Abstract
Despite recent studies indicating a significant correlation between somatosensory deficits and rehabilitation outcomes, how prevailing somatosensory deficits affect stroke survivors’ ability to correct their movements and recover overall remains unclear. To explore how major deficits in somatosensory systems impede stroke survivors’ motor correction to various external loads, we conducted a study with 13 chronic stroke survivors who had hemiparesis. An inertial, elastic, or viscous load, which was designed to impose perturbing forces with various force profiles, was introduced unexpectedly during the reaching task using a programmable haptic robot. Participants’ proprioception and cutaneous sensation were also assessed using passive movement detection, finger-to-nose, mirror, repositioning, and Weinstein pressure tests. These measures were then analyzed to determine whether the somatosensory measures significantly correlated with the estimated reaching performance parameters, such as initial directional error, positional deviation, velocity deviations, and speed of motor correction were measured. Of 13 participants, 5 had impaired proprioception, as they could not recognize the passive movement of their elbow joint, and they kept showing larger initial directional errors even after the familiarization block. Such continuously found inaccurate initial movement direction might be correlated with the inability to develop the spatial body map especially for calculating the initial joint torques when starting the reaching movement. Regardless of whether proprioception was impaired or not, all participants could show the stabilized, constant reaching movement trajectories. This highlights the role of proprioception especially in the execution of a planned movement at the early stage of reaching movement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Stroke survivors commonly experience impaired motor control after a stroke. The causes of this motor impairment are usually diverse, ranging from the loss of muscle strength to impaired motor coordination, and disrupt movement planning. Somatosensory loss after stroke is also common [1, 2]; however, how it contributes to motor impairment remains unclear.
Most clinical trials aimed at recovering motor impairments have concentrated on interventions that restore muscle strength. However, the effectiveness of interventions that solely focus on strengthening has been limited or has modest clinical outcomes. Conversely, recent studies have emphasized the importance of somatosensory systems in rehabilitation outcomes after stroke. Some studies have suggested that the extent of the somatosensory loss can even predict rehabilitation outcomes in stroke survivors [2, 3]. Despite this, it remains unclear which type of somatosensory deficit is most responsible for the impaired capacity for motor correction and therefore should be treated intensively to obtain better rehabilitation outcomes. Thus, in this study, we examined how somatosensory loss following hemispheric stroke correlates with the ability to execute correcting movements in response to an unexpected, external load imposed during a planar reaching task of the upper extremity.
Deficits in somatosensory systems can impair precise force generation and object manipulation after a stroke, as these systems play a crucial role in fine motor control and force generation [4, 5]. By contrast, healthy individuals can easily handle an object with an unknown weight and accomplish movements successfully [6] through their sensory systems, including vision, tactile sensation, static proprioception, and dynamic proprioception (kinesthesia). These sensory systems signal the difference between the predicted and actual movement outcomes, enabling individuals to correct their movements [7]. However, stroke survivors with sensory deficits may struggle to assess deviations from the predicted movement path and correct their movements, potentially leading to impaired motor learning and retention [8]. The underlying reasons for these impairments remain unclear [9].
Several case studies have shown that somatosensory loss can significantly affect motor performance, particularly in multi-joint movements and coordination, in patients with neurological disorders [4, 10, 11]. Inaccurate movement trajectories [10], numerous sub-movements for movement corrections [11], and different characteristics of motor learning [12] have been reported in patients with proprioceptive deafferentation. Interestingly, accurate adaptation performance has been observed even in participants with substantial proprioceptive deficits [4, 12, 13]. Nevertheless, these studies had some limitations, as they were based on case reports with relatively small sample size or included a lack of correlation between task-related performance parameters and somatosensory deficits. Thus, this study aimed to investigate how somatosensory loss affects motor control and the capability for motor correction to novel loads in stroke survivors.
