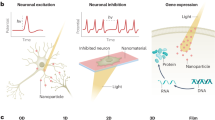
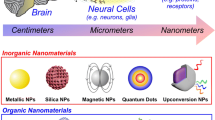
Abstract
Neuromodulation, as a fast-growing technique in neuroscience, has been a great tool in investigation of the neural pathways and treatments for various neurological disorders. However, the limitations such as constricted penetration depth, low temporal resolution and low spatial resolution hindered the development and clinical application of this technique. Nanotechnology, which refers to the technology that deals with dimension under 100 nm, has greatly influenced the direction of scientific researches within recent years. With the recent advancements in nanotechnology, much attention is being given at applying nanomaterials to address the limitations of the current available techniques in the field of biomedical science including neuromodulation. This mini-review aims to introduce the current state-of-the-art stimuli-responsive nanomaterials used for assisting thermally induced neuromodulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
1.1 Technologies for neuromodulation
Neuromodulation is defined as the application of various modalities (e.g. electricity, magnetic fields, light, or chemical agents) to interfere with the nervous system to regulate neural activity. It has not only been utilized in the neuroscientific investigation but also been clinically implemented to treat various neurological diseases, including depression, stroke, spinal cord injury, Parkinson's disease, chronic pain, etc. [1].
At the current time, therapeutic techniques for neuromodulation are mainly (1) oral medicines and (2) direct brain modulation. In terms of oral medicines, drugs used to modulate neural activities are limited owing to the blood–brain barrier (BBB), which is the first interface between the extracellular fluid of the central nervous system and the circulating blood. Moreover, with oral administration of medicines, time is needed for drug absorption which results in low temporal resolution for neuromodulation [2, 3].
In direct brain modulation, both invasive and non-invasive modulation techniques existed for effectively relieving the neurological disorder. Invasive stimulation, such as Deep brain stimulation (DBS) and intracranial cortical stimulation (ICS) possess high spatiotemporal precision. However, they require surgical implantation of electrodes to deliver an electrical pulse to targeted areas and present limited long-term efficacy [4]. On the other hand, there are non-invasive brain modulation techniques such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), which modulate brain activity by applying external stimuli, such as magnetic fields and electrical current [5]. However, magnetic stimulation lacks temporal resolution due to slow responsiveness efficiency, and the operation settings are relatively complicated. In addition, the use of TMS and tDCS in clinical settings is limited due to low spatial resolution and penetration depth as the magnetic or electric fields rapidly attenuate in-depth. Thus, a more efficient, non-invasive, cell-type specific, and less time-consuming neuromodulation technique is needed for the treatment of neurological disorders at the moment.
1.2 Advances of stimuli-responsive nanoparticles in thermal neuromodulation
Over the last decade, the advancement of nanotechnology has dramatically changed the lifestyle of human society in all kinds of ways. The incorporation of nanomaterials as transducers allow more efficient delivery of drugs and translation of external energy into the biological system. Moreover, it provides much needed benefits in the field of neuromodulation, such as higher spatiotemporal resolution and the usage of lower stimuli intensity, which is likely to minimize tissue damages [6]. The combination of nanotechnology and thermal neuromodulation provides great possibilities for manipulation of neural activities, followed by the discovery of the underlying mechanisms of neural systems and disease treatment. Among various thermal neuromodulation schemes, photothermal and magnetothermal neuromodulation are the two most frequently applied schemes for thermal neuromodulation. Hence, in this review, the recent advances in stimuli-responsive nanoparticles involved in photothermal and magnetothermal neuromodulation will be discussed.
1.3 Photothermal nanomaterials
The photothermal effect refers to the phenomenon in which photoexcitation of the materials activated by the light at specific wavelenghts results in the production of heat in the biological system. The photothermal sensitive nanomaterials function as nanotransducers to convert light into heat in order to generate localized hyperthermia for the stimulation of neurons. Currently, several photothermal nanomaterials, including gold nanomaterial [7,8,9,10,11,12,13], carbon nanomaterials [14, 15], liquid–metal nanocapsules [16], and semiconducting polymer nanobioconjugates (SPN) [17,18,19] have been shown to be able to modulate neural activities.
