Abstract
Objective
We aimed to evaluate the effect of vitexin on some hematologic parameters and oxidative stress markers in lead-induced toxicity in mice.
Methods
Forty adult male albino mice were divided into five groups of eight animals comprising: control; Pb; vitexin; Pb + vitexin; and Pb + vitexin (post) groups. Blood samples collected were analyzed using an auto hematology analyzer for sixteen parameters including red cell distribution width (RDW), red blood cell (RBC), and white blood cell (WBC). The levels of oxidative stress markers were also assessed.
Results
In Pb-treated group, RDW, RBC, MCV, Hb, Hct, granulocytes, and blood lead level were significantly different from the control group as well as Pb + vitexin (post) group. In Pb + vitexin groups, MCHC, platelets, and lymphocytes counts were significantly different from the control group. There was no difference in MPV, MID, and WBC between the groups. MDA level in Pb-treated group was significantly high while SOD and GPx levels were low. In Pb + vitexin-treated groups, SOD and GPx levels were significantly high while MDA was low.
Conclusion
Pb-induced toxicity caused significant changes in the values of hematologic parameters and oxidative markers measured but vitexin was able to mitigate some of the changes. Some of the values were inconsistent with Pb intoxication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is a toxic element that may cause acute, subacute, or chronic poisoning through environmental and occupational exposure [1,2,3]. A blood Pb level of < 10 μg/dL is enough to cause toxicity [4]. Multiple body organs and systems are affected by Pb poisoning. In neurological manifestations, Chronic Pb exposure among adults and children results in loss of short-term memory, inability to concentrate, increased excitability, depressive mood, paresthesia of extremities, loss of coordination, generalized abdominal pain, and nausea [3, 5, 6]. Also, neurophysiological changes such as difficulties in intelligence, memory, executive functioning, attention, processing speed, language, visuospatial skills, and motor skills have been associated with Pb toxicity [7]. Lead toxicity among other manifestations promotes changes in cardiac function and Cav1.2 ion channels [8] and also results in anemia [9, 10]. In animal models such as waterfowl, it has been reported that Pb toxicity induced significant changes in the values of hematologic parameters measured including PCV, hemoglobin concentration, and MCHC [11].
The underlying mechanisms involved in lead-induced neurotoxicity are complex. However, oxidative stress, membrane bio-physics alterations, deregulation of cell signaling, and impairment of neurotransmission have all been implicated in Pb neurotoxicity [6]. Pb toxicity results in oxidative stress via lipid peroxidation resulting in the production of reactive oxygen species (ROS) and direct depletion of antioxidant reserves [12]. One of the common consequences of oxidative stress is apoptosis [13, 14]. In order to combat free radicals and oxidative stress including those caused by Pb exposure, recent researches have focused on the benefits of flavonoids. This is because flavonoids have shown antioxidative activity, free radical scavenging capacity, hepatoprotective, anti-inflammatory, and antiviral and anticancer activities; in some cases, flavonoids prevent coronary heart diseases [15, 16]. Among the commonly known flavonoids is vitexin [16, 17].
Vitexin is an active flavonoid compound that is found in many plants including pearl millet, hawthorn, pigeon pea, mung bean, mosses, Passiflora, bamboo, mimosa, wheat leaves, and chasteberry [16]. Vitexin has many pharmacological and biological functions such as antioxidative, anti-tumor, antiviral, anti-inflammatory, anti-bacterial, antihypertensive, anti-nociceptive, antispasmodic, anti-diabetic, antidepressant, neuroprotective, and cognitive improving functions [18,19,20,21,22,23]. A hematologic study involving anti-inflammatory effect of vitexin on activated human peripheral blood neutrophils suggests that vitexin may be considered as a therapeutic strategy for treating patients with neutrophil-mediated inflammatory diseases [24].
Despite extensive studies on vitexin, the literature on its effect on hematologic and biochemical parameters is scanty and requires additional data. Also, the search for the best possible therapeutic control of oxidative stress caused by Pb toxicity is still on. It is because of these problems, that the study was aimed at evaluating the role of vitexin on hematologic and oxidative stress markers in lead-induced toxicity in mice.