For this study, we hypothesized that a decline in somatosensory system capacity, including static/dynamic proprioception in stroke survivors, would significantly impair their capability for motor correction in response to an unexpected change in the external load. To test this hypothesis, stroke survivors were categorized based on their functional levels of dynamic/static proprioception and cutaneous pressure sensory acuity. By correlating these functional levels of sensory systems with reaching performance parameters, we can assess how much the somatosensory deficit contributes to stroke survivors’ global motor deficits and capability for the motor correction in response to unexpected loads.
2 Materials and methods
This study enrolled 13 chronic stroke survivors with hemiparesis, with an average age of 60.08 ± 7.04 years and an average stroke onset of 10.77 ± 8.17 years ago. Before the experiment, all participants were informed about the experiment and signed a consent form, which was approved by the Northwestern Institutional Review Board (IRB # STU00208823). The participants involved in this experiment reported no history of upper body injuries that could interfere with performing reaching movements.
Before conducting the reaching task, the isometric elbow strength and functional level of somatosensory system acuity were evaluated on both sides of the upper limb of the participant (Fig. 1 and Table 1). The Weinstein monofilament pressure test (Fig. 1A) [14] was employed to measure cutaneous pressure sensation. Five monofilament tester pens with varying tip thicknesses were used to exert different pressures on the participant’s hand skin, and the minimum sensible pressure was recorded (Fig. 1A). If none of the monofilaments was recognized by the participant, the maximum weight of this test (300 g) was considered as the response [14, 15]. To assess dynamic proprioception (kinesthesia), a test based on the finger-to-nose test (Fig. 1B) [16, 17] and the passive elbow joint movement detection test (Fig. 1C) [18, 19] were performed. With the eyes closed, participants were asked to extend their elbow and then asked to touch their nose with the index finger. A positive outcome value was considered if a blindfolded participant could touch their nose successfully at least 3 out of 5 times during the finger-to-nose test (Fig. 1B). For the passive elbow joint movement detection test, the participant’s elbow joints were randomly flexed or extended using DORIS, a haptic robot with low mechanical impedance and noise [20], at a speed of 2 deg/s. The upper limbs of the participants were supported against gravity by the SaeboMAS (Saebo, Inc., Charlotte, NC) system. A positive outcome value was considered if the blindfolded participant could recognize the movement direction at least 3 out of 5 times (Fig. 1C).
Somatosensory and isometric force tests. A The Weinstein monofilament test for cutaneous pressure sensation, B finger to nose test and C passive movement detection test for dynamic (joint movement) proprioception, D mirror test and E repositioning test for static (joint position) proprioception, and F isometric elbow extension test for strength
Static proprioception was assessed using the mirror test (Fig. 1D) [21] and the repositioning test (Fig. 1E) [22]. During the tests, HapticMaster (Moog FCS Robotics, East Aurora, NY) [23] was used to passively move the paretic and nonparetic sides of the upper limb to a specific position in a plane. The participant was then asked to mimic the same position with the other side (Fig. 1D) or with the same side (Fig. 1E) of the upper limbs, which were supported by the SaeboMas system. This test was repeated three times, and the average positional deviation was considered the spatial error. The researchers also measured maximum elbow extension force by pushing the padded probe of the HapticMaster at a fixed position for 3 s, which was repeated three times and then averaged (Fig. 1F).
Before the actual loaded reaching task, participants underwent a baseline (familiarization) task consisting of 15 reaching trials to get familiar with the haptic device. During this baseline task, a 2 kg inertial load was applied to the handle of the Haptic Master held by the participant. The first three and last three reaching trials of this baseline task were analyzed to assess early and late familiarization, respectively (Fig. 2). During the experiments, the participants were required to grasp the handle of the HapticMaster and reach the target, which was positioned 20 cm from the starting point (Fig. 2). The starting point was located in front of the chest’s center. The target was always positioned in the direction away from the chest, mainly inducing elbow extension, and located in the direction of the moving arm (Fig. 2). The participants were provided with visual feedback of the current hand position and target position via a monitor.
