Abstract
Wet electrodes are widely used to perform biopotential measurements from the body, such as electroencephalogram, electrocardiogram, and electromyogram. These electrodes have certain disadvantages, including bacterial growth, irritation in long-term recordings, infection to patients’ skin caused by skin preparation techniques, and unpleasant feeling caused after their removal. Thus, a sprayable hydrogel (SH) was designed to avoid these problems. Five electrode configurations, namely, Zipprep™, wet Ag/AgCl, wipes, dry Ag/AgCl, and SH, were tested using an impedance analyzer. Measurements were obtained by placing each of the electrode systems on the forearm of five subjects, which comprised one Caucasian, two Indians, one Syrian, and one Cypriot aging between 23 and 60 years for 10 min. Impedance versus time and reactance versus resistance performance plots were compared and assessed. The performance of the SH sprayed under dry electrodes had lower impedance values compared with those of the dry Ag/AgCl and wipes. As a result, the SH electrode configuration can be used as an electrode set-up for acquiring and recording various physiological signals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The human body comprises many living tissues and produces electrical signals because of muscles and nerves that serve as sources for the functioning of the entire living system. Muscles usually contract with the help of the potential differences generated by migrating ions across cell membranes [1]. When cardiac cells are excited because of disturbed ionic concentrations across their cell membranes, they produce a characteristic pattern called electrocardiogram (ECG). Similarly, excited neurons and muscular cells in the brain produce characteristic patterns called electroencephalogram (EEG) and electromyogram (EMG), respectively. These generated biopotentials reach the skin because of the conductive property of the human body, wherein calcium, sodium, and chloride ions are the main contributors for the transport mechanism [2]. To make these potentials meaningful, the produced ionic current needs to be converted into an electron current by selecting a suitable transducer using appropriate electrodes.
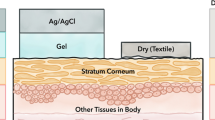
Meanwhile, the human skin acts as a barrier to prevent foreign bodies from entering into the human body. Thus, structural integrity is advantageous in the defense mechanism. However, recording biopotentials becomes a major challenge for biomedical engineers because of their varying impedance values [3]. Stratum corneum (SC), which refers to the outer layer of the skin, presents a major challenge for physicians because the ion transfer for measuring potentials is poorly allowed by this layer. The reason is that this layer has a very unstable and poorly defined capacitance. Capacitance is dependent on many factors, such as humidity, thickness [2], temperature, sweating [4], and type of electrode and gels used. Moreover, the impedance of the skin for frequencies in the range of 1 Hz to 1 MHz is higher than that of a metal electrolyte. Furthermore, impedance decreases with the increase in frequency because of the effect of SC [5].
When an electrolyte gel is applied onto the SC, its behaviour becomes similar to a semipermeable layer between applied gels that develops an ionic concentration gradient across, and the generated potential difference is called “contact potential”. However, the effect of contact potential on the signal of interest can be reduced by rubbing or puncturing the SC vigorously. However, this process is ineffective in long-term recording because the SC could regenerate in 24 h [6]. Furthermore, this process results in skin irritation [7] and signal degradation. The contact potential can also be eliminated by connecting the possible differences of electrode–electrolyte and electrolyte–skin interfaces in opposite directions during recording, thereby nullifying the effect of both potential differences. However, in practice, even if utmost care is taken in manufacturing the electrodes to reduce the electrode potential, other factors, such as hydration, temperature, sweating [4, 8], and electrolyte concentrations, will vary the contact potential, there by resulting in skin impedance. The produced noise can also be of the order of 1 to 15 microvolts [9].
The application of the electrode gel between the electrode and the skin will maintain the contact impedance as low as possible and reduce motion artefacts. In general, electrolyte (electrolyte jelly) comes in the form of electrolyte gel with chloride ions that maintain good contact [6]. Foams can also be used as electrode skin interface to match the surface geometry of the skin so as to accommodate the gaps of skin and hair [2].
Many research groups concentrated on measuring how these contact impedances vary for different conditions [4, 5, 9]. Moreover, some groups designed electrodes, such as CNT/PDM-based novel comb-shaped electrodes [10]. Silicon-based micro needle electrodes were also designed by using KoH etching technique to record hairy sites [11]. In 2014, Ha et al. devised an integrated circuit electrode interface to record biological signals to increase the common mode rejection and signal-to-noise ratios (SNR) [12]. In contrast to solid electrode configurations, researchers have also wax printed a 29.4 µm-thick conductive silver (Ag) flake ink. A Ag electrochemical sensor was screen printed on the wax based fabrication of the polypyrrole conductive polymer via electrochemical polymerization [13]; moreover, paper-based substrates and flexible non-woven fabrics were designed [14, 15].