The application of gold-based nanomaterials has substantially grown for the past few years due to their unique characteristics, including high biocompatibility, great optical property, easy synthesis process, and the possibility of surface modifications [6]. More importantly, their innate ability to cross the BBB opens up a wide range of possibilities for treating neuronal disorders of the central nervous system (CNS) [13, 20]. The photothermal property of gold nanomaterials is due to the stimulation of localized surface plasmon resonance (LSPR) with the light wavelengths between visible to near-infrared range. Upon exposure at the resonant frequency, electrons within the gold materials are excited and oscillate collectively, followed by mild heat generation, which activates the transient receptor potential cation channel subfamily V member 1 (TRPV1) (Fig 1).
Several studies have presented stimulation of neurons using gold nanomaterials in three different shapes, which are nanorod, nanosphere, and nanostar. The most frequently used morphology of gold nanomaterials for neural stimulation is gold nanorods (AuNR) as their optical absorption can be tuned by modifying the aspect ratio(length/width). In 2013, a group in Australia discovered that AuNR together with a 780 nm near-infrared (NIR) laser is able to induce NG108-15 neuronal cells outgrowth. Similar results were observed with AuNRs coated with poly-(4-styrenesulfonic acid) or silica [22]. In the following two years, the same group also presented that both coated and uncoated AuNRs are able to induce intracellular calcium transients and trigger differentiation of neuronal cells upon exposure to 780 nm laser light [9]. Contemporarily, other groups also demonstrated the use of AuNR for neural stimulation. Kim's group compared the effect of neural tissue stimulation with conventional infrared stimulation and AuNR-assisted infrared stimulation. Their results suggested that the neural stimulation with AuNR significantly increased the neuronal activation level and lowered the stimulation threshold of infrared, which is suggested to lessen the tissue damage [10]. However, a contradictory result published in 2014 claimed that AuNRs inhibited neural activities upon near-infrared exposure in the hippocampal neurons. The paper suggested that the inhibitory effect of photothermal stimulation underwent a different biological pathway with the involvement of the TREK-1 channel, a thermosensitive potassium channel, compared to other studies that claimed that photothermal activation of the TRPV1 channels [11] (Fig. 2b.).
Photothermal manipulation of membrane Ca2 + channel through nanomaterials. a Excitation of dorsal roots ganglion neurons with AuNPs with patch clamp recording setup. Comparison of the firing response of neuron induced by two stimuli types: electric and light. Neurons were responsive to light stimuli in the presence of AuNPs, while washing off AuNP did not trigger any neuronal response. Reproduced with permission [12]. Copyright 2015 Cell Press. b Photothermal inhibition of electrically evoked neural activity using AuNRs. Microelectrode array (MEA) for electrical stimulation and recording of the neural activity. Upon exposure to near infrared (NIR) irradiation, spike rates of neurons decreased in cells treated with AuNRs. Reproduced with permission [11]. Copyright 2014 American Chemical Society c Stimulation of ND7/23 cells and Hela cells by laser-induced liquid metal Nanocapsules. Upon exposure to laser, increased calcium influx can be observed through fluorescent microscopy. Calcium influx degree is positively correlated with the laser irradiation time. Reproduced with permission [16]. Copyright Nature Publishing Group d Nanomodulator in control of neural activity in ND7/23 cells upon NIR irradiation. Activated nanomodulators opened up protein channels for calcium and increased Ca2 + concentration in neurons can be observed in fluorescent microscopy. Reproduced with permission [21, 22]. Copyright 2014 John Wiley and Sons
Other than AuNR, golden nanoparticle (AuNP), which is known as golden nanospheres, and gold nanostar (AuNS) attracted researchers’ attention recently due to their smaller scales, which provide higher precision in neural modulation and less time-consuming synthesis procedures. One study shown in Fig 2a presented that AuNP at a 20 nm scale was able to excite dorsal roots ganglion neurons with a maximum firing rate of about 40 Hz [12]. In addition, the feasibility of AuNPs in the stimulation of a more complex neurological tissue was also shown in the paper. The authors demonstrated that the firing of action potentials was due to the heat-induced membrane capacitance change. Another study by the Yoo group presented that AuNS, which obtained better biocompatibility compared to AuNR, was capable of suppressing the neural activity when it was attached either directly on cell or on a microelectrode arrays (MEAs) chip [13].
