Abstract
Purpose
Meditation is renowned for its positive effects on cognitive abilities and stress reduction. It has been reported that the amplitude of electroencephalographic (EEG) infra-slow activity (ISA, < 0.1 Hz) is reduced as the stress level decreases. Consequently, we aimed to determine if EEG ISA amplitude decreases as a result of meditation practice across various traditions.
Methods
To this end, we analyzed an open dataset comprising EEG data acquired during meditation sessions from experienced practitioners in the Vipassana tradition—which integrates elements of focused attention and open monitoring, akin to mindfulness meditation—and in the Himalayan Yoga and Isha Shoonya traditions, which emphasize focused attention and open monitoring, respectively.
Results
A general trend was observed where EEG ISA amplitude tended to decrease in experienced meditators from these traditions compared to novices, particularly significant in the 0.03–0.08 Hz band for Vipassana meditators. Therefore, our analysis focused on this ISA frequency band. Specifically, a notable decrease in EEG ISA amplitude was observed in Vipassana meditators, predominantly in the left-frontal region. This reduction in EEG ISA amplitude was also accompanied by a decrease in phase-amplitude coupling (PAC) between the ISA phase and alpha band (8–12 Hz) amplitude, which implied decreased neural excitability fluctuations.
Conclusion
Our findings suggest that not only does EEG ISA amplitude decrease in experienced meditators from traditions that incorporate both focused attention and open monitoring, but this decrease may also signify a diminished influence of neural excitability fluctuations attributed to ISA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Infra-slow activity (ISA) of electroencephalogram (EEG), referring to EEG oscillations below 0.1 Hz, has been studied since the 1950s [1]. Essentially, the EEG ISA is considered to be associated with a fluctuation in neural excitability where neural excitability refers to the degree of net neural activity over the whole brain [2]. Neural excitability, which often fluctuates at infra-slow frequencies, has been shown to represent arousal variation. For example, the phase of the EEG ISA not only correlates with the amplitude fluctuation of fast oscillations (> 1 Hz) [3, 4] and muscle activity [5], but also with galvanic skin responses representing the arousal level [6]. Moreover, studies have revealed that behavioral performance of the somatosensory discrimination task [4] and the occurrence of different behaviors during playing the video game [7] vary with the phase of the EEG ISA.
The amplitude of the ISA oscillation is also related to physiological states and behavioral outcomes. The stress level [8] and reaction time (RT) in sensory discrimination tasks [9] increase as the amplitude of the ISA increases. Such modulation of the stress level and behavioral performance can also be seen as the effect of mindfulness meditation. Studies have indicated that mindfulness meditation can lower the stress level [10, 11; for a review, see 12] and improve cognitive functions [13]. Therefore, we can expect that the amplitude of the ISA would be correlated with the meditation effect. More specifically, we can expect that meditation would lower the amplitude of the ISA. Many meditation traditions can be organized into focused attention and open monitoring types [14], and mindfulness meditation includes both types of practice [15]. Therefore, it is conceivable that meditation traditions that include both types of practice may lead to decreases in the ISA amplitude.
Regarding relations of mediation with EEG, many studies have investigated mediation-induced modulation of the amplitude of relatively fast EEG oscillations over 1 Hz, such as alpha or theta oscillations, in both experienced and novice meditators. In novice meditators, the amplitude of alpha oscillations increased during meditation compared to mind wandering, while that of theta oscillations decreased [16]. In contrast, the amplitude of alpha oscillations decreased during meditation in experienced meditators [17]. Moreover, amplitude changes in fast oscillations vary depending on the tradition of meditation. The amplitude of alpha oscillations in experienced Vipassana meditators was found to be greater than that of experienced meditators in the Himalayan Yoga and Isha Shoonya traditions, as well as the novice control group [18].
Yet, few studies have explored the relationship between meditation and the EEG ISA. A study analyzed the amplitude of the EEG ISA during meditation, but did not investigate how it differed between experienced and novice meditators [19]. Another study compared the phase synchronization of the EEG ISA between experienced and novice meditators, showing that the phase synchronization was greater in novice than experienced meditators, but did not investigate the amplitude of the ISA [20].
Nonetheless, it would be worthy to study changes in the amplitude of the ISA by meditation. Knowing the relationship between meditation and the EEG ISA will extend EEG correlates of meditation to a wider frequency band of oscillations, enriching the neurophysiological basis of meditation. Also, the EEG ISA features reflecting the state of meditation can offer a new index to be used for the development of a neurofeedback system for meditation, as neurofeedback studies using the EEG ISA have recently increased [21,22,23].