ECG activity was taken by using liquid gallium as an electrode interface [16]. Electrode gels were formulated to reduce skin impedance so as to ensure the accuracy of the measured biopotentials [2, 7, 17]. Furthermore, other groups compared the performance of different electrodes and electrode gels to record biopotentials [18,19,20]. As a result, different types of electrodes and electrode gels were invented to reduce the impedance between the recording electrode and the skin. The development of new techniques for accurate measurements is continuous.
The gels used for measuring electrodermal activities should be ion-free, with the exception of sodium chloride (NaCl) ions, which should have a concentration similar to that of the human sweat (0.05 mol/l). In general, the gel should have two parts of Unibase and one part of physiological saline, which should be mixed using an electrical mixer and be left to stand before use [21]. Unibase contains ethylene polymer, takes up to 30% of its own water weight, and contains 0.02 mol/l of sodium ions. Furthermore, it contains preservatives, such as propyl hydroxy benzonate, sodium lauryl sulphate, and sodium citrate [22]. Cellulose-based electrode gels are made by adding 20 g of methylcellulose in 160 ml of 0.049 mol/l of NaCl solution, and then boiled for 4 min by rapid cooling under cool water. The final concentration of such mixtures will be 0.05 mol/l of NaCl and 11.1% methyl cellulose [22].
Freire et al. [23] provided a list of commercially available gels and their constituents. The selection of polarizable or non-polarizable electrodes can be conducted on the basis of the type of the recording site. Non-polarizable electrodes, particularly wet and gel electrodes, have minimal motion artefacts [6] and dissipate powerful charge imbalances induced by defibrillators during recordings [1]. Their polarization effects and contact impedances are low [17], economical, and have remarkable hygiene choices because of its disposable nature. Ag/AgCl electrodes also produce noise-free recording and are immune to long-term drifts, which are achievable, if potential differences between the electrodes are maintained to be as low as possible [1]. However, these electrodes also come with drawbacks for certain applications. When electrode gels are applied for non-polarizable electrodes [24], they become prone to dehydration and have reduced SNR [1, 17]. Furthermore, ionic current disturbances result in electrode movements or sweat formation [10, 17, 25]. They also take longer times for skin preparation [20, 26].
From the past 3 decades, large number of ultrasonic hydrogels was reported as tissue-mimicking (TM) materials and ultrasound scanner gels [27]. A TM phantom, which mimics a target tissue acoustically, is critical in quality assurance. Most popular ultrasonic TM phantoms that are made with agar and polyacrylamide gels are reported as TM hydrogel for high-intensity focused ultrasound lesion visualization for quality assurance tests [28,29,30,31]. Moreover, ultrasonic liquid gel for commercial scanners is made with high viscous active agent solutions that are similar to the proposed SH, which exhibits skin-sensitive characteristics, such as skin tightening and smoothing.
Gels and gel electrodes in long-term recordings are prone to bacterial growth, itching, irritation, infections, inflammation, and short shelf life [15]. Although polarizable electrodes have contact impedances that are initially high, contact impedance reduces three times with time because of perspiration [17]. At this point, contact impedance is close to non-polarizable electrodes. Meanwhile, dry electrodes show less motion artefacts than capacitive and wet electrodes [19].They also have disadvantages; such as high contact impedances during initial stages, long settling times for capacitive electrodes, and additional circuit is needed because of the high capacitive source impedance that increases the cost of the electrodes [19]. Moreover, electrode gels are quite thick and are unpleasant after removal of the electrode.
Thus, the preparation of a synthetic skin (e.g., SH) by considering the afore mentioned problems is a breakthrough idea; this idea is achieved by spraying SH on human skin while reducing the time taken for the dry electrodes to reach stable contact impedance from 3 to 8 min to the time similar to the wet electrodes, without solely relying on sweat buildup [18, 19].The synthetic skin should have an evaporative medium that leaves a hydrated layer with ionic properties that are similar to those of the natural sweat. After spraying the solution, the solution should be evaporated quickly to leave a hydrated layer.
This work aims to design a sprayable electrode gel (e.g., SH) that has chemical properties similar to those of commercially available electrode gels and pastes and to compare the contact impedances with floating sponge type, wet, and dry electrodes using impedance spectrometry. We further aim to reduce the initial contact impedance of the dry electrodes without relying solely on sweat buildup.
