan Y, Li DZ (2005) Isolation and ectopic expression of a bamboo MADS-box gene. Chin Sci Bull 50:217–224
Tian B, Chen Y, Li DZ, Yan Y (2006) Cloning and characterization of a bamboo Leafy Hull Sterile1 homologous gene. DNA Seq 17:143–151
Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309:1056–1059
Xu H, Chen LJ, Qu LJ, Gu HY, Li DZ (2010) Functional conservation of the plant EMBRYONIC FLOWER2 gene between bamboo and Arabidopsis. Biotechnol Lett 32:1961–1968
Zhang XM, Zhao L, Larson-Rabin Z, Li DZ, Guo ZH (2012) De Novo sequencing and characterization of the floral transcriptome of Dendrocalamus latiflorus (Poaceae: Bambusoideae). PLoS ONE 7, e42082
Acknowledgments
The authors thank two anonymous reviewers for critical reading and also thank Dr. Xuerong Shi for his critical reading of the manuscript. This work was supported by grant from the National Natural Science Foundation of China (grant no. 30901155), grant from the National Program on Key Basic Research Project (973 Program) (2012CB723008) and grant of Natural Science Foundation of Zhejiang Province (grant no. Y307499).
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiaoqin Guo and Yi Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, X., Wang, Y., Wang, Q. et al. Molecular characterization of FLOWERING LOCUS T(FT)genes from bamboo (Phyllostachys violascens). J. Plant Biochem. Biotechnol. 25, 168–178 (2016). https://doi.org/10.1007/s13562-015-0322-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-015-0322-x