Results
Comparison of hematologic parameters
Comparison of mean values of hematologic parameters in various groups of the experiment (Table 1). The red cell distribution width (RDW) was significantly higher in the control group when compared with the experimental groups except for Pb + Vx(post) group. Similarly, RDW was significantly higher in Pb + Vx(post) group when compared with the Pb group.
Regarding the level of red blood cell (RBC), it was significantly low in Pb and Vx groups when compared with control and Pb + Vx(post) groups. No significant difference was observed when the RBC level was compared between control, Pb + Vx and Pb + Vx(post) groups (Table 1).
Significantly low levels of hemoglobin (Hb) and hematocrit (Hct) were observed in both Pb and Vx groups when compared with control, Pb + Vx, and Pb + Vx(post) groups (Table 1). Also, mean corpuscular volume (MCV) was significantly low in Pb and Pb + Vx groups when compared with the control group. However, MCV was not different between control, Vx, and Pb + Vx(post) groups.
The mean corpuscular hemoglobin (MCH) level (Table 1) was significantly low in both Pb and Vx groups when compared with control and Pb + Vx groups. MCH level was not different when the comparison was made between control, Pb + Vx, and Pb + Vx(post) groups. Regarding mean corpuscular hemoglobin concentration (MCHC), it had a significantly higher level in the Pb + Vx group when compared with other groups including the control group.
Platelet distribution width (PDW), mean platelets volume (MPV), and minimum inhibitory dilution (MID) in the study (Table 1) were observed not to be significantly different across all the groups involved in the study. Procalcitonin (PCT) level was significantly high in both Vx and Pb + Vx groups when compared with the control group. In platelets count (PLT), the result (Table 1) shows a significantly high level of PLT in the Pb + Vx(post) group when compared with both control and Pb groups. No difference platelets count was observed between Vx, Pb + Vx, and Pb + Vx(post) groups.
The percentage of both lymphocytes (Lym) and granulocytes (Gran) in the study (Table 1) were significantly high Pb + Vx group when compared with other groups involved in the experiment including the control group. Also, percentage granulocyte in the Pb group was higher in comparison with control, Vx, and Pb + Vx(post) groups.
In Table 1, blood lead level (BLL) was significantly high in the Pb group when compared with control, Vx, and Pb + Vx(post) groups. Although the result shows a reduction in BLL in both Pb + Vx and Pb + Vx(post) groups, the reductions seen were not statistically significant.
Comparison of mean levels of oxidative stress markers in various groups of the experiment
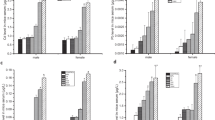
Malondialdehyde (MDA) level (Fig. 1) in Pb group was significantly higher (*P < 0.0001) than those in other groups including the control group. When MDA level was compared between control, Vx, Pb + Vx, and Pb + Vx(post) groups, there was no significant difference.
In Fig. 2, superoxide dismutase (SOD) level was significantly low in Pb group when compared with control, Vx, and Pb + Vx groups. Similarly, SOD level in Pb + Vx(post) group was significantly low when compared with control and Pb + Vx groups. No significant difference in SOD level was observed between control, Vx, and Pb + Vx groups neither were there any differences between Pb and Pb + Vx(post) groups.
Result in Fig. 3 shows glutathione peroxidase (GPx) level to be significantly low in the Pb group when compared with other groups including the control group and GPx level higher in the control group when compared with all the experimental groups. Furthermore, no significant difference in GPx level was observed between Vx, Pb + Vx, and Pb + Vx(post) groups.
Discussion
Hematologic parameters, comprising red and white blood cell counts, hemoglobin concentration, platelets, and others, are commonly utilized as clinical indicators of health and disease conditions [25, 26]. In mice, the previous study calculated mean total blood volume to be 5.85 mL per 100 g of body weight, the mean plasma volume to be 3.15 mL per 100 g and total blood volume to be 5.4–8.2% of body weight [27].