Therefore, we aimed to study a relationship between the amplitude of the ISA and meditation experiences. We hypothesized that the amplitude of the ISA in experienced meditators would be lower than that in novice meditators. Reduced stress levels and improved behavioral performance observed in experienced meditators [10, 11, 13] are associated with decreases in the amplitude of the ISA [8, 9]. In addition, the amplitude of blood-oxygen-level-dependent (BOLD) signals, which is known to correlate with the amplitude of the EEG ISA [24, 25], is modified in experienced meditators [26].
If, as per our hypothesis, the amplitude of the EEG ISA diminishes in experienced meditators, we further aimed to explore whether the effect of meditation on the EEG ISA would also be manifested in a relationship between the phase of the EEG ISA and neural excitability fluctuations [3,4,5,6]. It has been shown that the amplitude envelop of the alpha oscillations can represent neural excitability [27,28,29]. Thus, we computed phase-amplitude coupling (PAC) between the phase of the EEG ISA and the amplitude envelop of the alpha oscillations. Then, we examined if PAC differed between experienced and novice meditators.
Here, we explored changes in the amplitude of the ISA in experienced meditators compared to novice meditators using an open EEG dataset [30]. Given that differences exist between meditation traditions in alpha oscillations, we analyzed the EEG ISAs of experienced meditators from the Himalayan Yoga, Vipassana, and Isha Shoonya traditions. The Vipassana meditation tradition in this dataset follows the teachings of S. N. Goenka and, like mindfulness meditation, includes both focused attention and open monitoring practices. Therefore, the Vipassana tradition, which includes both types of practices taught by S. N. Goenka, such as mindfulness meditation, is suitable for studying ISA amplitude reduction. Himalayan Yoga and Isha Shoonya traditions underscore different characteristics of meditation such as focused attention and open monitoring, respectively [18].
2 Materials and methods
2.1 Dataset
We utilized an open EEG dataset recorded during a meditation and mind wandering experiment. Participants in the experiment belonged to one of the three experimental groups and one control group. Experienced meditators from the Himalayan Yoga (N = 24, 2 females, age: 49 ± 13 years old), Vipassana, as taught by S. N. Goenka, (N = 19, 5 females, age: 47 ± 15 years old), and Isha Shoonya (N = 20, 2 females, age: 40 ± 10 years old) traditions were divided into the three experimental groups. The control group (N = 31, 5 females, age: 45 ± 10 years old) consisted of individuals who were novice to any form of meditation. The Himalayan Yoga and Isha Shoonya traditions primarily emphasize focused attention and open monitoring, respectively. The Vipassana tradition, as taught by S. N. Goenka, involves the practice of focusing attention on the somato-sensation of the body, representing a type of focused attention. It can also be called an open monitoring type of meditation because the meditator practices to see what comes to one’s mind at advanced stages effortlessly (Fig. 1a). Each participant completed two 20-minute sessions of meditation and mind wandering [18].
During the first 10 min of the meditation session, all participants performed breath-focused meditation, a foundational practice across all three meditation traditions. In the last 10 min, participants in the experimental groups were engaged in the specific meditation practices of their respective traditions. Participants in the control group continued to perform breath-focused meditation in the last 10 min (Fig. 1b) [18].
In this study, we focused on data from the last 10 min of meditation sessions, assuming that participants would become immersed in the task. All participants kept their eyes closed during the entire meditation and wandering sessions. We analyzed 64-channel DC-EEG data (ActiveTwo, Biosemi, Netherlands) recorded in the dataset. The reference channel was located on the right mastoid, and data were recorded at a sampling rate of 1,024 Hz. This dataset is publicly available at: https://openneuro.org/datasets/ds003969/versions/1.0.0 [30].
Participants and behavioral task. (a) Participants from three meditation traditions. The Himalayan Yoga and Isha Shoonya traditions primarily emphasize focused attention and open monitoring, respectively. Vipassana meditation, as taught by S. N. Goenka, involves the practice of focusing attention on the somato-sensation of the body, representing a type of focused attention. It can also be called an open monitoring type of meditation because the meditator practices to see what comes to one’s mind at advanced stages effortlessly. For more information, see, Braboszcz et al. [18]. (b) Meditation task. In this study, we focused on data from the last 10 min of meditation sessions, assuming that participants would become immersed in the task
2.2 EEG preprocessing
We began the EEG analysis by removing the bad channels indicated in the dataset notes. As EEG in this dataset was recorded with the participants’ eyes closed, we did not consider eye-blink artifacts. Ocular-motor and muscle activity artifacts are known to occur in frequency ranges above 20 Hz [31, 32], which is distant from the ISA frequency band (< 0.1 Hz) Nonetheless, we removed potential artifacts by median filtering EEG signals with a 1-second window size to reduce artifacts. Because artifacts in the ISA frequency band are not well known, when analyzing time-domain amplitudes, we used the time-domain median, which is robust to outliers, instead of the time-domain mean, to avoid biasing the results due to potential artifacts.
