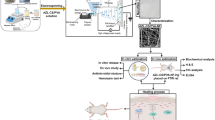
Abstract
The current study aimed to develop a potential wound dressing using vitamin B12-loaded polycaprolacton/gelatin nanofibrous scaffold. In order to produce wound dressings, 1000 mcg of vitamin B12 was added to polycaprolacton/gelatin solution and the nanofibrous scaffolds were fabricated through electrospinning method. The obtained scaffolds were studied regarding their hydrophobicity, microstructure, amount of water absorption, water vapor permeability, tensile strength, release test, and cellular proliferation assay. In vitro studies revealed that the incorporation of vitamin b12 into polycaprolacton/gelatin scaffolds could significantly augment L929 cells proliferation at 1 and 3 days post-seeding. However, there was not statistically significant difference between Vitamin B12-containing and polymer-only scaffolds in tensile strength study, surface wettability measurement, water vapor transmission test, the capacity for water absorption, and nanofiber’s diameter. Both vitamin containing and free dressings were applied on the full-thickness excisional wound in rat model to compare their healing potential. Our results showed that after 14 days, vitamin B12 containing dressing could significantly enhance wound closure compared to vitamin B12 free scaffolds (92.27 ± 6.84% vs. 64.62 ± 2.96%). Furthermore, histopathological examinations showed significantly greater epithelial thickness in polycaprolacton/gelatin/vitamin B12 group compared to other experimental groups. This preliminary study suggest potential applicability of the proposed dressing to treat skin wounds in clinic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Skin as the largest organ in the body plays a fundamental role in body hemostasis. It serves as the first line of defense against environmental hazards, the source for vitamin D production, and protects body against dehydration, and temperature fluctuations [1]. Although this tissue has an inherent capacity to repair following injury, in case of extensive skin injuries body mechanisms fail to replace the damaged tissue and the wound turns into either chronic non-healing wound or fibrosis tissue [2]. The healing of skin wounds involves series of interrelated phases including inflammation, proliferation, maturation, and remodeling. In chronic wounds, a persisting inflammatory environment is established which contribute to matrix metalloproteinases production and remodeling of the existing matrix. In such cases, the application of a proper wound dressing can significantly augment the healing process. Among various wound care products, electrospun nanofibrous wound dressings have gained therapeutic appeal during the past decade [3]. In this method a positive high voltage is applied to a polymer solution to form a polymer jet which travels towards the collecting mandrel to produce nanofibers [4]. The produced scaffolds have a high potential to be used for engineering of various tissues since the parameters of fabrication process can be tuned to form desired fiber diameter and morphology [5]. High surface to volume ratio, resemblance to extracellular matrix architecture, high capacity for drug delivery, and stimulation of skin cells proliferation are possible therapeutic footholds of electrospun wound dressings in wound healing [6, 7]. To prepare an ideal wound dressing, material selection and optimization is a key step [8]. Polymer blend of polycaprolacton (PCL) and gelatin has been successfully utilized in different biomedical fields [9, 10]. Both polymers are biocompatible, biodegradable, and non-immunogenic [11]. The reason why polymer blend of PCL and gelatin is more favorable compared to individual use of each polymer is that PCL lacks cell recognition sites and has a high surface hydrophobicity. On the other hand, gelatin provides sufficient cytocompatiblity and suitable surface wettability but it has rapid degradation rate and low mechanical strength. By combining PCL and gelatin we can take advantages of both polymers and produce a new biomaterial with higher potential for biomedical applications [12, 13]. Despite having several benefits, nanofibrous scaffolds produced from PCL and gelatin does not have sufficient bioactivity for a successful wound healing. Therefore, in this study we hypothesized adding vitamin B12 into the scaffolds to enhance healing activity. Vitamin B12 also knowns as cobalamin is involved in DNA, protein, and fatty acids metabolism in every cell [14]. Depletion of this vitamin contributes to hyperpigmentation and psoriasis disease. It can enhance skin cells proliferation and can aid in angiogenesis phase of wound healing [15, 16]. The aim of the current study is to produce a vitamin B12 loaded PCL/gelatin nanofibrous scaffold in order to produce a potential wound care product.