A Types of unexpected loads, B experimental setup, and C protocols. A While subjects reached toward the target, either the inertial, elastic, or viscous load was applied without notice immediately after starting the reaching movement. B Subjects could see the home, target, and current position of the cursor from the monitor while holding the end effector of the HapticMaster, and their upper arm was supported by the SaeboMAS.
Following the baseline task, all participants completed two loaded reaching sessions, each consisting of three blocks of reaching experiments. Each block comprised 15 reaching trials, with one of three different load types, i.e., an inertial, elastic, or viscous load [24, 25]. After each block of 15 reaching trials, a 10-min rest was given. In total, the participants completed 15 unloaded baseline (familiarization) trials and 2 of 45 loaded reaching trials, totaling 105 reaching trials in a day. Both blocks had the same protocol except for the random order of mechanical loads.
For the loaded reaching trials, all three types of mechanical loads (inertial, elasticity, and viscosity), which was designed to arouse various force profiles (as depicted in Fig. 2), were implemented virtually (Fig. 2), without prior notification, using the HapticMaster [25]. All load types acted on a reaching plane and did not have a vertical direction component against gravity. In the inertial load condition, a 5-kg load was applied before the start, which differed from the 2-kg load used in the baseline task. Under the elastic load condition, since a subject was asked to reach the target by extending an elbow joint only, a torsional spring with a single degree of freedom was used to apply the elastic load. This elastic load \(({f}_{k})\) was negatively proportional to \({\theta }_{elbow}\) and was expressed as follows:
where the k and r were the torsional stiffness (1.2 N-m/rad) and distance from the elbow joint to the handle, respectively, measured on a plane (Fig. 2). The elastic load resisting the change in \({\theta }_{elbow}\) was 0 when the hand was in the starting position \(({\theta }_{elbow}=0)\) and increased as the hand approached the target. In the viscous load condition, a load proportional to the speed of the handle (m/s) and opposite to the direction of motion was applied before the reaching movement began. The resulting viscous load \(({f}_{c})\) can be expressed as follows:
where c was the viscosity (10 N-s/m). A 10-min break was provided between the first and second sessions. For more information on the experimental protocol, please refer to the study published earlier by the authors [25].
The load types used in previous studies were designed to have similar integral values of contact force [25]. However, if a participant was unable to handle the load and could not reach the target during the practice session, the load magnitude was reduced by 25% (subject #3 and #5 from Table 1). This approach resulted in all participants completing the reaching tasks. The participants were instructed to reach the target at a self-selected, submaximal speed and maintain this speed throughout the experiment session. The magnitudes of the additional loads and their corresponding peak resisting forces were limited to not exceed 30% of the maximum voluntary elbow extension force. All loads were controlled to have the same magnitude of the time integral of manipulating contact forces, as measured from the baseline task without an additional load [24, 25].
The Haptic Master was used to record kinematic and kinetic data, including reaching movement trajectories and manipulating contact forces, with sampling frequencies of 2 kHz. The recorded kinematic/kinetic data were then filtered using a fifth-order Butterworth low-pass filter with a cutoff frequency of 10 Hz.
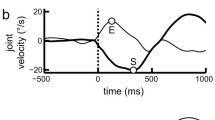
The reaching performance parameters were estimated from the first three reaching trials to examine the participant’s immediate response when perturbation was provided. The initial directional error, and positional deviation (Fig. 3). The initial directional error (\({\theta }_{i}\) from the third row of Fig. 3) was calculated as the angular deviation at 10% of the reaching time from the straight line connecting the starting point and the displayed target (Fig. 3). To assess the efficiency of the reaching trajectory, the lateral positional deviation from the straight line connecting the starting point and the displayed target was calculated (red-shaded area from the third row of Fig. 3) and was then divided by the length of the straight, shortest line connecting the home and target positions, which indicated an average of the lateral positional deviation.