In addition to gold nanomaterials, Fig. 3a presents other inorganic nanomaterials accommodated with photothermal properties are also utilized to modulate neural activity.Carbon nanomaterials present a good photothermal conversion efficiency and high energy absorbance in the NIR range [20]. Dye-functionalized carbon nanohorns (CNH), with conjugated fluorescent IRDye800CW = presented higher photothermal conversion efficiency compared to bare CNHs since the excitation energy of the dye upon the NIR irradiation would transfer to the CNH, resulting in a lower stimulation energy level. It was demonstrated that dye-CNH induced calcium influx in Hela, RAW264.7, and ND7/23 cells and triggered paw movement in a living frog model remotely [21, 22](Fig. 2d). Also, another carbon-made nanotube hybrid with thermoresponsive ligands was able to trigger integrin receptor clustering and control the differentiation of human neural stem cells via photothermal effect [14]. Other than carbon nanomaterials, liquid metal nanocapsules, which were formed by light-induced morphological transformation, generated heat in response to CERTAIN Light and induced calcium influx in ND7/23 cells on a microseconds timescale [16] (Fig. 2c).
Other photothermal nanomaterials for neuromodulation. A schematic illustration (left) and TEM (right) image of a Transformable LM nanocapsule Reproduce with permission. [16] Copyright 2017 Nature Publishing Group b Carbonanohorns Reproduce with permission [21, 22]. Copyright 2014 John Wiley and Sons c SPN. Reproduce with permission [17]. Copyright 2016 American Chemical Society
In addition to inorganic nanomaterials, organic nanomaterial as shown in Fig. 3b and c, which were nanobioconjugates made of semiconducting polymer (SP), has also been used as a photothermal transducer in neuromodulation.This organic nanoconstruct exhibited better biocompatibility and higher photothermal conversion efficiency compared to GNR. Not surprisingly, it was reported in the study that their designated variants of SP nanobioconjugates increased the intracellular calcium influx by twofold within the first 100 ms and the calcium channel activities could be controlled reversibly by switching the NIR laser on and off at an interval of 0.5 s [17]. Another macromolecular infrared nanotransducers for deep-brain stimulation (MINDS), which composed of organic SP core showed efficient heat generation upon NIR-II illumination. Even at a distance of 50 cm above the mouse head, deep brain neurons were activated at a low incident power density [18]. Moreover, NIR responsive nanoparticles proposed by the Xing group obtained not only excellent photothermal efficiency but also an ability to penetrate the blood brain barrier as well as drug loading capability, which presented a new concept of multifunctional nanotransducers for neuromodulation [19].
1.4 Magnetothermal nanomaterials
The penetration of visible light into biological tissue is limited due to light scattering, thus the optical approach in modulating neural activities in the deep brain region remains a problem. Magnetic fields are deemed as promising external stimuli for controlling neural activities due to their relatively low interaction with biological molecules and ability to penetrate deep brain regions. Superparamagnetic nanoparticles, which are the most widely used magnetic nanoparticles (MNPs), are small particles made from ferromagnetic materials. They are usually tiny enough to be counted as only single-domain magnetic particles, thus no interactions or orderings can be observed. In other word, superparamagnetic nanoparticles do not retain any net magnetization in the absence of the external magnetic field, which makes them suitable for biomedical usage [15].
In recent years, in order to strengthen the modulation effect and provide precise localization, more studies have used MNPs as transducers to translate magnetic fields into different stimuli such as thermal energy. The first study that applied MNPs for remote neuromodulation was carried out in 2010. Huang et. al presented that manganese ferrite (MnFe2O4) nanoparticles with a diameter of 6 nm were able to convert the magnetic energy imposed by a radio-frequency (RF) magnetic field into thermal energy. By conjugating streptavidin with nanoparticles, MNPs were able to target cells of interest that expressed genetically engineered proteins both in hippocampal neurons and in C.elegans. Moreover, the study demonstrated that with the application of MNPs, a RF magnetic field strength at 0.67kA m-1, which fulfills the Food and Drug Administration’s standard for RF fields’ strength for clinical use, was able to induced calcium influx and led to cell membrane depolarization in hippocampal neurons. Moreover, the MNPs together with magnetic fields were able to trigger a thermal avoidance reaction in C. elegans [23](Fig. 5b).