2 Methods and materials
2.1 Materials
The materials and solvents were purchased from Sigma-Aldrich (St. Louis, USA) and Merck (Darmstadt, Germany), respectively unless otherwise noted.
3 Preparation of vitamin B12 loaded PCL/gelatin scaffolds
Firstly PCL (Mw 70,000) at the final concentration of 12% was added to HFIP solvent and dissolved at room temperature for 24 h. Then gelatin (type A) at weight ratio of 20:80 was added to PCL solution and stirred at room temperature for 6 h. After preparing the polymer solution, 1000 mcg of vitamin B12 was added to PCL/gelatin solution. For electrospinning the polymer samples were transferred to disposable syringes and fixed in the feeding pump of electrospinning device. The feeding rate was set at 1 ml/h and 20 kV voltage was applied to the tip of the syringe. Needle to mandrel distance was 15 cm and the turning rate of the mandrel fluctuated between 550 and 600 rpm.
4 Scanning electron microscopy analysis of the scaffolds
To evaluate the microstructure of the scaffold they were imaged under a scanning electron microscope (SEM; DSM 960A, Zeiss, Germany) at 15 kV after coating with gold for 250 s using a sputter coater (SCD 004, Balzers, Germany).
5 Tensile strength analysis
The mechanical strength of the samples was studied on dry rectangular specimens (80 mm × 10 mm) by using an Instron 5566 universal testing machine (Instron, MA) at a strain rate of 10 mm/min.
6 Contact angle measurement
To evaluate the surface wettability of the scaffolds they were studied using a contact angle measuring device (G10, KRUSS, and Germany).
7 Water vapor permeability (WVP)
The capacity of the dressings to let water vapor passage was analyzed using the flexible bottles permeation technique. 10 ml of water was poured into the bottles and their opening was covered with different scaffolds. The bottles were kept in a shaker incubator for 12 h and weight loss of water was measured as the function of time. The rate of WVP was determined using the following equation:
where w stands for the water weight loss, A is the area (1.18 cm2), and T is the exposure time.
8 Water uptake capacity
For the assessment of the capacity of the samples to uptake water, they were immersed into the water for 24 h and then taken out and immediately weighted. Water uptake capacity was calculated using the following equation.
where w0 is the initial weight of dry scaffolds and w1 is the weight of samples after taking out of water.
9 Cellular proliferation assay
In vitro cytotoxicity of the samples on L929 cells was evaluated using MTT assay. After proper sizing, the scaffolds were put into the wells of 96-well plate. The samples were first incubated with 70% ethanol then ethanol was discarded and the samples were dried under sterile condition followed by UV radiation for 15 min. After sterilizing, 10,000 L929 cells were seeded onto the scaffolds and cultured for 3 days in an incubator. MTT assay was performed 1 and 3 days after cell seeding. At each time step, supernatant in the wells was removed and cell-scaffold construct was washed with PBS three times. Then MTT solution in PBS was added to each well and incubated for 4 h. After 4 h, the MTT solution was discarded and DMSO was added to dissolve any formed formazan crystals. The absorption value of each sample was read at 570 nm using a microplate reader.
10 Microbial penetration
The ability of the dressings to deter microbial invasion was assessed by placing the scaffold on an open container filled with 5 ml of Brain heart infusion (BHI) broth (Merck, Germany) (test area: 0.8 cm2). Containers closed by cotton and open vials served as negative and positive controls respectively. The samples were maintained in room temperature for 7 days. The cloudiness of the media as the reflection of microbial invasion was studied at 3 and 7 days time-steps in which the absorbance values of the samples was determined by a microplate spectrophotometer at 600 nm.
11 Release study
Vitamin B12-loaded scaffolds were cut and put in the beaker containing 10 ml of distilled water and kept on a vertex shaker. At predetermined time intervals 1 ml of samples was taken out and replaced with an equal amount of distilled water. The absorbance of the collected samples was measured using a UV visible spectrophotometer at a wavelength of 372 nm. Vitamin B12 concentrations were studied according to absorbance values of pre-calibrated data from known concentrations.