For EEG signals in fast frequency bands (> 1 Hz), we removed artifacts through three steps: firstly, bandpass filtering was performed using a finite impulse response (FIR) filter with a Hamming window at 1–40 Hz (or 60–110 Hz); secondly, median filtering was applied with a 1/16-second window size to reduce artifacts (for 1–40 Hz band-passed data); and finally, we used the Artifact Subspace Reconstruction method implemented in the EEGLAB toolbox (using “EEG = clean_asr( EEG, 20 )” [33]), to remove other remaining artifacts. EEG data were downsampled to 512 Hz during these three steps.
We applied the surface Laplacian filtering to EEG signals to reduce the effect of volume conduction. The Current Source Density (CSD) Toolbox [34,35,36] was utilized to apply the surface Laplacian filtering to EEG data. We set the filtering parameters, m = 4 and λ = 10− 5, similar to other EEG oscillation studies [37, 38].
2.3 EEG analysis
Using EEG signals at each channel after preprocessing above, we analyzed EEG signals in three ways to estimate: (1) the amplitude of the ISA via the fast Fourier transform (FFT); (2) the amplitude of the ISA via the bandpass filtering (0.03–0.08 Hz); and (3) PAC between the phase of the ISA (0.03–0.08 Hz) and the amplitude envelope of the alpha and high gamma oscillations. First, we employed FFT to measure the amplitude of the ISA. EEG signals were downsampled to 8 Hz before applying the FFT. The reason for downsampling to 8 Hz was to obtain a sufficient number of data samples per cycle (> 80, for the EEG amplitude and phase analyses) even at the fastest frequency of ISA (0.1 Hz), while also ensuring rapid data processing. Then, we created a time window with a 100-second width randomly placed within the period of interest (i.e., the last 10 min of the sessions) and applied the FFT to EEG signals in this window. The amplitude at each frequency in the infra-slow band (0.01–0.1 Hz) was then measured by taking the absolute value of the FFT results. We repeated this procedure 1,000 times and took the median amplitude. Due to the utilization of extended time windows with uniformly distributed random starting points, our data sampling for FFT resulted in overlaps across time windows, which consequently led to more frequent sampling of data in central time segments. However, we opted for this approach since we assumed that the ISA amplitude would now vary substantially throughout the 10-minute meditation period. This approach is akin to assessing the ISA amplitude via a moving window with regular intervals of 0.5 s, albeit with the introduction of variability in the starting points of the windows. As such, this approach essentially highlights the concept of random sampling in the estimation of the ISA amplitude.
Next, we employed bandpass filtering to measure the amplitude of the ISA. EEG data were downsampled to 8 Hz and bandpass filtered using a FIR filter with a Hamming window at 0.03–0.08 Hz. This infra-slow frequency band was determined empirically by assessing the amplitude of the ISA above (see Sect. 3.1). The resulting infra-slow signals were Hilbert transformed to estimate the amplitude envelope of the ISA (0.03–0.08 Hz). We took absolute value to the Hilbert transformed ISA data and took the median amplitude envelope.
Lastly, PAC between the phase of the ISA and the amplitude envelope of the alpha (8–12 Hz) and high gamma (60–110 Hz) oscillations was also analyzed. EEG data were downsampled to 8 Hz and bandpass filtered using a FIR filter with a Hamming window at 0.03–0.08 Hz. The resulting infra-slow signals were Hilbert transformed to estimate the phase of the ISA. To compute PAC, the phases of the ISA were divided into eight intervals: [-π,-3π/4], [-3π/4,-2π/4], [-2π/4,-π/4], [-π/4,0], [0,π/4], [π/4,2π/4], [2π/4,3π/4], and [3π/4,π] radian. The amplitude envelope of the alpha and theta oscillations was calculated using Hilbert transform as described above. Then, we collected the values of the amplitude envelope of the alpha and theta oscillations corresponding to each ISA phase interval and averaged all the collected values in each phase interval. The averaged amplitudes were then normalized to make the sum of the amplitudes over eight intervals equal to one, similar to a probability distribution. PAC was quantified using the modulation index (MI) based on the Kullback–Leibler divergence between the normalized average amplitudes and the uniform distribution [39]. The statistical significance of the PAC was verified by calculating the MI of 1,000 surrogate data obtained by randomly shuffling the phases of the ISA.