RBCs number varies according to physiological (e.g., pregnancy, aging, or physical exercise) and pathological (e.g., anemia, thalassemia) conditions [28]. A previous study [29] reported a normal reference range of RBCs (× 106/µL) in mice to be 6.9–11.76 (male) and 8.6–11.3 (female). In this study, the result shows that Pb-administered and vitexin-administered groups have low RBCs when compared with the control group. This suggests that the animals in those groups (Pb and Vx) may perhaps be anemic. In groups where vitexin was concurrently administered with Pb or post-treated (Pb + Vx and Pb + Vx(post) groups), RBC numbers were maintained at the same level as seen in the control group. This suggests that the Pb +vitexin combination could maintain normal RBCs number. The reason for this outcome is not fully understood, but it is possible that the antioxidant activity of vitexin may have increased the antioxidant capacity of the cells thereby neutralizing pro-oxidant generated due to Pb toxicity and in turn reduced the rate of apoptosis. The reason behind better red blood cell indices in Pb + Vx and Pb + Vx(post) groups when compared with vitexin group is not fully understood at the moment. However, it is possible that the animals in the vitexin group may have suffered trauma or infection caused by the IP administration procedure which was not detected in the study. It is also possible that vitexin only administration may accelerates red blood cell turnover in the mice spleen. This part of the result will be a focus in our feature studies.
The red blood cell distribution width (RDW) is a degree of RBC size heterogeneity, which is calculated by dividing the standard deviation (SD) of erythrocyte volumes for the mean corpuscular volume (MCV) [28, 30]. RDW points out the degree of anisocytosis of the red blood cell population [28, 31]. In mouse, rat, and hamster, RBCs with a diameter of one-third of the standard RBC size were seen to have moderate anisocytosis [29]. The result from this study shows that the Pb-treated group has significantly small RDW when compared with the control group and other groups where vitexin was involved in treatment. This result suggests that treating animals with Pb in this study may have resulted in anisocytosis. However, this condition may have been corrected with the intervention of vitexin.
Mouse platelets (thrombocytes) are round to oval in form and are just about 1–4 µm in diameter. An earlier study [29] has reported the reference number of mouse platelets (× 103/µL) to be 157–412 (male) and 170–410 (female). This study (Table 1) shows that platelets count in Pb-treated group was lower than the control group as well as other groups particularly the two Pb + vitexin-treated groups. This result suggests that Pb treatment could result in low platelets count probably because of the abnormally high cell death rate due to Pb toxicity. The result also suggests that Pb plus vitexin treatment could maintain normal platelets count by reducing the rate of rapid cell death caused by Pb toxicity through ROS. This is probably done through an increase in the antioxidant capacity of the cells to neutralize ROS generated.
Hemoglobin (Hb) level in this study (Table 1) was significantly low in the Pb group and vitexin group when compared with both control and Pb + Vx(post) group. The result suggests that the combination of both Pb and vitexin could maintain a steady Hb level in mice but on the contrary, Pb alone or vitexin alone causes a reduction. The reason for this result is not fully understood but would be a focus for further study. According to Lindstrom et al., [29], normal mice Hb (g/dl) level are 11.1–11.5 (male) and 10.7–11.1 (female). The Hb levels seen in this study is higher than the level earlier reported probably due to differences in the route of blood collection.
White blood cells (WBCs) concentration is affected by different factors including physiological and pathological conditions. In this study, there was no significant difference in WBCs concentration between the groups including the control group. However, the result of WBCs concentration in this study is similar to the result reported by Lindstrom et al., [29]. In that report, the range of mice WBCs count was 12.5–15.9 (× 103/µl) for male and then 12.1–13.7 (× 103/µl) female. The result of WBCs in this study suggests that all the groups have equal capacity to fight infection or immune response.
In mice, roughly 75% of the WBC is made up of lymphocytes with the previous report putting the normal value of lymphocytes at 82.4% for males and 77.9% for females [29]. However, the values seen in this study were higher probably due to inflammatory responses by the animals which may persist weeks prior to the experiment [32].
Oxidative stress arises as a result of a physiological imbalance between the levels of antioxidants and oxidants in favor of oxidants. One of the end products of lipid peroxidation and a widely recognized oxidative stress marker is malondialdehyde (MDA) [33, 34]. In this study, the MDA level in Pb-treated group was significantly higher than other groups including the control. This probably suggests that the level of oxidative damage was higher in Pb-treated group than other groups. Earlier reports [35, 36] had also suggested that Pb increases the level of tissue MDA. On the contrary, vitexin has been reported to significantly reduce MDA level [37].