12 In vivo study
A full thickness excisional wound model was exploited to assess the healing capacity of the fabricated dressings. Nine male Wistar rats were purchased from Pasteur institute and kept at standard conditions at the time of study. For the wound model creation, the animals were anesthetized by intraperitoneal injection of Ketamine 100 mg/kg/Xylazine 10 mg/kg. The skin in the back of the animals was shaved and disinfected using povidone iodine and 1.5 × 1.5 cm2 of their skin was removed. The rats were divided into three groups including PCL/gelatin group, PCL/gelatin/Vit B12 group, and control group (in which the wounds were covered by sterile gauze). After 7 and 14 days post-surgery, the macroscopic changes in the wounds were studied by imaging using a digital camera. The wound size reduction was calculated using an image analysis software (Digimizer, Ostend, Belgium) from the corresponding images using the following equation:
After 14 days, the rats were humanely killed and the wound samples were harvested for histopathological examinations. The specimens were fixed in 10% buffered formalin and then processed and embedded in paraffin. The samples were then cut and stained with hematoxylin-eosin (H&E) and visualized under a light microscope.
12.1 Statistical analysis
The results were statistically analyzed by Graph pad prism version 5 software using Student’s t-test and the data were expressed as the mean ± standard deviation (SD). All experiments were performed at least three times and in all evaluations, p < 0.05 was considered as the statistically significant.
13 Results
13.1 Characterization of the scaffolds
The results of SEM imaging (Fig. 1) showed that the scaffold had randomly-oriented fibers with smooth morphology. 30 random fibers were selected for size measurement using image analysis software and the results showed that there is no statistically significant difference between vitamin B12 containing and vitamin B12 free scaffolds (673.68 ± 11.64 nm vs. 710.19 ± 9.70 nm, for PCL/gelatin and PCL/gelatin/Vit B12 scaffolds respectively). Tensile strength plays a vital role in the easy applicability of the wound dressings. Our data showed that tensile strength for PCL/ gelatin and PCL/gelatin/Vit B12 scaffolds was 2.67 ± 0.29 Mpa and 2.80 ± 0.96 Mpa respectively. Statistically no significant difference was observed between groups. Surface wettability of the dressings is a determining factor for a successful wound healing since it affects the ability of the dressing to absorb wound exudates and maintaining the wet environment of the wound. The contact angle measurement for PCL/gelatin and PCL/gelatin/ Vit B12 scaffolds showed that there was no statistically significant difference between two dressings (59.08 ± 2.37° vs. 60.93 ± 4.51°). The ability of the dressing to allow for gas exchange can have a profound impact in wound healing. Higher WVP causes rapid dehydration which results in scar tissue formation while on the other hand low WVP results in accumulation of wound exudates and predispose the wound for infections [17]. Our results showed that the WVP for PCL/gelatin and PCL/gelatin/ Vit B12 scaffolds was 13.98 ± 3.41 mg/cm2 and 12.97 ± 2.38 mg/cm2 respectively. The differences were not statistically significant. The capacity of the scaffolds to absorb water was measured since it reflects the ability of the wound dressing to regulate wound exudate [18]. The results showed that water-uptake capacity of the PCL/gelatin and PCL/gelatin/Vit B12 scaffolds was 11.32 ± 1.09% and 12.06 ± 2.13% respectively. The differences were not statistically significant. MTT assay was performed to evaluate the toxicity of the dressings towards L929 cells. As shown in Fig. 2 the absorbance values for PCL/gelatin/Vit B12 scaffolds was significantly higher compared to PCL/gelatin scaffolds in 1 and 3 day after cell seeding implying that vitamin B12 incorporation has augmented L929 cells proliferation. Wound dressings should prevent wound infection by stopping the bacteria from migration to the wound bed. This ability was assessed by microbial penetration test. The results (Fig. 3) showed that microbial contamination of the bottles that ware capped by PCL/gelatin or PCL/gelatin/Vit B12 scaffolds was comparable with negative control group in which the container was sealed with airtight cap. This finding implies that the produced dressings have considerably prevented microbial penetration. The absorbance values for positive control group was significantly higher than negative control group and both scaffolds. There was not statistically significant difference between vitamin-loaded or vitamin-free scaffolds indicating that Vitamin B12 had no effect on bacterial migration through the dressing. The release study (Fig. 4) showed that the scaffolds displayed an initial burst release of 26% within the 24 h and a relatively slower release which was sustained after 120 h. The initial burst release can be due to presence of Vitamin B12 on nanofiber’s surface while the sustained release could be due to gradual degradation of gelatin within the incubation period [19].