2 Materials and methods
2.1 Preparation of SH
2.1.1 Materials
To prepare an electrode gel, it should contain three components: a thickening agent, a saline solution with ionic concentration similar to sweat, and a preservative. During the preparation of SH, Hydroxypropyl methylcellulose (HPMC) is used as a thickening agent. Its basic form is Cellulose ether, which forms by hydrated matrix layers when treated with water. It is also widely used in pharmaceuticals for making capsules and other clinical applications [25].It is commercially available under the name of METHOCEL (A4M Premium EP, Dow Chemical Company, USA). To make the saline solution, NaCl was used, and ethanol was added to act as an evaporative and sprayable medium. The preservatives, such as ethyl hydroxyl benzoates and tragacanth, that are used by Grey and Smith were not utilized in the making of SH [22].
2.1.2 SH preparation protocol
The preparation of SH involves the mixing of the saline solution with the HPMC dispersed in ethanol.
-
1.
NaCl was used to prepare a saline solution with a molarity of 100 mM. This saline solution was prepared by adding 2.92 g of NaCl into 100 ml of deionized water and was mixed thoroughly to avoid any crystals. In the 100 ml of NaCl solution, 400 ml of deionized water was added to make a 500 ml final saline solution.
-
2.
A total of 5 g of HPMC was dispersed slowly and steadily for 5 min (time was considered to achieve constant mixing rate and avoid lumping) in 150 ml of ethanol while mixing using a magnetic stirrer at 400 rotations per minute. At this stage, the HPMC particles in the ethanol were still dispersed but not hydrated.
-
3.
A total of 5 ml of saline solution prepared in Step 1was added to the solution obtained from Step 2. Adding the saline solution will hydrate the HPMC molecules and solution isotonic. The solution obtained at this stage was called SH. The process was carried out at room temperature without heating the solutions.
The SH prepared at this stage was too viscous for spraying, and the viscosity of the solution was estimated to be approximately 10 KPas. To make it appropriate for spraying, the SH was further diluted with ethanol at a ratio of 0.0625:1 (SH: ethanol). Mixing SH with ethanol that is higher than the 0.0625 ratio would block the spray head of the spraying bottle used, thereby causing difficulty in pressing after 2 to 3 clicks. Additionally, wipes were prepared by soaking soft perforated cloth in SH and sealed in grip seal bags for storing purposes.
2.1.3 Testing the sprayed area
A spread area test was performed to determine the optimum spraying area by varying the distance between the spray nozzle and the target area. Initially, 5 ml of commercially available food color was mixed in a spray bottle that contains 20 ml of SH. The resultant mixture was sprayed after shaking it thoroughly (to avoid HPMC deposits in spray bottle) on a graph paper by varying the distance from the spray bottle nozzle at 3, 5, and 10 cm. The graph paper was then left to dry for some time, and the stained area and gaps were calculated. From the test results, optimum distance to obtain an even spread area of SH was selected at 3 cm. Spraying at distances more than 3 cm created gaps in the spread area, whereas spraying at a distance below 3 cm made the sprayed surface wetter and took more time for drying.
2.2 Electrical characterization of SH
The method used for the electrical characterization of SH was adopted from [2, 18,19,20], which measures electrode and electrode gel characteristics on the basis of their application on skin. Searle and Kirkup [18] described the performance of wet Ag/AgCl electrodes, and dry stainless steel electrodes by applying them on human skin. The impedance measurements were obtained by plotting |X| versus R in the frequency range of 1–500 Hz. The resulting plot indicates the actual performance of the electrodes and assumes them as the Cole–Cole model (Fig. 1), which is a series resistor, Rs, connected in series with a parallel combination of Rd and Cd.
Searle and Kirkup [19] also compared the performance of the wet Ag/AgCl, dry, and insulating electrodes by placing them on the forearms of five subjects. However, their performance was tested on the basis of the changes of impedances with time. The wet Ag/AgCl electrode was taken as a reference electrode, and the other electrode configurations were compared with it by constructing an electrode attachment case to ensure constant distance among all the electrodes. All the afore mentioned methods were made using a purpose-built analog system. However, Gruetzmann et al. [2] used HP4192A impedance analyzer to calculate impedances.