Verification of the effectiveness of MNP was first applied on mammalians in 2012. Stanley et.al demonstrated with the expression of genetically engineered protein and application of MNPs with exposure of magnetic field were able to remotely control the release of insulin in mice [24]. In 2015, Chen et al. presented the use of the magnetothermal effect to modulate of neural activities in a mammalian system. In this work, with the introduction of genetic engineering protein receptors into the ventral tegmental area (VTA), followed by MNPs injection and exposure to magnetic fields, the magnetothermal excitation was observed both in vitro, in which the membrane depolarization was induced in hippocampal cells, as shown in Fig. 4a, and in vivo, in which a specific neuronal population was activated in the deep brain region in mice, as shown in Fig. 5c. In addition, the researchers showed the long term effectiveness of neural stimulation with MNP for more than one month [25]. Later in 2016, bidirectionally targeted temporal neuromodulation through magnetothermal effect in mice was presented by Stanley et. al. In the study, the TRPV1 receptors were targeted in a subpopulation of glucose-sensing neurons in ventromedial hypothalamus (VMH) through ferritin nanoparticles injection and magnetic field stimulation. Both remote excitation and inhibition of the modified TRPV1 channels successfully adjusted the blood glucose levels and altered the feeding behavior in mice [26].
Magnetothermal manipulation of neural activities in vitro through MNPs. a Alternating magnetic fields induced action potentials with co-existence of TPRV1 and MNPs. Reproduce with permission [25]. Copyright 2015 American Association for the Advancement of Science b Inhibition of neural activity in hippocampal neurons expressing anoctamin 1 with MNP upon exposure to the magnetic field. Reproduce with permission [28]. Copyright 2018 Frontier Scientific Publishing c Increased firing rates induced by magnetic fields and MNPs was observed through GCaMP6f fluorescence. Reproduce with permission [27]. Copyright 2017 ELife Sciences Publications d Calcium concentration changed in adrenal cells in response to capsaicin and magnetothermal stimulation by MNPs. Reproduce with permission [30]. Copyright 2020 American Association for the Advancement of Science
Magnetothermal manipulation of neural activities in vivo through MNPs. a Magnetothermal stimulation in the motor cortex induced running behavior in awake mice. The activeness corresponded to the onset-time period of magnetic fields. Reproduce with permission [27]. Copyright 2017 ELife Sciences Publications. b Thermal avoidance response in C. elegans was evoked by MNP upon application of magnetic fields. A threshold temperature for behavioral response was measured to be at 34 °C. Reproduce with permission [23]. Copyright 2010 Nature Publishing Group c Magnetothermal stimulation was able to excite neurons in vivo represented by the c-fos staining under fluorescence microscopy. Reproduce with permission [25]. Copyright 2015 American Association for the Advancement of Science
In 2017, magnetothermal neural stimulation was validated in an awake, freely moving animal model. (Fig. 5a) In order to confirm the capability of magnetothermal stimulation in modulating any behaviors in an freely moving animal, Pralle group activated the motor cortex and observed the running behavior in mice. The motor cortex of mice was expressed with uniform TPRV1 with a viral infection to ensure robust magnetothermal activation of neurons. It was reported that the magnetic field induced a mice behavior of running along the periphery of the observation arena, compared to resting or slow locomotion in the absence of a magnetic field [27]. Over the years, TRPV1 has been genetically modified in cells or animals to active neurons and evoke animal behaviors. In 2018, the Pralle group demonstrated that the anoctamin 1 (TMEM16A), a temperature gated chloride channel, could suppress 80% of the neuronal activities upon magnetothermal stimulation [28]. Yet TMEM16A has only shown its capability to inhibit neuronal activity in vitro, and therefore, more work needs to be done to validate the effect of TMEM16A in the mammalian system (Fig. 4b).