14 In vivo study
To quantify the wound healing process wound size reduction was determined according to macroscopic images. Data showed that wounds treated with PCL/gelatin/Vit B 12 scaffold had 92.27 ± 6.84% of wound closure at 14 days post-surgery while wounds covered with PCL/gelatin group had 64.62 ± 2.96% of wound size reduction. At 7 days post-surgery PCL/gelatin/Vit B12 group could reach to 59.38 ± 3.37% of wound closure while PCL/gelatin group could close the wounds for about 42.18 ± 2.69% at this time step. Statistical analysis showed that there was a statistically significant difference at both time steps between Vitamin B12-containing and Vitamin B 12-free groups. Sterile gauze group had 19.38 ± 2.98% and 42.34 ± 1.87% of wound closure at 7 days and 14 days post-surgery. H8E staining (Fig. 5) showed that the wounds treated with PCL/gelatin/Vit B12 dressing had significantly higher epithelial thickness compared to PCL/gelatin group (39.31 ± 2.09 µm vs. 24.18 ± 3.65 µm). This value for negative control group was 7.21 ± 1.38 µm. These results signifies better re-epithelialization process in the PCL/gelatin/Vit B12 group.
15 Discussion
Vitamins are of paramount importance in skin care and wound healing. Therefore, they have been supplemented with various cosmetic products. However, due to fast oxidation process their bioavailability is rather low [20, 21]. Therefore, the use of an efficient drug delivery vehicle which releases these agents in a sustained manner can circumvent vitamins low bioavailability. A variety of drug delivery vehicles have been devised for vitamins delivery [22, 23]. However, the way these delivery vehicles can be exploited to produce a wound dressing is an intense area of research. In a previous study conducted by Li et al. Vitamin A and E were successfully incorporated into gelatin nanofiber by electrospinning technique in order to produce a potential wound dressing. The scaffolds loaded with vitamins exhibited a sustained release over 2 months. Antibacterial studies revealed that vitamin E-loaded dressings could successfully inhibit the growth of E. coli and S. aureus. Cell culture studies showed that the scaffold s could enhance the proliferation of fibroblasts and significantly improve the expression of collagen-related genes. In vivo analysis confirmed higher healing potential of vitamin loaded dressings compared to vitamin-free ones [24]. In another study Najafi et al. loaded vitamin C in a core shell system of polyvinyl alcohol/chitosan nanofibers to fabricate a nanofibrous wound dressing. Encapsulation is an efficient method that inhibits rapid oxidation of vitamin C hence increase its bioavailability [25]. Madhaiyan et al. fabricated a transdermal delivery system based on PCL for vitamin B12. They produced the scaffolds using electrospinng method. The physicochemical and biological properties of the scaffolds were studied. To reduce the high hydrophobicity of PCL plasma treatment was exploited. High surface to volume ration and high drug encapsulation efficacy have added to nanofiber-based drug delivery vehicles biomedical application [26]. Abubakr et al. investigated the effects of encapsulation process parameters of calcium alginate beads on Vitamin B12 drug release kinetics. They produced the beads by ionotropic gelation method followed by oven air drying. Among various parameters, thermal effects were found to play the major role in tuning the release profile of Vitamin B12 from the alginate beads [27]. In the current study we successfully fabricated an electrospun PCL/gelatin wound dressing for sustained delivery of Vitamin B12 into the wound bed. This delivery vehicle could significantly augment L929 cells proliferation which was evidenced by MTT assay. This is in accordance with previous studies that have reported proliferative effects of this vitamin on various cell lines [28, 29]. Various physicochemical analysis were performed in this study and they proved that this dressing is not cytotoxic and has suitable mechanical properties for easy application on the wound site. Furthermore, surface wettability study was performed since this feature determines the adhesiveness of the dressing to the wound site which is unfavorable and can reopen the closed wound upon wound dressing’s removal [17]. Polymer blend of PCL and gelatin provided a suitable