In the current study of SH electrical characterization, HP 4284A Precision LCR meter (Hewlett-Packard Company, 1000 NE circle Blvd, Corvallis, Oregon, USA) was used. The method used in this work is similar to that of Searle and Kirkup [18] for measuring the performance of different electrode configurations with time. It also uses the method suggested by Searle and Kirkup [18, 19] for electrode placement. The HP 4284A Precision LCR meter is a digital impedance analyzer that can take point measurements over the 20 Hz to 1 MHz frequency range. It uses the test voltage level between 5mVrms and 2Vrms and current levels between 50µArms and 100mArms. Measurements are performed by applying a constant voltage through a pair of injecting leads (e.g., Lcurrent and Lpotential) and by measuring the induced current in the device under test by using a second pair of electrode (e.g., Hpotential and Hcurrent). The lead can either be set up in a 4-lead configuration or a 2-lead configuration. In the current study, a 2-lead configuration was used (Fig. 2).
2.3 Electrode configuration setup
To place the electrodes on the subjects’ forearms and to ensure a constant distance between each electrode pair during the measurement, an electrode assembly was constructed. The dimensions of the plastic assembly were set to 9 cm × 3 cm. To mount the electrodes on the forearm with a fixed separation distance between their centers, holes were punched at a distance of 3 cm. The plastic assembly was stapled with Velcro strap to tie around the forearm securely (Fig. 3a, b). The holes are useful for keeping the separation between the electrodes constant throughout the measurement, as well as in establishing connection with the leads of the LCR meter.
a Physical layout of applying electrodes, b plastic assembly sheet for electrode placement, c assembly of Zipprep™ electrode pair on a plastic electrode assembly sheet, d Assembly of wet Ag/AgCl electrode pair on plastic electrode assembly sheet, e assembly of dry Ag/AgCl electrode pair on plastic electrode assembly sheet
The impedance values of the five electrode configurations were compared. Their construction is explained as follows:
-
1.
A commercially available Zipprep™ electrode supplied by (Aspect medical systems Inc. Norwood, MA, USA.) was used as a reference electrode. It comprises an adhesive layer combined with a small amount of electrode gel and sponge-like material in a grove to puncture the SC gently. The metal part of the electrode is made up of Ag/AgCl with a diameter of 14 mm. Subsequently, the electrodes were attached to the plastic assembly sheet before measurement (Fig. 3c).
-
2.
The second type of electrode configuration used was the wet Ag/AgCl. To prepare this type of electrode configuration, the sponge and gel that come with the Zipprep™ electrode were removed. To remove any traces of gel, ethanol was used without damaging the AgCl layer on the metal part of the electrode. Subsequently, 1.5 ml of electrode gel (Electrocap International, Inc., Horsham, West Sussex, UK) was applied for the wet Ag/AgCl electrode configuration (Fig. 3d).
-
3.
The third type of electrode configuration is the dry Ag/AgCl. To make an electrode pair, a method similar to the one used for preparing the wet Ag/AgCl configuration was used here. However, instead of applying electrode gels, the adhesive layer that surrounds the metal area was cut and removed (Fig. 3e).
-
4.
The fourth electrode configuration is the dry Ag/AgCl electrode configuration, but with the SH applied on the skin.
-
5.
The final electrode configuration measures the contact impedance by wiping SH on the skin using the dry Ag/AgCl electrode configuration. The wipes were prepared by immersing them in high viscous SH and packing them in a sealed grip bag. The grip seal bag prevents the ethanol from drying and guarantees long storage periods.
2.4 Testing the HP 4284A precision LCR meter
Before taking any subject measurements, a Cole–Cole model was constructed and plotted for |X| versus R. The circuit was constructed by connecting a 270 KΩ series resistor with a parallel combination of 390 KΩ resistor and a 1µF capacitor (Fig. 1). The applied voltage was set to 1 V, and the injected frequency was varied from 20 Hz to 1 MHz. Reactance and resistance values were then measured accordingly.