Superoxide dismutase (SOD) and glutathione peroxidase (GPx) are first-line defense antioxidant enzymes that dismutate superoxide radicals and breakdown hydrogen peroxides into harmless molecules [38, 39]. In this study, the levels of both SOD and GPx were significantly low in Pb-treated group when compared with the control and other experimental groups (particularly Pb + Vx). This result suggests that vitexin may have boosted the antioxidant enzyme defense of the cells to fight against excessive ROS generated as a result of Pb toxicity. This in turn probably reduced cellular injury and apoptosis. Findings from earlier studies [37, 40] observed an increase in the level of SOD and GPx when vitexin was administered. Vitexin was also noted to have reduced apoptosis [41].
Materials and methods
Chemicals and reagents
Vitexin extract, Pb acetate, malondialdehyde (MDA), glutathione peroxidase (GPx), and superoxide dismutase (SOD) test kit were purchased from Sigma-Aldrich (St Louis, MO, USA). Vitexin was dissolved in normal saline (NaCl, 0.9%).
Animals
Forty adult male albino mice weighing 19–20 g were procured from the Temidale animal center, Ogbomosho, Nigeria. The animals were acclimatized for 7 days on a 12 h light/dark cycle at approximately 23 °C at the animal holdings of Central Research Lab, University of Ilorin. The animals were provided with food and water ad libitum. The animals were maintained under the National Institutes of Health guide for the care and use of laboratory animals (NIH Publication 8th edition). The experimental protocols involved in the study were approved by the University of Ilorin Ethical and Review Committee with approval number: UERC/ASN/2018/1257.
Animal grouping and administration
The animals were randomly divided into five groups of eight animals (n = 8). Control was administered 0.2 ml normal saline through oral gavage; Pb group (oral gavage of Pb acetate 100 mgkg/b.wt./d for 7 days); vitexin group (intraperitoneal injection of vitexin 10mgkg/b.wt./d for 7 days); Pb + vitexin group (concurrent treatment with 100 mgkg/b.wt./d of Pb + 10 mgkg/b.wt./d vitexin for 7 days). Pb + vitexin group (post-treatment with 10 mgkg/b.wt./d of vitexin 6 h after the administration of 100 mgkg/b.wt./d of Pb for 7 days).
Collection and analysis of blood
On the 7th day of administration, animals were anesthetized with an intraperitoneal injection of ketamine (50 mg/0.2 ml) and blood from all the animals according to the group was collected via cardiac puncture into EDTA test tubes. Blood samples collected were immediately stored at − 20 °C before the estimation of hematologic parameters using the auto hematology analyzer (Seattle, USA). The parameters analyzed include the red blood cell count (RBC), hemoglobin (Hb), hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelet distribution width (PDW), procalcitonin (PCT), and platelets (PLT). Others include minimum inhibitory dilution (MID), white blood cell (WBC), granulocytes (Gran), lymphocytes (Lym), platelets large cell ratio (P-LCR), and mean platelets volume (MPV). Also, the estimation of blood lead level (BLL) was done.
Biochemical assay
The prefrontal cortex tissue was dissected and homogenized with an ice-cold buffer to prepare a homogenate solution. The homogenate was centrifuged at 10,000 g for 10 min and the supernatant obtained was used for the estimation of lipid peroxidation (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GPx) levels. Estimation was done according to instruction on each assay kit purchased from Sigma-Aldrich (MO, USA). The results were expressed as U/mg protein.
Statistical analysis
All statistical analyses and graph fitting were performed using GraphPad Prism 5 for Windows. The significance of difference was determined using a one-way ANOVA followed by Tukey’s post hoc test for all parameters. All data were expressed as the mean ± standard error of the mean (SEM), with n representing the number of animals used in each experiment. Statistical significance was defined at the level of P < 0.05 to P < 0.0001.
Conclusion
Pb-induced toxicity caused significant changes in the values of hematologic parameters and oxidative markers measured in mice but vitexin was able to mitigate some of the changes. Some of the values were inconsistent with Pb intoxication. However, the values on hematologic parameters obtained will provide a platform for additional studies on the potentials of vitexin against the effect of Pb intoxication on hematologic parameters.