Reaching movement (left column), velocity (middle column), and acceleration (right column) profiles from a representative subject with intact (two upper lows) and impaired proprioception (two lower rows), which was determined using the movement detection test. An initial directional error (\({\theta }_{i}\), green), positional deviation (blue shaded area) from the straight line connecting the home and target, and velocity deviation from the average of the last three reaching trials (red shaded area and black dashed line, respectively,) from the fully adapted trials were also determined. Line color indicates the number of the reaching trial. Reaching (%) refers to its time
To assess the speed and consistency of the participant’s reaching movements while adapting to unexpected loads, we first determined the velocity deviation from the average velocity profile of the three most recent, or most stabilized, reaching trials for each load type (blue-shaded area in the third row of Fig. 3). The velocity deviation was plotted over 15 reaching trials and then fitted with a motor learning curve as follows:
which was used to derive the inverse of the exponential rate of decay (the parameter b from Eq. (3)) [26] and the speed of motor adaptation, where y, a, e, b, and x are the reaching performance parameter (defined as the velocity deviation in this study), initial value of the velocity deviation at the first reaching trial, Euler’s number (exponential function), adaptation speed indicating a decrease in the velocity deviation, and reaching trial number, respectively [25].
To investigate potential effect of the functional level of the muscular and somatosensory systems on the reaching performance parameters, we used a linear mixed-effect model. In this statistical model, the reaching performance parameters (Table 2) from the upper limbs, such as the initial directional error, and positional deviation, and speed of motor correction (parameter b from the learning curve) (Eq. 3) were considered response variables. Sensory measures from the upper limbs (Table 2) and other experiment conditions (hemiparesis, number of experimental sets, and load type) were considered fixed effects. After the data were fitted to such linear mixed-effect model, the analysis of variance was utilized to find a significant effect of each fixed effect. Possible interaction effects between the fixed effects and random effects between participants were also taken into account. If a significant interaction between the sensory measures and load types was found, we conducted an analysis of variance as a post-hoc test (multiple t-tests for the impaired/intact dynamic proprioception or correlation analysis for other sensory measures with continuous scores) to determine which load type had a significant effect. Since this study has a multiple number of fixed effects (n = 6), the Bonferroni correction was also used so the corresponding p values were multiplied by 6 (Table 2). The statistical model enabled us to identify the experimental conditions and the sensory acuity that had a significant effect on the reaching performance parameters, including initial direction error, lateral positional deviation, and speed of motor correction (parameter b). To check if the collected data follow a normal distribution, due to the relatively small number of participants in this study, the Shapiro-Wilk test has been done prior to the above statistical tests. The null hypothesis that initial directional error (p = 0.1841), lateral positional deviation (p = 0.0952), and motor correction speed (p = 0.0.2581) all follow a normal distribution was not rejected. All data analysis and statistical analysis were performed using a customized MATLAB (Mathworks, Natick, MA, USA) software and a linear mixed-effects model function installed in the same software.
To clearly observe the effects of proprioception on the reaching performance parameters, we divided the participants into two distinct groups based on the proprioception test results. For this comparison, we utilized the passive movement detection test, which categorizes subjects into either positive or negative outcomes, as its test results were not continuous.
3 Results
All 13 chronic stroke survivors completed the planar reaching task and readily adapted their responses to the applied loads, as evidenced by consistent reaching trajectories (Fig. 3) and the reduced velocity deviation (Fig. 4) after a few reaching trials with each load type. In the course of adapting to a given load in the reaching task, the participants were consistently able to show more consistent, bell-shaped reaching velocity profiles (Fig. 3).