surface wettability that did not adhere to the wound bed. Generally synthetic biomaterials are more hydrophobic compared to naturally-derived materials and this hampers their tissue engineering applications. One of the most effective strategies to improve their surface wettability is to use a hybrid material of synthetic and natural polymers. In the current study we used a polymer blend of PCL and gelatin to fabricate the nanofibers and the produced scaffold s exhibited an optimal surface hydrophilicity. WVP and capacity for water uptake studies was performed to evaluate the ability of the dressings to absorb wound exudates and keeping the wound wet. In addition, ease of nutrients diffusion through the wound dressing to the cells which have been seeded on them in the culture system would be facilitated by proper water holding capacity which will prevent water loss in the wound site. The MTT assay confirmed that the scaffolds could supply cultured L929 cells. In vivo study proved that the proposed wound dressing could successfully regulate wound exudate and protected the wound from infection. In vivo study was conducted to compare the healing potential of Vitamin B12-loaded wound dressings with the same scaffold but without Vitamin. All in all, wound closure study and histopathological examinations showed that vitamin-containing dressings outperformed the polymer only scaffold. This could be because of the following reasons. Firstly, Vitamin B12 may have increased the proliferation of resident fibroblast cells and keratinocytes as its proliferative effects on various cell lines have been well documented in other studies [30, 31]. Secondly, this agent can enhance the protein production and metabolism therefore it is expectable that Vitamin B12 has increased the collagen synthesis which plays an integral role in wound closure [32]. Finally, vitamin B12 is heavily involved in angiogenesis therefore it can aid in granulation tissue formation which is one of the important phases of wound healing [33]. This preliminary results provide evidence suggesting the potential applicability of proposed wound dressing to treat skin injuries in clinic.
16 Conclusions
In summary, Vitamin B12 was incorporated into polycaprolacton/gelatin nanofibers in order to fabricate a potential wound dressing. The addition of Vitamin B12 significantly enhanced L929 cells proliferation. Macroscopic and histopathological examinations proved that Vitamin B12-loaded dressings had higher healing potential compared to polymer-only scaffolds.
References
Stojic M, et al. Skin tissue engineering. In: García-Gareta E, editors. Biomaterials for skin repair and regeneration. Amsterdam: Elsevier; 2019, p. 59-99.
Qu J, et al. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem Eng J. 2019;362:548–60.
Zahedi P, et al. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Technol. 2010;21(2):77–95.
Bognitzki M, et al. Nanostructured fibers via electrospinning. Adv Mater. 2001;13(1):70–2.
Subbiah T, et al. Electrospinning of nanofibers. J Appl Polym Sci. 2005;96(2):557–69.
Zhao R, et al. Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int J Biol Macromol. 2014;68:92–7.
Gamez E, et al. Antimicrobial electrospun polycaprolactone-based wound dressings: an in vitro study about the importance of the direct contact to elicit bactericidal activity. Adv Wound Care. 2019;8(9):438–51.
Xue J, et al. Bioinspired multifunctional biomaterials with hierarchical microstructure for wound dressing. Acta Biomater. 2019;100:270–9.
Heidari M, et al. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater Sci Eng C. 2019;103:109768.
Gil-Castell O, et al. Polycaprolactone/gelatin-based scaffolds with tailored performance: in vitro and in vivo validation. Mater Sci Eng C. 2020;107:110296.
Bahcecioglu G, et al. Hydrogels of agarose, and methacrylated gelatin and hyaluronic acid are more supportive for in vitro meniscus regeneration than three dimensional printed polycaprolactone scaffolds. Int J Biol Macromol. 2019;122:1152–62.
Hu Y, et al. Electrospun gelatin/PCL and collagen/PCL scaffolds for modulating responses of bone marrow endothelial progenitor cells. Exp Therap Med. 2019;17(5):3717–26.
Kannaiyan J, et al. Fabrication of electrospun polycaprolactone/gelatin composite nanofibrous scaffolds with cellular responses. Am J Nano Res Appl. 2019;7(2):11–20.