2.5 Measurement of the impedances with five electrode configurations
The contact impedance values of the five electrode configurations, explained above, were measured on five subjects, which comprise one Caucasian, two Indians, one Syrian, and one Cypriot. The subjects were males with mean age of 33.6 years. The experiment was performed with the approval of the ethics committee of the University of Manchester, U.K, and the subjects provided their informed consent to their inclusion in the study as required. To avoid electrical shocks caused by the LCR meter, the injected current was limited to 50 µA. To know the contact impedance of the skin under real conditions, measurements were taken without any skin abrasions or hair shaving. A total of 10 frequencies in the range of 20–5 KHz were used to obtain the reactance and resistance parts of the electrode systems. Each measurement lasted 10 min for a single electrode pair for every subject. As a result, the total time spent on a single subject was 50 min, given that 5 electrode configurations are being tested. Only one electrode pair was measured at any given time per single subject. Plans for applying the different electrode configurations for every subject were made before the measurements were taken. Measurements were taken by placing the electrodes on the anterior right and left forearms (Figs. 4a–e). The Zipprep™ electrode was placed on the anterior side to the proximal end of the forearm (Fig. 5). To measure the wet Ag/AgCl electrode configuration, the electrodes were positioned below the location used for the Zipprep™ electrodes (Fig. 4a). The dry Ag/AgCl electrodes were similarly placed below the wet Ag/AgCl electrode configuration (Fig. 4a). SH was sprayed on the subject’s proximal left forearm by positioning the nozzle 3 cm above the skin. The spraying bottle was well shaken before spraying, and the sprayed site was left to dry for 1 min to form a hydrated layer before placing electrodes on it. Similarly, wipes with SH were also tested on the left forearm on a position slightly below the SH electrode configuration (Fig. 4a). To avoid the differences in impedance values caused by electrode placement on the forearm, the electrode positions were rotated anti clock wise from one subject to another before measurement (Fig. 4b–e). To avoid any electrical shocks, each subject was asked to remove any metal objects and cautioned to avoid any contact with metal objects nearby.
The built-in frequency sweep mechanism on the LCR meter was used. This step enabled quick measurements of R and |X|, which were stored automatically on one screen. However, the speed at which the screen is updated prevented the user from noting down the measured values. Therefore, a camcorder was positioned in front of the screen, and the values were recorded in a video format. The acquired impedance values were in the form of video clips that lasted 250 min for the 5 subjects. Each electrode configuration measurement lasted 10 min. The clips were paused at 30-s intervals, and the values were noted in an Excel sheet, there by providing 1000 values for each subject with 200 values for every pair of electrodes.
3 Results
The values obtained by measuring the spread and gaps that resulted from applying the SH from various distances are plotted as histogram (Fig. 6). A uniform spread area of SH was obtained at 3 cm. However, spraying at distances more than 3 cm created gaps in the spread area. The simple Cole–Cole model of reactance against the resistance of the model components and the obtained impedance at various frequencies were plotted in Fig. 7a and b, respectively. The results are consistent with those obtained by Searle and Kirkup [18].
To know the performance of all the five electrode configurations, the impedances at 45 Hz for any given electrode configuration for all the five subjects were averaged and compared. The comparison was conducted by plotting the relationship between the impedance versus time (Fig. 8). To obtain an acceptable curve-fit, a polyexponential function in the form of Eq. (1) was used [19],
where an, bn, and c are constants; the curve fitting of the impedance against time was plotted in MATLAB, and the results were shown in Fig. 8.
To obtain the reactance against resistance, all the values for any given electrode configuration were averaged over the five subjects with respect to frequency along the time scale (0–10 min) were recorded. To observe the behavior of the reactance and resistance changes with respect to time for the individual electrode configuration, every frequency was plotted in MATLAB. The resulting plots for the dry Ag/AgCl, the Zipprep™, the wet Ag/AgCl, SH, and wipes electrode configurations are shown in Figs. 9, 10, 11, 12, and 13, respectively. The results indicate the changes in impedance values for recorded frequencies for the five electrode configurations.
Figure 9 presents the resistance against reactance of the dry electrode configuration at different frequencies. At 20, 150, and 400 Hz frequencies, the resistance component has decreased but the reactance component has increased, and the electrode configuration has longer stability time at 20, 1000, and 2000 Hz. At 45 Hz, the reactance and resistance components were reduced approximately by 10 KΩ. Figure 10 demonstrates the resistance against reactance of Zipprep™ electrode configuration at various frequencies. At frequencies of 20, 45, and 4000 Hz, longer time was spent to attain stability compared with the remaining frequencies. The resistance and reactance components at 45 Hz and 4000 Hz remain constant but significantly reduced at 20 Hz by an approximate value of 175 KΩ.
In Fig. 11, the reactance component at 20, 150, and 400 Hz has increased, and resistance component has decreased for wet electrode configuration. Moreover, impedance components at 45 Hz have less variation by approximately 10 KΩ throughout the recording.
Figure 12 shows the resistance against reactance of SH electrode configuration at various frequencies. The frequencies at 20, 45, and 400 Hz displayed longer stability time, whereas the reactance component remains constant throughout the recording time at 45 Hz and 4000 Hz.
Figure 13 demonstrates the resistance against the reactance of wipes electrode configuration at various frequencies. The impedance components significantly reduced, except that at 20 Hz. Moreover, this electrode configuration took longer time to attain stable impedance value between frequencies 20 Hz to 400 Hz.