Velocity error (deviation from the fully adapted reaching trial) as a function of the reaching trial from the nonparetic (top row) and paretic (bottom row) side of subjects with intact (circle) and impaired (triangle) proprioception with the inertial (left), elastic (center), and viscous (right) load. Each color indicates a different subject
Functional levels of static proprioception, dynamic proprioception, and cutaneous pressure sensation were quantitatively assessed (Fig. 1), and all results are presented in Table 1. From the dynamic proprioception test (passive movement detection test), 5 of 13 participants were unable to recognize passive elbow movements at an angular speed of 2 deg/s, indicating their impaired dynamic proprioceptive system after stroke. Notably, one participant could not recognize the passive elbow movement but could touch the nose 3 of 5 times with jerky movements. The median values of the recognizable monofilament force for the nonparetic and paretic sides were 0.07 and 2 g, respectively. An averaged isometric elbow extension force from the nonparetic and paretic sides was 77.6 ± 31.4 N and 32.8 ± 27.7 N, respectively. During the monofilament test, two participants (participants #7 and #13 from Table 1) could not recognize any pressure level with the affected side of the upper limbs.
Hemiparesis (paretic/nonparetic side) showed a significant effect on the reaching performance parameters estimated in this study (Table 2 and Fig. 5), including a significant larger initial directional error (Fig. 6) and lateral positional deviation (Fig. 7). No significant effect was found with the number of experiment set (Table 2). The load type also showed a significant effect on the initial directional error and lateral positional deviation (Table 2).
The sensory measures from the passive movement detection, finger-to-nose, repositioning, and cutaneous tests showed significant correlations with the initial directional error (p = 0.0139, 0.0061, 0.0104, and 0.0012, respectively) (Table 2, Figs. 5 and 6). The movement detection measure also showed a significant effect on the positional deviation (p = 0.0497) (Table 2). In addition, the parameter b from the learning curve based on the velocity deviation (Eq. 3 and Fig. 8), which indicates the speed of motor correction, were not significantly different between the intact and impaired dynamic proprioception groups for all load types tested in this study (p > 0.05, Table 2 and Fig. 8).
Comparison of the parameter b from the learning curve (Eq. 3) between the nonparetic and paretic sides (left) and between the intact and impaired proprioception groups with the paretic side of the upper limbs (right). Error bars indicate 95% CIs (* p < 0.05, ** p < 0.01, *** p < 0.001)
Significant interaction effects were found between the movement detection measure and before/after familiarization on the initial directional error (p = 0.0147), between the hemiparesis and before/after familiarization on the positional deviation (p = 0.0147), and between the movement detection and experiment set on the positional deviation (p = 0.0223) (Table 2 and Fig. 6). No significant difference in the initial directional error (p = 0.5288) was found between the impaired and intact proprioception groups before the familiarization (early baseline), however, after the familiarization, a significant difference was found (p = 0.0252) (Fig. 6). Similarly, after the familiarization, a significant difference in the lateral positional deviation was found between the impaired and intact proprioception groups (p = 0.0001) (Fig. 7).
4 Discussion
Somatosensory deficits after stroke, especially proprioceptive deficits, appear to significantly affect reaching performance, especially at the very beginning of the reaching movement. However, these somatosensory deficits did not appear to significantly impair the capability for motor correction while stabilizing and correcting the subsequent reaching movements in response to novel loads. Most notably, initial directional error was significantly larger in the impaired proprioception group than in the intact proprioception group. The participants with impaired proprioception kept showing a larger initial directional error even after the familiarization session (baseline), while another group with intact proprioception could reduce the initial directional error after the familiarization session (Table 2 and Fig. 6). Proprioceptive loss appeared to be strongly correlated with poor spatial accuracy at the very beginning of the reaching movement, which is related to the initial execution of planned movements.
Interestingly, the results of this study appear to contradict the possible explanation representing the possibility that the proprioceptive loss limited the ability to build and update the human internal models. In this study, no statistical evidence supported the idea that proprioceptive loss negatively affected the establishment of internal models in a human brain. None of the somatosensory measures showed significant correlations with the parameter b from the learning curve (Eq. 3), regardless of the load types. In addition, all participants even with the impaired proprioception could show the stabilized, constant reaching movement trajectories, which can be seen from the reduced velocity deviation (Fig. 5). In line with this explanation, several studies have also reported that even with proprioceptive deficits, patients could adapt to the external force or visual field using their visual or vestibular inputs [27, 28]. These results imply that stroke survivors could remember (or store) the previously exerted joint torques and movement errors, correct the next movement, and finally update internal models for subsequent movement trials.