Mendonça N, et al. Plasma vitamin B12, supplementation and mortality. J Gerontol Ser A. 2019;74(1):138–8.
Rathod RS, et al. Maternal omega-3 fatty acid supplementation to a vitamin B12 deficient diet normalizes angiogenic markers in the pup brain at birth. Int J Dev Neurosci. 2015;43:43–9.
Aroni K, et al. Skin hyperpigmentation and increased angiogenesis secondary to vitamin B12 deficiency in a young vegetarian woman. Acta Derm Venereol. 2008;88(2):191–2.
Ehterami A, et al. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int J Biol Macromol. 2018;117:601–9.
Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care. 2014;3(8):511–29.
Salehi M, et al. Sciatic nerve regeneration by transplantation of Schwann cells via erythropoietin controlled-releasing polylactic acid/multiwalled carbon nanotubes/gelatin nanofibrils neural guidance conduit. J Biomed Mater Res Part B Appl Biomater. 2018;106(4):1463–76.
Yu Y, et al. Vitamin metal-organic framework-laden microfibers from microfluidics for wound healing. Mater Horiz. 2018;5(6):1137–42.
Mohammed BM, et al. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int Wound J. 2016;13(4):572–84.
Starr NJ, et al. Enhanced vitamin C skin permeation from supramolecular hydrogels, illustrated using in situ ToF-SIMS 3D chemical profiling. Int J Pharm. 2019;563:21–9.
Batchelor R, et al. (–)-Riboflavin (vitamin B2) and flavin mononucleotide as visible light photo initiators in the thiol-ene polymerisation of PEG-based hydrogels. Polym Chem. 2017;8(6):980–4.
Li H, et al. Electrospun gelatin nanofibers loaded with vitamins A and E as antibacterial wound dressing materials. RSC Adv. 2016;6(55):50267–77.
Najafi-Taher R, et al. Preparation of an ascorbic acid/PVA-chitosan electrospun mat: a core/shell transdermal delivery system. RSC Adv. 2015;5(62):50462–9.
Madhaiyan K, et al. Vitamin B12 loaded polycaprolactone nanofibers: a novel transdermal route for the water soluble energy supplement delivery. Int J Pharm. 2013;444(1–2):70–6.
Abubakr N, et al. Effects of encapsulation process parameters of calcium alginate beads on Vitamin B12 drug release kinetics. Asia Pac J Chem Eng. 2010;5(5):804–10.
Kim G, et al. Effects of vitamin B12 on cell proliferation and cellular alkaline phosphatase activity in human bone marrow stromal osteoprogenitor cells and UMR106 osteoblastic cells. Metabolism. 1996;45(12):1443–6.
Urban K, et al. Influence of B vitamins on proliferation and differentiation of osteoblastic bovine cell cultures: an in vitro study. In: Urban K, Auer J, Bürklein S, Plate U, editors. Biomineralization. Springer; 2018. p. 121–8.
Cantatore P, et al. Alteration of mitochondrial DNA and RNA level in human fibroblasts with impaired vitamin B12 coenzyme synthesis. FEBS Lett. 1998;432(3):173–8.
Wang Y-P, et al. High frequencies of vitamin B12 and folic acid deficiencies and gastric parietal cell antibody positivity in oral submucous fibrosis patients. J Formos Med Assoc. 2015;114(9):813–9.
Rembe J-D, Fromm-Dornieden C, Stuermer EK. Effects of vitamin B complex and vitamin C on human skin cells: is the perceived effect measurable? Adv Skin Wound Care. 2018;31(5):225–33.
Saghiri MA, et al. Vitamins and regulation of angiogenesis:[A, B1, B2, B3, B6, B9, B12, C, D, E, K]. J Funct Foods. 2017;38:180–96.
Acknowledgements
This study was supported by AJA University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no financial or other potential conflicts of interest exist with regard to this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional research committee. Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Farzanfar, S., kouzekonan, G.S., Mirjani, R. et al. Vitamin B12-loaded polycaprolacton/gelatin nanofibrous scaffold as potential wound care material. Biomed. Eng. Lett. 10, 547–554 (2020). https://doi.org/10.1007/s13534-020-00165-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-020-00165-6