References
Alli LA (2015) Blood level of cadmium and lead in occupationally exposed persons in Gwagwalada, Abuja, Nigeria. Interdiscip Toxicol 8(3):146–150
Kim HC, Jang TW, Chae HJ, Choi WJ, Ha MN, Ye BJ et al (2015) Evaluation and management of lead exposure. Ann Occup Environ Med 27:30
Wani AL, Ara A, Usmani JA (2015) Lead toxicity: a review. Interdiscip Toxicol 8(2):55–64
Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL (2008) Blood lead concentrations < 10 microg/dL and child intelligence at 6 years of age. Environ Health Perspect 116(2):243–248
von Stackelberg K, Guzy E, Chu T, Claus Henn B (2015) Exposure to mixtures of metals and neurodevelopmental outcomes: a multidisciplinary review using an adverse outcome pathway framework. Risk Anal 35(6):971–1016
Sharma P, Chambial S, Shukla KK (2015) Lead and neurotoxicity. Indian J Clin Biochem IJCB 30(1):1–2
Mason LH, Harp JP, Han DY (2014) Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int 2014:840547
Ferreira de Mattos G, Costa C, Savio F, Alonso M, Nicolson GL (2017) Lead poisoning: acute exposure of the heart to lead ions promotes changes in cardiac function and Cav1.2 ion channels. Biophys Rev 9(5):807–825
Pearce JM (2007) Burton’s line in lead poisoning. Eur Neurol 57(2):118–119
Hegazy AA, Zaher MM, Abd El-Hafez MA, Morsy AA, Saleh RA (2010) Relationship between anemia and blood levels of lead, copper, zinc, and iron among children. BMC Res Notes 3:133
Katavolos P, Staempfli S, Sears W, Gancz AY, Smith DA, Bienzle D (2007) The effect of lead poisoning on hematologic and biochemical values in trumpeter swans and Canada geese. Vet Clin Pathol 36(4):341–347
Sharma B, Singh S, Siddiqi NJ (2014) Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res Int 2014:640754
Redza-Dutordoir M, Averill-Bates DA Activation of apoptosis signaling pathways by reactive oxygen species Biochimica et Biophy (2016) Activation of apoptosis signaling pathways by reactive oxygen species. Biochim et Biophys Acta (BBA) Mol Cell Res 1863(12):2977–2992
Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X et al (2007) Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 9(1):49–89
Shashank Kumar, Pandey Abhay K (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J 2013:16
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:e47
He M, Min JW, Kong WL, He XH, Li JX, Peng BW (2016) A review of the pharmacological effects of vitexin and isovitexin. Fitoterapia 115:74–85
Choi JS, Nurul Islam M, Yousof Ali M, Kim EJ, Kim YM, Jung HA (2014) Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem Toxicol 64:27–33
Hritcu L, Ionita R, Postu PA, Gupta GK, Turkez H, Lima TC et al (2017) Antidepressant flavonoids and their relationship with oxidative stress. Oxid Med Cell longev 2017:5762172
Yang L, Yang ZM, Zhang N, Tian Z, Liu SB, Zhao MG (2014) Neuroprotective effects of vitexin by inhibition of NMDA receptors in primary cultures of mouse cerebral cortical neurons. Mol Cell Biochem 386(1–2):251–258
Zhu Q, Mao L-N, Liu C-P, Sun Y-H, Jiang B, Zhang W et al (2016) Antinociceptive effects of vitexin in a mouse model of postoperative pain. Sci Rep 6:19266
Rosa SIG, Rios-Santos F, Balogun SO, Martins DTdO (2016) Vitexin reduces neutrophil migration to inflammatory focus by down-regulating pro-inflammatory mediators via inhibition of p38, ERK1/2 and JNK pathway. Phytomedicine 23(1):9–17
Lima LKF, Pereira SKS, Junior R, Santos F, Nascimento AS, Feitosa CM et al (2018) A brief review on the neuroprotective mechanisms of vitexin. Biomed Res Int 2018:4785089
Bahareh Nikfarjam, Farid Hajiali, Mohtaram Adineh, Marjan Nassiri-Asl (2017) Anti-inflammatory effects of quercetin and vitexin on activated human peripheral blood neutrophils: the effects of quercetin and vitexin on human neutrophils. J Pharmacop 20:127–131