Rather, our results highlight the effect of proprioceptive deficits on the early stage of generating initial joint torques. This explanation can be supported by the finding that nearly all somatosensory measures showed significant correlations with the initial directional error (Table 2), although the measure from the mirror test did not survive based on the Bonferroni correction. Especially, a significant difference in the initial directional error between the paretic and non-paretic side (p = 0.0164) (Table 1) can be explained by an interaction effect between the movement detection test measure and before/after familiarization on the same parameter (initial directional error) (p = 0.0147) (Table 2). While the participants with their paretic side with the intact proprioception could reduce the initial directional error after a few trials by updating their internal model of body map, others with the impaired proprioception could not even after the familiarization, and also for all load types (right panel of Fig. 6). These results imply the possibility that the proprioceptive loss might have a significant role during the calculation or execution of the joint torques before or right after the onset of the reaching movements. However, as one of the limitations of this study, it is still unknown whether proprioceptive loss affects the accuracy of the calculation of the required joint torques or the execution of the calculated joint torques.
The results of this study are in line with those of several studies, and some are novel findings. Several previous studies have shown that patients with deafferentation show larger positional deviations in reaching movements in case reports. Similarly, the Sainburg group reported that participants with proprioceptive deficits showed larger movement deviations [10, 29, 30]. Our study showed similar results to these previous studies, but it is novel because it showed the origins of such larger movement deviations with proprioceptive deficits. From this study, proprioceptive loss only showed a significant correlation with the initial directional error regardless of the magnitude of perturbation at the beginning of the movement. Therefore, the larger positional deviation with impaired proprioception as seen in previous studies might be mainly due to inaccurate movement direction in the very early stages of movement execution, not while correcting the ongoing movements and counteracting for external perturbations.
Cutaneous tactile (pressure) sensation might also influence the capability for movement correction; however, our study could not address the effect of cutaneous tactile impairment over proprioception on the capability to manipulate limb load. Cutaneous sensory deficits, including poorer friction discrimination after neurological disorders, appeared to be associated with grip force generation [31, 32]; however, our study did not show any significant correlation with reaching performance parameters, except for the initial directional error. Notably, with the limited number of study participants, some of the participants showed both impaired proprioception and impaired cutaneous sensation; thus, it was difficult to distinguish each effect of one type of sensory deficit on motor performance from another. In addition, our scale for tactile sensation might not be that sensitive or we might need more participants; thus, further investigation is needed to determine the effect of tactile sensation on load manipulation.
Although weaker muscle strength poststroke can predict the largest portion of reaching inaccuracy and poorer performance [33, 34], the magnitudes of the additional loads in this study were already scaled down based on the muscular capacity of each stroke survivor. In addition, the impaired proprioception group (Figs. 5, 6, 7 and 8) showed a comparable, or even larger, average value of elbow extension torque than the intact proprioception group from their paretic side of the upper limbs. Thus, the potential effects of reduced muscle strength on the speed of motor correction might well be canceled out. Notably, the maximum isometric elbow extension torque was not significantly correlated with the estimated speed of motor correction (Table 2). Interestingly, one of the participants with the lowest Fugl–Meyer upper extremity motor score (12 of 66) due to the inability to abduct the shoulder joint showed one of the fastest movement correction speeds. This participant had relatively good dynamic proprioception and intact tactile sensation, which emphasizes the role of dynamic proprioception and the presence of other factors likely affecting the capability of manipulating the mechanical impedances.
This study has some limitations. Statistically, especially with the limited number of human subjects, the independent variables in this study (sensory measure) might not be independent of each other and may be dependent variables because it tests motor performance (response of the statistical model). For example, since the finger-to-nose and repositioning tests obviously involve voluntary movements, it may be unreasonable to view these measures as independent variables. From that point of view, the passive movement detection test or mirror test may provide independent variables with better accuracy. However, further study is needed on this concern.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kessner SS, Schlemm E, Cheng B, Bingel U, Fiehler J, Gerloff C, et al. Somatosensory deficits after ischemic stroke: time course and association with Infarct Location. Stroke. 2019;50(5):1116–23.