2.4 Statistical tests
In this study, we used the one-tailed Wilcoxon rank-sum test to compare the outcomes of the EEG data analysis (see Sect. 2.3) between the experimental and the control groups. Utilizing the Wilcoxon rank-sum test eliminates the need to assume that the data follow a specific distribution. The decision to utilize a one-tailed statistical test was driven by our hypothesis that the ISA amplitude would decrease, supported by preliminary observations of a tendency of ISA amplitude reduction (Fig. 2a). To tackle the issue of multiple comparisons, we used a false discovery rate (FDR) corrected p-value of 0.05 over 64 channels. A one-tailed permutation test was used to determine the statistical significance of the Modulation Index (MI) in Phase-Amplitude Coupling (PAC). In this instance, we applied an FDR-corrected p-value of 0.05 across participants in the experimental groups.
2.5 Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used Google Translate and ChatGPT in order to improve readability and language. After using this tool/service, the authors reviewed and edited the texts as needed and take a full responsibility for the contents of the publication.
3 Results
3.1 Reduced amplitude of EEG infra-slow activity in experienced meditators of the vipassana tradition
First, we observed that the amplitudes of the EEG ISA in experienced meditators were lower than those of novices (Fig. 2a). Such lowered amplitudes of the EEG ISA were observed in experienced meditators of all three traditions during meditation. Therefore, in order to select an ISA frequency band in which the ISA amplitude is significantly lower, the ratio of EEG channels in which the ISA amplitude of experienced meditators is significantly lower than that of novices was calculated for each frequency (One tailed Wilcoxon rank-sum test, FDR-corrected p < 0.05). As a result, it was confirmed that the ISA amplitude of experienced meditators who practiced Vipassana was significantly lowered in 1–10% of channels at 0.03–0.08 Hz (Fig. 2b). In other experimental groups, this significant reduction was very small or absent (Fig. 2b). Therefore, in the following analyses, the ISA frequency band was defined as 0.03–0.08 Hz.
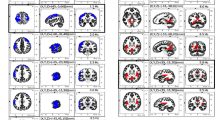
We assessed the difference in the amplitude of the EEG ISA between the experimental groups and the control group on the selected ISA frequency band (0.03–0.08 Hz). In the group of Vipassana meditators, the amplitude of the ISA at a frequency band of 0.03–0.08 Hz in the left frontal region during meditation was lower than in the control group (One tailed Wilcoxon rank-sum test, FDR-corrected p < 0.05) (Fig. 3). The group of Vipassana meditators showed lower amplitudes of the ISA at 13 out of 64 EEG channels at 0.03–0.08 Hz than the control group (One tailed Wilcoxon rank-sum test, FDR-corrected p < 0.05) (Fig. 4). In the group of Himalayan Yoga and Isha Shoonya meditators, none of the EEG channels showed a significant difference in the amplitude of the ISA compared to the control group during meditation (One tailed Wilcoxon rank-sum test, FDR-corrected p < 0.05) (Fig. 3).
Infra-slow activity (ISA) frequency band selection for reduced electroencephalogram (EEG) ISA in experienced meditators. (a) Reduced EEG ISA observed in experienced meditators during meditation. Left: channel F7. Right: all channels. (b) Percentage of EEG channels with significantly reduced amplitude relative to controls (novices) (One tailed Wilcoxon rank-sum test, FDR-corrected p < 0.05). Note that the significance reductions were observed in 0.03–0.08 Hz band of Vipassana meditators
3.2 Reduced coupling between the phase of infra-slow activity and amplitude of alpha oscillations in experienced meditators of the vipassana tradition
We examined phase-amplitude coupling (PAC) between the phase of the ISA and the amplitude envelope of the alpha (8–12 Hz) and high gamma (60–110 Hz) oscillations. The reason for using these frequency bands is that in a previous study using the same dataset as the current study, the alpha and high-gamma frequency bands showed differences between experienced and novice meditators [18]. The infra-slow frequency band was determined as 0.03–0.08 Hz, in which the amplitudes of the ISA were most significantly different between experienced and novice meditators (Fig. 3).