Kelada SNP, Aylor DL, Peck BCE, Ryan JF, Tavarez U, Buus RJ et al (2012) Genetic analysis of hematological parameters in incipient lines of the collaborative cross. G3 (Bethesda) 2(2):157–165
Okada Y, Kamatani Y (2012) Common genetic factors for hematological traits in humans. J Hum Genet 57(3):161–169
Pilny AA (2008) Clinical hematology of rodent species. Vet Clin N Am Exot Anim Pract 11(3):523–533
Danese E, Lippi G, Montagnana M (2015) Red blood cell distribution width and cardiovascular diseases. J Thorac Dis 7(10):E402–E411
Lindstrom NM, Moore DM, Zimmerman K, Smith SA (2015) Hematologic assessment in pet rats, mice, hamsters, and gerbils: blood sample collection and blood cell identification. Clin Lab Med 35(3):629–640
Feng GH, Li HP, Li QL, Fu Y, Huang RB (2017) Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol 2(3):172–175
Vaya A, Rivera L, Todoli J, Hernandez JL, Laiz B, Ricart JM (2014) Hematological, biochemical and inflammatory parameters in inactive Behcet’s disease. Its association with red blood cell distribution width. Clin Hemorheol Microcirc 56(4):319–324
Bolliger AP, Everds N (2012) Chapter 2.9—Haematology of the mouse. In: Hedrich HJ (ed) The laboratory mouse, 2nd edn. Academic Press, Boston, pp 331–347
Yonny ME, García EM, López A, Arroquy JI, Nazareno MA (2016) Measurement of malondialdehyde as an oxidative stress biomarker in goat plasma by HPLC-DAD. Microchem J 129:281–285
Ayala A, Muñoz MF, Argüelles S et al (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014. https://doi.org/10.1155/2014/360438
Barkur RR, Bairy LK (2015) Assessment of oxidative stress in the hippocampus, cerebellum and frontal cortex in rat pups exposed to lead (Pb) during specific periods of initial brain development. Biol Trace Elem Res 164(2):212–218
Xu J, Lian L-j, Wu C, Wang X-f, Fu W-y, Xu L-h (2008) Lead induces oxidative stress, DNA damage, and alteration of p53, Bax and Bcl-2 expressions in mice. Food Chem Toxicol 46(5):1488–1494
Nurdiana S, Goh YM, Hafandi A, Dom SM, Nur Syimal’ain A, Noor Syaffinaz NM et al (2018) Improvement of spatial learning and memory, cortical gyrification patterns and brain oxidative stress markers in diabetic rats treated with Ficus deltoidea leaf extract and vitexin. J Tradit Complement Med 8(1):190–202
Deponte M (2013) Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim et Biophys Acta (BBA) Gener Subj 1830(5):3217–3266
Ighodaro OM, Akinloye OA (2018) First-line defense antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defense grid. Alex J Med 54(4):287–293
An F, Yang G, Tian J, Wang S (2012) Antioxidant effects of the orientin and vitexin in Trollius Chinensis Bunge in D-galactose-aged mice. Neural Regen Res 7(33):2565–2575
Lyu Z, Cao J, Wang J, Lian H (2018) Protective effect of vitexin reduces sevoflurane-induced neuronal apoptosis through HIF-1α, VEGF and p38 MAPK signaling pathway in vitro and newborn rats. Exp Ther Med 15(3):3117–3123
Acknowledgements
The authors thank Mr Leviticus Arietarhire and Dr Emeka of the central research and diagnostic laboratory, Ilorin, for their technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nathaniel Ohiemi Amedu and Gabriel Olaiya Omotoso declare that they have no conflict of interest.
Ethical approval
The experimental protocols involved in the study were approved by the University of Ilorin Ethical and Review Committee with Approval Number: UERC/ASN/2018/1257.
Rights and permissions
About this article
Cite this article
Amedu, N.O., Omotoso, G.O. Evaluating the role of vitexin on hematologic and oxidative stress markers in lead-induced toxicity in mice. Toxicol. Environ. Health Sci. 12, 257–263 (2020). https://doi.org/10.1007/s13530-020-00039-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-020-00039-5