Ingemanson ML, Rowe JR, Chan V, Wolbrecht ET, Reinkensmeyer DJ, Cramer SC. Somatosensory system integrity explains differences in treatment response after stroke. Neurology. 2019;92(10):e1098–108.
Turville M, Carey LM, Matyas TA, Blennerhassett J. Change in functional arm use is associated with somatosensory skills after sensory retraining poststroke. Am J Occup Ther. 2017;71(3):7103190070p1-71031900709.
Miall RC, Kitchen NM, Nam S-H, Lefumat H, Renault AG, Ørstavik K, et al. Proprioceptive loss and the perception, control and learning of arm movements in humans: evidence from sensory neuronopathy. Exp Brain Res. 2018;236(8):2137–55.
Nowak DA, Hermsdörfer J, Topka H. Deficits of predictive grip force control during object manipulation in acute stroke. J Neurol. 2003;250(7):850–60.
Crevecoeur F, Thonnard J-L, Lefevre P. A very fast time scale of human motor adaptation: within movement adjustments of internal representations during reaching. Eneuro. 2020. https://doi.org/10.1523/ENEURO.0149-19.2019.
Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269(5232):1880–2.
Vidoni ED, Boyd LA. Preserved motor learning after stroke is related to the degree of proprioceptive deficit. Behav Brain Funct. 2009;5(1):1–10.
Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19(1):84–90.
Sainburg RL, Ghilardi MF, Poizner H, Ghez C. Control of limb dynamics in normal subjects and patients without proprioception. J Neurophysiol. 1995;73:820–35. https://doi.org/10.1152/jn.1995.73.2.820.
Sarlegna FR, Gauthier GM, Bourdin C, Vercher J-L, Blouin J. Internally driven control of reaching movements: a study on a proprioceptively deafferented subject. Brain Res Bull. 2006;69(4):404–15.
Yousif N, Cole J, Rothwell J, Diedrichsen J. Proprioception in motor learning: lessons from a deafferented subject. Exp Brain Res. 2015;233(8):2449–59.
Lefumat HZ, Miall RC, Cole JD, Bringoux L, Bourdin C, Vercher J-L, et al. Generalization of force-field adaptation in proprioceptively-deafferented subjects. Neurosci Lett. 2016;616:160–5.
Suda M, Kawakami M, Okuyama K, Ishii R, Oshima O, Hijikata N, et al. Validity and reliability of the Semmes-Weinstein Monofilament test and the thumb localizing test in patients with stroke. Front Neurol. 2021;11:1957.
Ambron E, Liu Y, Grzenda M, Medina J. Examining central biases in somatosensory localization: evidence from brain-damaged individuals. Neuropsychologia. 2022;166:108137.
Louis ED, Applegate LM, Borden S, Moskowitz C, Jin Z. Feasibility and validity of a modified finger-nose‐finger test. Mov Disord Off J Mov Disord Soc. 2005;20(5):636–9.
Rodrigues MR, Slimovitch M, Chilingaryan G, Levin MF. Does the finger-to-nose test measure upper limb coordination in chronic stroke? J Neuroeng Rehabil. 2017;14(1):1–11.
Larsen R, Lund H, Christensen R, Røgind H, Danneskiold-Samsøe B, Bliddal H. Effect of static stretching of quadriceps and hamstring muscles on knee joint position sense. Br J Sports Med. 2005;39(1):43–6.
Janwantanakul P, Magarey ME, Jones MA, Dansie BR. Variation in shoulder position sense at mid and extreme range of motion. Arch Phys Med Rehabil. 2001;82(6):840–4.
Keonyoung O, Rymer WZ, Plenzio I, Mussa-Ivaldi F, Park S, Choi J. Development of a planar haptic robot with minimized impedance. IEEE Trans Biomed Eng. 2020;68(5):1441–9.