We first verified whether the PAC outcomes in the dataset analyzed in this study were significantly different from the chance level, as demonstrated in previous studies [3, 4]. As for alpha oscillations, during meditation, PAC was significantly stronger than the chance level in 84.73%, 79.36%, 78.45%, and 84.77% of participants from the Himalayan Yoga, Vipassana, Isha Shoonya, and control groups, respectively (Permutation test, FDR-corrected p < 0.05). As for high gamma oscillations, during meditation, PAC was significantly stronger than the chance level in 46.32%, 41.80%, 46.05%, and 51.41% of participants from the Himalayan Yoga, Vipassana, Isha Shoonya, and control groups, respectively (Permutation test, FDR-corrected p < 0.05).
Having confirmed that PAC outcomes were significant in many participants (especially, alpha oscillations), we compared PAC outcomes between the experimental and control groups. As for alpha oscillations, PAC was significantly weaker in the group of Vipassana meditators than in the control group at 22 out of 64 EEG channels encompassing the left frontal region during meditation (One tailed Wilcoxon rank-sum test, FDR-corrected p < 0.05) (Fig. 4). On the other hand, significant differences in PAC were barely presented between the Himalayan Yoga meditators (3 out of 64 EEG channels) or Isha Shoonya meditators (0 out of 64 EEG channels) group and the control group during meditation (One tailed Wilcoxon rank-sum test, FDR-corrected p < 0.05) (Fig. 4).
As for high gamma oscillations, significant differences in PAC were not presented between an experimental and the control group during meditation (Himalayan Yoga meditators: 0 out of 64 EEG channels; Vipassana meditators: 0 out of 64 EEG channels; Isha Shoonya meditators: 0 out of 64 EEG channels) (One tailed Wilcoxon rank-sum test, FDR-corrected p < 0.05) (Fig. 4).
Changes in phase-amplitude coupling (PAC) of alpha and high gamma oscillations. The phase originated from the ISA of 0.03–0.08 Hz, while the amplitude came from the alpha (8–12 Hz) and high gamma (60–110 Hz) oscillations. The figure shows changes from the control group to each experimental group during meditation. Black dots indicate statistically significant channels (One tailed Wilcoxon rank-sum test, FDR-corrected p < 0.05)
4 Discussion
Previous research has revealed that the amplitude of fast EEG oscillations (> 1 Hz) was reduced in experienced meditators [16,17,18]. In contrast, how the amplitude of EEG ISA (< 0.1 Hz) would be altered in experienced meditators is largely unknown. In this study, we investigated the amplitude of EEG ISA in experienced meditators of various types, including the Himalayan Yoga, Vipassana, and Isha Shoonya traditions. We found the lowered amplitude of EEG ISA in Vipassana meditators, as taught by S. N. Goenka, compared to the novice group (Figs. 2 and 3). Specifically, the amplitude of EEG ISA (0.03–0.08 Hz) over left frontal region was reduced during meditation in Vipassana meditators. Also, no apparent reduction of the amplitude of EEG ISA was found in Himalayan Yoga and Isha Shoonya meditators. Therefore, the reduction of the amplitude of EEG ISA by meditation may vary with meditation traditions in terms of practice and brain region.
ISA represents intrinsic neural excitability fluctuations [3,4,5,6], and the correlation between behavior and ISA intensifies when behavioral performance diminishes [4, 7]. This suggests that a state heavily influenced by fluctuations unrelated to task performance is not conducive to effective task execution. A condition characterized by a high ISA amplitude may indicate that the impact of intrinsic neural excitability fluctuations is significant. This interpretation aligns with existing research findings [9], which suggest improved behavioral performance when the ISA amplitude is reduced. Thus, the reduced ISA amplitude (Fig. 3) can be perceived as beneficial for the meditation task, mitigating the influence of intrinsic neural excitability fluctuations that are not related to task performance.
Decreased ISA amplitudes are known to reflect positive effects with mindfulness meditation, including stress reduction [10–11; for a review, see 12] and enhancement of cognitive abilities [8, 9, 13]. Mindfulness meditation encompasses both focused attention and open monitoring practices [15]. Such a meditation category also includes Vipassana meditation as taught by S. N. Goenka in addition to mindfulness meditation. In this study, the reduction in ISA amplitude was primarily observed in the Vipassana meditation condition, suggesting that the combination of focused attention and open monitoring might be essential for reducing ISA amplitude. Training of both focused attention and open monitoring reportedly increases the amplitude of alpha oscillations compared to training that focuses on only one of these aspects [18]. This suggests that neural systems may respond differently depending on the type of training. Consequently, the observed reduction in ISA amplitude during meditation among experienced meditators (Fig. 3) could be associated with the beneficial effects of meditation practices that integrate both focused attention and open monitoring.