Other than their role as primary transducers, MNPs have also been applied as secondary transducers for neuromodulation. In a study conducted by Rao et al., they presented a remotely controlled chemo-magnetic neuromodulation in freely moving animals. In the study, the heat produced by MNPs upon magnetic field exposure triggered the drug release of thermal-sensitive liposomes which in turn led the chemogenetics activation of specific neurons that have been genetically modified. The magnetically gated liposomes successfully alternated both social and motivation behaviors of mice through the activation of different pathways [29].
2 Summary and perspectives
In this review, recent progresses with respect to two promising approaches, photothermal and magnetothermal, in nanomaterials-assisted thermally-induced neuromodulation have been introduced. These new techniques not only provide minimal invasiveness, high spatial–temporal resolution as well as cell-type specificity, but also bring neuromodulation techniques into a new safer stage. However, there still remain several challenges to be answered for clinical usage, including the safety of nanomaterials and transfection with viral vectors. Thereby, future works should consider the feasibility of implementing their techniques on human subjects.
References
Johnson MD, Lim HH, Netoff TI, Connolly AT, Johnson N, Roy A, Holt A, Lim KO, Carey JR, Vitek JL, He B. Neuromodulation for brain disorders: challenges and opportunities. IEEE Trans Biomed Eng. 2013;60:610–24.
Wong KH, Riaz MK, Xie Y, Zhang X, Liu Q, Chen H, Bian Z, Chen X, Lu A, Yang Z. Review of current strategies for delivering Alzheimer’s disease drugs across the blood-brain barrier. Int J Mol Sci. 2019;20:381.
Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412–a020412.
Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, Matthews K, McIntyre CC, Schlaepfer TE, Schulder M, Temel Y, Volkmann J, Krauss JK. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15:148–60.
Polanía R, Nitsche MA, Ruff CC. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci. 2018;21:174–87.
Paviolo C, Stoddart PR. Gold nanoparticles for modulating neuronal behavior. Nanomaterials. 2017;7:1–14.
Rizvi SMD, Hussain T, Ahmed ABF, Alshammari TM, Moin A, Ahmed MQ, Barreto GE, Kamal MA, Ashraf GM. Gold nanoparticles: a plausible tool to combat neurological bacterial infections in humans. Biomed Pharmacother. 2018;107:7–18.
Raliya R, Saha D, Chadha TS, Raman B, Biswas P. Non-invasive aerosol delivery and transport of gold nanoparticles to the brain. Sci Rep. 2017;7:1–8.
Paviolo C, Haycock JW, Cadusch PJ, McArthur SL, Stoddart PR. Laser exposure of gold nanorods can induce intracellular calcium transients. J Biophotonics. 2014;7:761–5.
Eom K, Kim J, Choi JM, Kang T, Chang JW, Byun KM, Jun SB, Kim SJ. Enhanced infrared neural stimulation using localized surface plasmon resonance of gold nanorods. Small. 2014;10:3853–7.
Yoo S, Hong S, Choi Y, Park JH, Nam Y. Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers. ACS Nano. 2014;8:8040–9.
Carvalho-de-Souza JL, Treger JS, Dang B, Kent SBH, Pepperberg DR, Bezanilla F. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron. 2015;86:207–17.
Lee JW, Jung H, Cho HH, Lee JH, Nam Y. Gold nanostar-mediated neural activity control using plasmonic photothermal effects. Biomaterials. 2018;153:59–69.
Kim HW, Yang K, Jeong WJ, Choi SJ, Lee JS, Cho AN, Chang GE, Cheong E, Cho SW, Lim YB. Photoactivation of noncovalently assembled peptide ligands on carbon nanotubes enables the dynamic regulation of stem cell differentiation. ACS Appl Mater Interfaces. 2016;8:26470–81.
Wallyn J, Anton N, Vandamme TF. Synthesis, principles, and properties of magnetite nanoparticles for in vivo imaging applications—A review. Pharmaceutics. 2019;11:1–29.