Gurari N, Drogos JM, Dewald JP. Investigation of how accurately individuals with hemiparetic stroke can mirror their forearm positions. PLoS ONE. 2021;16(4):e0250868.
Goble DJ, Lewis CA, Hurvitz EA, Brown SH. Development of upper limb proprioceptive accuracy in children and adolescents. Hum Mov Sci. 2005;24(2):155–70.
Linde RVD, Lammertse P, Frederiksen EB, The HapticMaster, a new high-performance haptic interface. In: Proceedings of EuroHaptic Edinburgh UK. 2002;1–5.
Stoeckmann TM, Sullivan KJ, Scheidt RA. Elastic, viscous, and mass load effects on Poststroke muscle recruitment and co-contraction during reaching: a pilot study. Phys Ther. 2009;89:665–78. https://doi.org/10.2522/ptj.20080128.
Oh K, Rymer WZ, Choi J. The speed of adaptation is dependent on the load type during target reaching by intact human subjects. Exp Brain Res. 2021;239(10):3091–104.
Lipták BG. Instrument engineers’ handbook, volume two: process control and optimization. Boca raton: CRC Press; 2006.
Bringoux L, Di Scotto C, Borel L, Macaluso T, Sarlegna FR. Do visual and vestibular inputs compensate for somatosensory loss in the perception of spatial orientation? Insights from a deafferented patient. Front Hum Neurosci. 2016;10:181.
Sarlegna FR, Malfait N, Bringoux L, Bourdin C, Vercher J-L. Force-field adaptation without proprioception: Can vision be used to model limb dynamics? Neuropsychologia. 2010;48(1):60–7.
Findlater SE, Mazerolle EL, Pike GB, Dukelow SP. Proprioception and motor performance after stroke: an examination of diffusion properties in sensory and motor pathways. Hum Brain Mapp. 2019;40(10):2995–3009.
Gordon J, Ghilardi MF, Ghez C. Impairments of reaching movements in patients without proprioception. I. spatial errors. J Neurophysiol. 1995;73(1):347–60.
Blennerhassett JM, Matyas TA, Carey LM. Impaired discrimination of surface friction contributes to pinch grip deficit after stroke. Neurorehabilit Neural Repair. 2007;21(3):263–72.
Nowak DA, Hermsdörfer J. Selective deficits of grip force control during object manipulation in patients with reduced sensibility of the grasping digits. Neurosci Res. 2003;47(1):65–72.
Mercier C, Bertrand AM, Bourbonnais D. Differences in the magnitude and direction of forces during a submaximal matching task in hemiparetic subjects. Exp Brain Res. 2004;157(1):32–42.
Wagner JM, Lang CE, Sahrmann SA, Hu Q, Bastian AJ, Edwards DF, et al. Relationships between sensorimotor impairments and reaching deficits in acute hemiparesis. Neurorehabilit Neural Repair. 2006;20(3):406–16.
Acknowledgements
Not applicable.
Funding
This research was supported by Basic Science Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2023-00252471). This project was also supported by the National Institutes of Health grant 5R01HD089952-05. This work was also supported by the Korea Institute of Science and Technology (KIST) Institutional Program (Project no. 2E32341).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design and writing this manuscript. Material preparation, data collection and analysis were performed by KO, WZR, and JC.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflict of interest related to this work.
Ethical approval
This study was approved by the Northwestern Institutional Review Board (IRB # STU00208823).
Consent to participate
All participants were informed about the experiment and signed a consent form, which was approved by the Northwestern Institutional Review Board (IRB # STU00208823).
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oh, K., Rymer, W.Z. & Choi, J. A pilot study: effect of somatosensory loss on motor corrections in response to unknown loads in a reaching task by chronic stroke survivors. Biomed. Eng. Lett. 14, 523–535 (2024). https://doi.org/10.1007/s13534-024-00348-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-024-00348-5