PAC between the phase of ISA and the amplitude of fast oscillations differed between meditation traditions (Fig. 4). In Vipassana meditators, both the amplitude of the ISA and PAC between the ISA phase and the alpha amplitude decreased during meditation. But in Himalayan Yoga and Isha Shoonya meditators, the amplitude of ISA, and PAC between the ISA phase and the alpha amplitude were barely changed during meditation. We speculate that the PAC between the ISA and alpha oscillations changed in Vipassana meditators, because alpha oscillations are reported to be modulated only in Vipassana meditators among the 3 traditions [18]. This suggests that changes associated with alpha oscillations may be related to meditation traditions that include both focused attention and open monitoring practices, for example, the Vipassana meditation, as taught by S. N. Goenka. In previous studies using the same dataset as this study, high gamma activity did not differ by meditation tradition [18]. In this study, there were no changes in the coupling between the ISA phase and high gamma amplitude across all meditation traditions, supporting the idea that ISA-related changes may be associated only with meditation traditions that include both focused attention and open monitoring practices.
PAC between ISA and alpha oscillations is recognized as a natural phenomenon, evidenced by previous studies of sleep [3] and task performance [4]. Given that the amplitude of alpha oscillations is linked to neural excitability [27,28,29], PAC between ISA and alpha oscillations suggests that the ISA phase reflects the fluctuations of neural excitability. Additionally, the decrease in PAC observed in this study (Fig. 4) might indicate the reduced influence of intrinsic excitability fluctuations. Such a reduction in intrinsic neural excitability fluctuations could lead to a more stable brain state. A stable brain state is likely beneficial in promoting the positive effects on meditation [8, 9] associated with a decrease in ISA amplitude. This result also sheds light on the role of alpha oscillations, which are widespread throughout the brain [27,28,29].
This perspective can be confirmed in future neurofeedback studies that are based on the amplitude of EEG ISA, such as recent studies [21,22,23]. In other words, it is worthwhile to investigate whether neurofeedback training that reduces the amplitude of the ISA will have various positive effects.
4.1 Limitations
However, the generalization of the results of this study to other forms of meditation is necessary. For instance, it remains unclear how other well-known forms of meditation, such as mindfulness, which lies somewhere between focused attention and open monitoring, might affect the amplitude of ISA. Thus, future studies need to address the generalizability of the amplitude of ISA as an index of meditation experiences.
4.2 Conclusions
EEG ISA is related to the meditation experience. In experienced meditators, the amplitude of the EEG ISA is reduced. Additionally, the correlation between EEG ISA and neuronal excitability fluctuations is reduced. This reduction in EEG ISA may be related to the positive effects of meditation.
References
Aladjalova NA. Infra-slow rhythmic oscillations of the steady potential of the cerebral cortex. Nature. 1957;179(4567):957–9. https://doi.org/10.1038/179957a0.
Ly JQM, Gaggioni G, Chellappa SL, Papachilleos S, Brzozowski A, Borsu C, Rosanova M, Sarasso S, Middleton B, Luxen A, Archer SN, Phillips C, Dijk D-J, Maquet P, Massimini M, Vandewalle G. Circadian regulation of human cortical excitability. Nat Commun. 2016;7(1):11828. https://doi.org/10.1038/ncomms11828.
Vanhatalo S, Palva JM, Holmes MD, Miller JW, Voipio J, Kaila K. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc Natl Acad Sci U S A. 2004;101(14):5053–7. https://doi.org/10.1073/pnas.0305375101.
Monto S, Palva S, Voipio J, Palva JM. Very slow EEG fluctuations predict the dynamics of stimulus detection and oscillation amplitudes in humans. J Neurosci. 2008;28(33):8268–72. https://doi.org/10.1523/JNEUROSCI.1910-08.2008/.
de Goede AA, van Putten MJAM. Infraslow activity as a potential modulator of corticomotor excitability. J Neurophysiol. 2019;122(1):325–35. https://doi.org/10.1152/jn.00663.2018.
Sihn D, Kim S-P. Brain infraslow activity correlates with arousal levels. Front Neurosci. 2022;16:765585. https://doi.org/10.3389/fnins.2022.765585.
Sihn D, Kim S-P. Differential modulation of behavior by infraslow activities of different brain regions. PeerJ. 2022;10:e12875. https://doi.org/10.7717/peerj.12875.
Brinkman SL. Infra-slow electroencephalographic activity during stress. Master thesis. University of Twente. 2018. https://purl.utwente.nl/essays/77030.