Chechetka SA, Yu Y, Zhen X, Pramanik M, Pu K, Miyako E. Light-driven liquid metal nanotransformers for biomedical theranostics. Nat Commun. 2017;8:1–19.
Lyu Y, Xie C, Chechetka SA, Miyako E, Pu K. Semiconducting polymer nanobioconjugates for targeted photothermal activation of neurons. J Am Chem Soc. 2016;138:9049–52.
Wu X, Jiang Y, Rommelfanger NJ, Yin R, Liu J. Through-scalp deep-brain stimulation in tether-free , naturally-behaving mice with widefield NIR-II illumination. bioRxiv. 2020; 1–35.
Li B, Wang Y, Gao D, Ren S, Li L, Li N, An H, Zhu T, Yang Y, Zhang H, Xing C. Photothermal modulation of depression-related ion channel function through conjugated polymer nanoparticles. Adv Funct Mater. 2021;2010757:1–9.
Maiti D, Tong X, Mou X, Yang K. Carbon-based nanomaterials for biomedical applications: a recent study. Front Pharmacol. 2019;9:1–16.
Miyako E, Russier J, Mauro M, Cebrian C, Yawo H, Ménard-Moyon C, Hutchison JA, Yudasaka M, Iijima S, De Cola L, Bianco A. Photofunctional nanomodulators for bioexcitation. Angew Chemie. 2014;126:13337–41.
Paviolo C, Haycock JW, Yong J, Yu A, Stoddart PR, Mcarthur SL. Laser exposure of gold nanorods can increase neuronal cell outgrowth. Biotechnol Bioeng. 2013;110:2277–91.
Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat Nanotechnol. 2010;5:602–6.
Dordick S, Friedman JM. Plasma glucose in mice. 2013; 336:604–608.
Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P. Wireless magnetothermal deep brain stimulation. Science. 2015;347:1477–80.
Stanley SA, Kelly L, Latcha KN, Schmidt SF, Yu X, Nectow AR, Sauer J, Dordick JS, Friedman JM. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature. 2016;531:1–20.
Munshi R, Qadri SM, Pralle A. Transient magnetothermal neuronal silencing using the chloride channel anoctamin 1 (TMEM16A). Front Neurosci. 2018;12:1–13.
Munshi R, Qadri SM, Zhang Q, Rubio IC, del Pino P, Pralle A. Magnetothermal genetic deep brain stimulation of motor behaviors in awake, freely moving mice. Elife. 2017;6:1–26.
Rao S, Chen R, LaRocca AA, Christiansen MG, Senko AW, Shi CH, Chiang PH, Varnavides G, Xue J, Zhou Y, Park S, Ding R, Moon J, Feng G, Anikeeva P. Remotely controlled chemomagnetic modulation of targeted neural circuits. Nat Nanotechnol. 2019;14:967–73.
Rosenfeld D, Senko AW, Moon J, Yick I, Varnavides G, Gregureć D, Koehler F, Chiang PH, Christiansen MG, Maeng LY, Widge AS, Anikeeva P. Transgene-free remote magnetothermal regulation of adrenal hormones. Sci Adv. 2020;6:1–12.
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2020R1C1C1007589), Korea Medical Device Development Fund grant funded by the Korean government (Ministry of Science and ICT, Ministry of Trade, Industry and Energy, Ministry of Health & Welfare, Ministry of Food and Drug Safety) (NTIS no. 9991006805), National R&D Program through the National Research Foundation of Korea (NRF) funded by Ministry of Science and ICT (2021M3H4A1A03048648/2021M3F3A2A01037365), the Smart Project Program through KAIST–Khalifa Joint Research Center (KK-JRC), KAIST College of Engineering Global Initiative Convergence Research Program, KI Meta Convergence Research Program, and KAIST Post-AI Research Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yang C declares that s/he has no conflict of interest in relation to the work in this article. Park S declares that s/he has no conflict of interest in relation to the work in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, C., Park, S. Nanomaterials-assisted thermally induced neuromodulation. Biomed. Eng. Lett. 11, 163–170 (2021). https://doi.org/10.1007/s13534-021-00193-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-021-00193-w