4 Discussion
4.1 Sprayable hydrogel
In this study, the minimum distance for spraying the SH was 3 cm. This distance was optimum because the SH will not be evenly distributed on the skin at higher distances. Furthermore, when the SH was sprayed with distance less than 3 cm, it formed droplets and took more time for drying. At the optimum distance, the ethanol in the SH takes long time to evaporate before a hydrated matrix layer is deposited.
4.2 Comparison of impedances of SH with different electrode configurations
Results were obtained as expected for the dry Ag/AgCl, wet Ag/AgCl, Zipprep™, and SH electrodes. However, the impedance values of the wipes at the initial times were lower than that of the SH (Fig. 8). Instead, it showed slightly higher values, which could be caused by the blockage of sweat pores due to high density of HPMC in the wipes. The performance obtained from the first three types of electrodes (i.e. dry Ag/AgCl, wet Ag/AgCl, and Zipprep™) in the current study matched the results presented by Searle and Kirkup [19]. Moreover, the dry electrode impedances (stainless steel, aluminum, and titanium electrodes) reported by Searle and Kirkup were compared with the proposed SH [12]. The initial contact impedance of SH is approximately 0.63 MΩ, but when compared with stainless steel, aluminum, and titanium electrodes, it becomes 2.2, 3.1, and 2.0 MΩ, respectively. The average value of the initial contact impedance of stainless steel, aluminum, and titanium electrodes is 2.43 MΩ, which is 4 times higher than the SH impedances.
The dry electrodes at 20 Hz took longer time to stabilize compared with the resistance component at 45 Hz, which arrived at a stable rate more rapidly (Fig. 9). Slight increase in the capacitance component may be due to increased capacitance in electrode and epidermis. Moreover, in the settlement in the case of Zipprep™ electrodes, stability was achieved more rapidly at 45 Hz, whereas it took longer for the wet Ag/AgCl electrode configurations to attain stability (Fig. 10). SH and wipes for the frequencies range 20 Hz to 150 Hz attained stability longer (Figs. 11, 12, 13), but their resistance component is lower when compared with the dry Ag/AgCl configuration. Moreover, among all the electrode configurations, the impedances of the Zipprep™ electrodes were low and remained nearly constant throughout the measurement. The low values of this particular type of electrode configuration is due to the reduction of the effect of SC caused by the sponge-like material on the Zipprep™ electrodes that slightly perforates the skin during the application, there by resulting in a large decrease in skin impedance.
The impedances of the wet Ag/AgCl electrode configuration were slightly higher than those of the Zipprep™ electrodes and decreased exponentially with time because of the hydration of the SC by the electrode gel itself. A huge difference in impedance (approximately 30 KΩ) between the dry Ag/AgCl and SH electrodes can be observed in the initial contact with the skin (Fig. 8). This difference can be seen more clearly from 0 to 4 min due to the presence of hydrated HPMC matrix layer on the skin surface. After that period, the impedance values were stabilized without touching the fit line of the dry Ag/AgCl electrodes. The reason behind comparing the impedances at 45 Hz is that EEG and ECG frequency ranges include 45 Hz. The reactance versus resistance plots denote the changes in the resistive and reactance components of the electrode and the electrolyte interface and tissue present between the electrode pairs [6]. Stability can be compared by looking at the length of each line for a given frequency for the entire measuring period.
The Zipprep™ and wet electrodes showed lower impedance values than the rest of the electrode configurations. Furthermore, they attained their steady values rapidly (Fig. 11). The dry Ag/AgCl, SH, and wipes electrode impedance values were higher and took longer time to achieve stability. Such results are consistent with the results obtained by Searle and Kirkup [18, 19]. In the present study, the impedance values may appear slightly higher than those obtained by Searle and Kirkup [18, 19]. The reason is that in the current study, no skin preparation was performed, which would perhaps have made the recorded values higher.
The errors in the measured impedance values depend upon the pressure applied by the electrodes on the skin [2], the separating distance between the electrode pair, the surface area of the applied electrodes [19], and the subjects’ skin type. These factors may have altered the results and made them slightly different to the results obtained by Searle and Kirkup [19]. In the present study, emphasis was given to compare the performance of SH with dry Ag/AgCl electrodes. The results show that although the stability attainment is longer for SH configuration, the reactance component of the SH is lower than the dry Ag/AgCl electrode configuration, thereby proving its advantage over the dry Ag/AgCl electrodes (Figs. 9 and 12).