Sato N, Katori Y. (2019). Infra-slow electroencephalogram power associates with reaction time in simple discrimination tasks. Proceedings of the International Conference on Neural Information Processing; Sydney, NSW, Australia. https://doi.org/10.1007/978-3-030-36708-4_41.
Zarenejad M, Yazdkhasti M, Rahimzadeh M, Tourzani ZM, Esmaelzadeh-Saeieh S. The effect of mindfulness-based stress reduction on maternal anxiety and self-efficacy: A randomized controlled trial. Brain Behav. 2020; 10(4):e01561. Doi: e01561.
Kirk U, Axelsen JL. Heart rate variability is enhanced during mindfulness practice: a randomized controlled trial involving a 10-day online-based mindfulness intervention. PLoS ONE. 2020;15(12):e0243488. https://doi.org/10.1371/journal.pone.0243488.
Ghawadra SF, Abdullah KL, Choo WY, Phang CK. Mindfulness-based stress reduction for psychological distress among nurses: a systematic review. J Clin Nurs. 2019;28(21–22):3747–58. https://doi.org/10.1111/jocn.14987.
Gupta SS, Manthalkar RR, Gajre SS. Mindfulness intervention for improving cognitive abilities using EEG signal. Biomed Signal Process Control. 2021;70:103072. https://doi.org/10.1016/j.bspc.2021.103072.
Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. TRENDS COGN SCI. 2008;12(4):163–9. https://doi.org/10.1016/j.tics.2008.01.005.
Van Dam NT, van Vugt MK, Vago DR, Schmalzl L, Saron CD, Olendzki A, Meissner T, Lazar SW, Kerr CE, Gorchov J, Fox KCR, Field BA, Britton WB, Brefczynski-Lewis JA, Meyer DE. Mind the hype: a critical evaluation and prescriptive agenda for Research on Mindfulness and Meditation. Perspect Psychol Sci. 2018;13(1):36–61. https://doi.org/10.1177/1745691617709589.
Rodriguez-Larios J, Alaerts K. EEG alpha–theta dynamics during mind wandering in the context of breath focus meditation: an experience sampling approach with novice meditation practitioners. Eur J Neurosci. 2021;53:1855–68. https://doi.org/10.1111/ejn.15073.
Rodriguez-Larios J, Bracho Montes de Oca EA, Alaerts K. The EEG spectral properties of meditation and mind wandering differ between experienced meditators and novices. NeuroImage. 2021;245:118669. https://doi.org/10.1016/j.neuroimage.2021.118669.
Braboszcz C, Cahn BR, Levy J, Fernandez M, Delorme A. Increased gamma brainwave amplitude compared to control in three different meditation traditions. PLoS ONE. 2017;12(1):e0170647. https://doi.org/10.1371/journal.pone.0170647.
Rodin E, Bornfleth H, Johnson M. DC-EEG recordings of mindfulness. Clin Neurophysiol. 2017;128(4):512–9. https://doi.org/10.1016/j.clinph.2016.12.031.
Jo H-G, Naranjo JR, Hinterberger T, Winter U, Schmidt S. Phase synchrony in slow cortical potentials is decreased in both expert and trained novice meditators. Neurosci Lett. 2019;701:142–5. https://doi.org/10.1016/j.neulet.2019.02.035.
Leong SL, Vanneste S, Lim J, Smith M, Manning P, De Ridder D. A XXXandomized, double-blind, placebo-controlled parallel trial of closed-loop infraslow brain training in food addiction. Sci Rep. 2018;8(1):11659. https://doi.org/10.1038/s41598-018-30181-7.
Gabrielsen KB, Clausen T, Haugland SH, Hollup SA, Vederhus J-K. Infralow neurofeedback in the treatment of substance use disorders: a randomized controlled trial. J Psychiatry Neurosci. 2022;47(3):E222–9. https://doi.org/10.1503/jpn.210202.
Mathew J, Adhia DB, Smith ML, De Ridder D, Mani R. Source localized infraslow neurofeedback training in people with chronic painful knee osteoarthritis: a randomized, double-blind, sham-controlled feasibility clinical trial. Front Neurosci. 2022;16:899772. https://doi.org/10.3389/fnins.2022.899772. PMID: 35968375; PMCID: PMC9366917.
Grooms JK, Thompson GJ, Pan W-J, Billings J, Schumacher EH, Epstein CM, Keilholz SD. Infraslow electroencephalographic and dynamic resting state network activity. Brain Connect. 2017;7(5):265–80. https://doi.org/10.1089/brain.2017.0492.