The scope of this paper in formulating SH was limited to sprayable consistency. The electrical stability of the SH is demonstrated in Fig. 12 via Cole–Cole plots. It takes longer duration to attain a stable state below 200 Hz. Temperature and humidity affects the impedances of different electrode configurations. The present work was performed at a room temperature of 25 °C. Cornish B et al., 1998 reported that humidity and temperature affect the impedances in the recording environment [4]. As the temperature and humidity of the recording person or the recording environment increase, the impedances decrease irrespective of the type of electrodes used. This phenomenon is a result of perspiration build-up between skin and electrodes, thereby reducing the impedances.
Temperature and agitation affect the gelation of the aqueous-treated HPMC, as mentioned in a technical handbook [25]. Rapid heating and agitation of hydrated HPMC would result in altered strength and texture of the HPMC and may influence the SH properties in turn. During the formulation of SH, HPMC was hydrated with saline water at 20 °C in ethanol using a magnetic stirrer set to 400 rpm. At this temperature, the hydration of HPMC molecules did not form or delaminate into gel.
The formula ratio affects the contact impedances, as explained by the impedances from the wipes electrode configuration. The wipes electrode configuration is a resultant of high viscous SH with ethanol percentage reduced by 50%. During the measurements, SH did not irritate or damage human skin. This phenomenon maybe due to two reasons. First, The constituents of SH, such as HPMC, which was available under the trade name of METHOCEL (Dow chemical company USA), was used as the base material for formulating SH. Second, USFDA accepted this constituent as a food grade product. SH was prepared to keep its tonicity equal to the natural sweat tonicity, which is 0.05 mol/l. For this reason, SH did not cause any irritation or damage to the skin after its application.
4.3 Limitations and future work
The current study evaluated the measured impedance over a period of 10 min. To understand the performance of the SH better and assess whether it would cause any skin irritation, it has to be tested for a longer period of time. ECG measurements for the features of the electrodes are not taken in this aspect. ECG measurements can also be performed to compare the performance of SH with other types of electrodes. Furthermore, motion artefacts, which are commonly encountered in long-term recordings, can also be tested using SH. Simultaneous measurements can be performed to avoid any differences in impedances by connecting all the electrode pairs to a multiplexer circuit.
5 Conclusions
The performance of the SH and wipes were compared with those of the Zipprep™, wet Ag/AgCl, and dry Ag/AgCl electrodes. SH provided good results by lowering the initial contact impedance of the skin when compared with the dry electrodes. However, the impedances measured using wipes were slightly higher than those obtained by using SH and lower than those when using the dry electrode configuration. Thus, SH electrodes could be used for electrode applications, such as EEG, ECG, and surface EMG. However, the problem with SH is that the bottle should be shaken thoroughly to agitate HPMC deposits and to avoid blocking the spray nozzle. Alternatively, wipes, which have a longer shelf life, can be used.
References
Kahanpur RS. Handbook of biomedical instrumentation. 3rd ed. New York: McGraw Hill Professional; 2014.
Gruetzmann A, Hansen S, Müller J. Novel dry electrodes for ECG monitoring. Physiol Meas. 2007;8(11):375.
Scanlon VC, Sanders T. Essentials of anatomy and physiology. 5th ed. Philadelphia: FA Davis; 2018.
Cornish B, Thomas B, Ward L. Effect of temperature and sweating on bioimpedance measurements. Appl Radiat Isot. 1998;49(5–6):475–6.
Woo E, Hua P, Webster J, Tompkins W, Pallas-Areny R. Skin impedance measurements using simple and compound electrodes. Med Biol Eng Comput. 1992;30(1):97–102.
Webster JG. Medical instrumentation application and design. Hoboken: Wiley; 2009.
Baek JY, An JH, Choi JM, Park Lee SH. Flexible polymeric dry electrodes for the long-term monitoring of ECG. Sens Actuators A. 2008;143(2):423–9.
Clar E, Her C, Sturelle C. Skin impedance and moisturization. J Soc Cosmet Chem. 1975;26:337–53.
Hewson D, Hogrel JY, Langeron Y, Duchêne J. Evolution in impedance at the electrode-skin interface of two types of surface EMG electrodes during long-term recordings. J Electromyogr Kinesiol. 2003;13(3):273–9.
Jung HC, Moon JH, Baek DH, Lee JH, Choi YY, Hong JS, Lee SH. CNT/PDMS composite flexible dry electrodesfor long-term ECG monitoring. IEEE Trans Biomed Eng. 2012;59(5):1472–9.
Mahony CO, Pini F, Blake A, Webster C, Brien JO, McCarthy KG. Microneedle-based electrodes with integrated through-silicon via for biopotential recording. Sens Actuators A. 2012;186:130–6.
Ha S, Kim C, Chi YM, Akinin A, Maier C, Ueno A, Cauwenberghs G. Integrated circuits and electrode interfaces for noninvasive physiological monitoring. IEEE Trans Biomed Eng. 2014;61(5):1522–37.
Zhou Y, Ding X, Zhang J, Duan Y, Hu J, Yang X. Fabrication of conductive fabric as textile electrode for ECG monitoring. Fibers Polymers. 2014;15(11):2260–4.
Maddipatla D, Narakathu BB, Bazuin BJ, Atashbar MZ. Development of a printed impedance based electrochemical sensor on paper substrate. In: IEEE sensors. 2016; p. 1–3.
Yokus JS. Fabric-based wearable dry electrodes for body surface biopotential recording. IEEE Trans Biomed Eng. 2016;63(2):423–30.
Yu Y, Zhang J, Liu J. Biomedical implementation of liquid metal ink as drawable ECG electrode and skin circuit. PLoS ONE. 2013;8(3):e58771.
Taheri BA, Knight RT, Smith RL. A dry electrode for EEG recording. Electroencephalogr Clin Neurophysiol. 1994;90(5):376–83.
Searle A, Kirkup L. Real time impedance plots with arbitrary frequency components. Physiol Meas. 1999;20(1):103.
Searle A, Kirkup L. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol Meas. 2000;21(2):271.
Xu S, Dai M, Xu C, Chen C, Tang M, Shi X, Dong X. Performance evaluation of five types of Ag/AgCl bio-electrodes for cerebral electrical impedance tomography. Ann Biomed Eng. 2011;39(7):2059–67.
Fowles DC, Christie MJ, Edelberg R, Grings WW, Lykken DT, Venables PH. Publication recommendations for electrodermal measurements. Psychophysiology. 1981;18(3):232–9.
Grey SJ, Smith BL. A comparison between commercially available electrode gels and purpose made gel, in the measurement of electrodermal activity. Psychophysiology. 1984;21:551–7.
Freire Becchi M, Ponti S, Miraldi E, Strigazzi A. Impedance spectroscopy of conductive commercial hydrogels for electromyography and electro-encephalography. Physiol Meas. 2010;31(10):S157.
Chi YM, Cauwen Berghs G. Micro power non-contact EEG electrode with active common mode noise suppression and input capacitance cancellation. In: Conference in proceedings on IEEE engineering in medicine and biology society. 2009; p. 4218–21.
Ethers MC. Technical handbook. Midland, MI: Dow Chemical Company; 1997.
Cédric A, Burke MJ. Low-frequency response and the skin-electrode interface in dry-electrode electrocardiography. Book: advances in electrocardiograms-methods and analysis. London: Intech Open; 2012. p. 23–52.
Al-Nima AM, Alkotaji M, Aliraqi O, Ali ZH. Preparation and evaluation of ultrasound transmission gel. Asian J Pharm Clin Res. 2019;12(1):422–7.
Park SK, Guntur SR, Lee KI, Paeng DG, Choi MJ. Reusable ultrasonic tissue mimicking hydrogels containing nonionic surface active agent for visualizing thermal lesions. IEEE Trans Biomed Eng. 2010;57:194–202.
Choi MJ, Guntur SR, Lee KI, Paeng DG, Coleman AJ. A tissue mimicking polyacrylamide hydrogel phantom for visualizing thermal lesions generated by high intensity focused ultrasound. Ultrasound Med Biol. 2013;29:439–48.
Guntur SR, Choi MJ. An improved tissue mimicking polyacrylamide gel for visualization of thermal lesions produced by HIFU. Ultrasound Med Biol. 2014;40:2680–91.
Guntur SR. Tissue mimicking phantoms for visualizing thermal lesions by high intensity focused ultrasound. Ph.D. thesis. Jeju, Korea: Jeju National University; 2013.
Acknowledgements
Authors would like to thank Dr. Paul Beatty, Centre for imaging Science and Biomedical Engineering, The University of Manchester, U.K. for providing research support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boppudi, S.T., Belal, S. & Guntur, S.R. Preparation and characterization of a novel sprayable hydrogel for skin preparation to record ECG and other biopotentials. Biomed. Eng. Lett. 10, 533–546 (2020). https://doi.org/10.1007/s13534-020-00164-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-020-00164-7