Keinänen T, Rytky S, Korhonen V, Huotari N, Nikkinen J, Tervonen O, Palva JM, Kiviniemi V. Fluctuations of the EEG-fMRI correlation reflect intrinsic strength of functional connectivity in default mode network. J Neurosci Res. 2018;96(10):1689–98. https://doi.org/10.1002/jnr.24257.
Berkovich-Ohana A, Harel M, Hahamy A, Arieli A, Malach R. Alterations in task-induced activity and resting-state fluctuations in visual and DMN areas revealed in long-term meditators. NeuroImage. 2016;135:125–34. https://doi.org/10.1016/j.neuroimage.2016.04.024.
Sauseng P, Klimesch W, Gerloff C, Hummel FC. Spontaneous locally restricted EEG alpha activity determines cortical excitability in the motor cortex. Neuropsychologia. 2009;47(1):284–8. https://doi.org/10.1016/j.neuropsychologia.2008.07.021.
Thies M, Zrenner C, Ziemann U, Bergmann TO. Sensorimotor Mu-alpha power is positively related to corticospinal excitability. Brain Stimul. 2018;11(5):1119–22. https://doi.org/10.1016/j.brs.2018.06.006.
Iemi L, Gwilliams L, Samaha J, Auksztulewicz R, Cycowicz YM, King J-R, Nikulin VV, Thesen T, Doyle W, Devinsky O, Schroeder CE, Melloni L, Haegens S. Ongoing neural oscillations influence behavior and sensory representations by suppressing neuronal excitability. NeuroImage. 2022;247:118746. https://doi.org/10.1016/j.neuroimage.2021.118746.
Delorme A, Braboszcz C. Meditation vs thinking task. OpenNeuro. [Dataset]. 2021. https://doi.org/10.18112/openneuro.ds003969.v1.0.0.
Keren AS, Yuval-Greenberg S, Deouell LY. Saccadic spike potentials in gamma-band EEG: characterization, detection and suppression. NeuroImage. 2010;49(3):2248–63. https://doi.org/10.1016/j.neuroimage.2009.10.057.
Muthukumaraswamy SD. High-frequency brain activity and muscle artifacts in MEG/EEG: a review and recommendations. Front Hum Neurosci. 2013;7:138. https://doi.org/10.3389/fnhum.2013.00138.
Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009.
Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. evaluation with auditory oddball tasks. Clin Neurophysiol. 2006;117(2):348–68. https://doi.org/10.1016/j.clinph.2005.08.034.
Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clin Neurophysiol. 2006;117(2):369–80. https://doi.org/10.1016/j.clinph.2005.08.03332.
Kayser J. (2009). Current source density (CSD) interpolation using spherical splines - CSD toolbox (Version 1.1). New York State Psychiatric Institute: Division of Cognitive Neuroscience. http://psychophysiology.cpmc.columbia.edu/Software/CSDtoolbox.
Tenke CE, Kayser J, Manna CG, Fekri S, Kroppmann CJ, Schaller JD, Alschuler DM, Stewart JW, McGrath PJ, Bruder GE. Current source density measures of electroencephalographic alpha predict antidepressant treatment response. Biol Psychiatry. 2011;70(4):388–94. https://doi.org/10.1016/j.biopsych.2011.02.016.
Sihn D, Kim JS, Kwon O-S, Kim S-P. Breakdown of long-range spatial correlations of infraslow amplitude fluctuations of EEG oscillations in patients with current and past major depressive disorder. Front Psychiatry. 2023;14:1132996. https://doi.org/10.3389/fpsyt.2023.1132996.
Tort ABL, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104(2):1195–210. https://doi.org/10.1152/jn.00106.2010.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. RS-2023-00213187 and No. RS-2023-00302489).
Author information
Authors and Affiliations
Contributions
Duho Sihn: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision, Project administration, Funding acquisition. Junsuk Kim: Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision. Sung-Phil Kim: Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision, Project administration, Funding acquisition.
Corresponding authors
Ethics declarations
Ethical approval
The authors conducted this study using the publicly available dataset of human subjects. This dataset is publicly available at: https://openneuro.org/datasets/ds003969/versions/1.0.0 [30]. Experiments on this dataset were approved by the ethical committee of the University of California San Diego (IRB project # 090731) [18].
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sihn, D., Kim, J. & Kim, SP. Meditation-type specific reduction in infra-slow activity of electroencephalogram. Biomed. Eng. Lett. 14, 823–831 (2024). https://doi.org/10.1007/s13534-024-00377-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-024-00377-0