Abstract
Background
Oxidative stress is suggested as a potential contributary factor for feto-maternal complications in gestational diabetes mellitus (GDM) and the understanding of oxidative stress and antioxidant levels in GDM still remains obscure.
Objective
This study aimed to investigate the serum levels of oxidants and antioxidants in women with GDM in a Sri Lankan context, and a potential diagnostic marker panel for GDM.
Methods
This pilot case–control study included 30 untreated GDM patients, and 30 age-matched healthy pregnant women (controls) in their second or third trimesters. After collection of demographic and anthropometric data from all study participants, their serum levels of nitric oxide derivative (NOx) concentration, lipid peroxidation (LPO) level, total antioxidant capacity (TAC), and catalase enzyme (CAT) activity were measured. The CombiROC web tool assessed the diagnosis accuracy of potential biomarkers of GDM.
Results
Significantly higher levels of serum NOx (p < 0.001), LPO levels (p < 0.01), and significantly lower TAC (p < 0.001) and CAT activity (p < 0.05) were observed in GDM-afflicted women compared to controls. LPO level:TAC and LPO level:CAT activity ratios were significantly increased in GDM patients (p < 0.001). CombiROC analysis identified five potential diagnostic marker panels with the highest discriminatory power: (NOx-TAC-LPO), (BMI-NOx-TAC-LPO), (BMI-NOx-LPO-CAT), (NOx-TAC-LPO-CAT), and (BMI-NOx-TAC-LPO-CAT). Body mass index (BMI) was identified as an important noninvasive marker of GDM with the cut off of 22.4 kg/m2.
Conclusion
This pilot study demonstrated increased oxidative stress and weaker antioxidant defenses in Sri Lankan women with GDM. The identification of potential diagnostic markers, including BMI, may improve GDM diagnosis in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is a condition of impaired glucose tolerance that manifests or is identified for the first time during pregnancy [1]. A meta-analysis conducted in 2019 reported that the South Asian region (Bangladesh, India, and Sri Lanka) has the highest prevalence of GDM (11.4%) which is notably higher than the rest of the world (3.6–6.0%) [2].
The consequences of GDM affect both maternal and fetal health. Women with GDM are at high risk of experiencing life-long type 2 diabetes, metabolic syndrome, and cardiovascular disease. The fetus of a GDM mother has been associated with respiratory distress, fetal macrosomia, and fetal anomalies. Although the exact mechanisms causing these complications remain poorly understood, recent evidence suggests that oxidative stress plays a major role in the pathogenesis of GDM [3]. Oxidative stress (OS) arises when there is an imbalance between pro-oxidants (free radicals) and the body’s capacity to eliminate them through antioxidants [4].
Existing evidence elucidates that pregnancies complicated by diabetes disrupt both the generation of free radicals and the functioning of antioxidant defenses. Hyperglycemia induces the production of oxygen radicals through various mechanisms: non-enzymatic glycation, glucose auto-oxidation, altering mitochondrial electron transport chain, and activation of polyol pathways. Oxidative degradation of membrane lipids due to excess oxygen radicals results in lipid peroxidation (LPO) [5].
Nitric oxide (NO) is a highly reactive free radical synthesized by the enzyme nitric oxide synthase, from L-arginine. NO is crucial in extensive metabolic, vascular, and cellular processes. Changes in the synthesis and bioavailability of nitric oxide in diabetes including GDM have been reported [3, 6].
Antioxidants counteract oxidative damage by preventing the formation of free radicals, inhibiting free radical activity, repairing damage caused by free radicals, and increasing the excretion or absorption of damaged molecules. Antioxidants can be divided into either enzymatic antioxidants or non-enzymatic antioxidants. Catalase enzyme (CAT) is responsible for scavenging hydrogen peroxide (H2O2). Antioxidants in the body have a cumulative effect. Hence, total antioxidant capacity (TAC) is an important parameter to evaluate the combined action of all antioxidants in body fluids [7].
The correlation between oxidative stress and type 2 diabetes has been well documented. However, studies on GDM, a condition with similar pathogenesis, are limited and conflicting. Several studies reported a decrease in TAC [8] and an increase in LPO levels among women with GDM [9, 10]. Lower CAT activity has been observed in women with late-onset GDM [9]. Contradictory results, where no changes in LPO levels [11], TAC [12], and CAT activity in GDM women [5], have also been reported. Increased NO levels were observed in diabetic patients [13] whereas others reported the converse [14].
Based on existing knowledge, the oxidant-antioxidant status of women with GDM may not follow a global pattern. Furthermore, as such studies are non-existent in Sri Lanka, this pilot case–control study was undertaken to fill this research gap, with a double pronged aim, (i) to examine oxidative stress in GDM and, (ii) to establish a potential diagnostic marker panel for GDM.
Materials and methods
Study design
The Ethics Review Committees of the Faculty of Graduate Studies, University of Colombo (No: FGS/ERC/2022/010) and of the De Soysa Hospital for Women, Sri Lanka (No: 023), approved the study in accordance with principles outlined in the Declaration of Helsinki and its later modifications. This study was carried out based on a case–control design at the De Soysa Hospital for Women in Colombo, Sri Lanka, from December 2022 to April 2023.
Thirty pregnant women with untreated GDM and 30 age- and gestational age-matched healthy pregnant women were enrolled as cases and controls, respectively. Inclusion criteria for the case group required the age range between 18 and 35 years and diagnosis of GDM during the second or third trimester of pregnancy. Pregnant women were screened for GDM using a 75 g oral glucose tolerance test (OGTT) according to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) consensus criteria. GDM was defined if any one of the plasma glucose thresholds was met or exceeded: fasting ≥ 92 mg/dl (≥ 5.1 mmol/l) or 1 h ≥ 180 mg/dl (≥ 10 mmol/l) or 2 h ≥ 153 mg/dl (≥ 8.5 mmol/l). Controls were selected from a cohort of pregnant women with similar characteristics as cases but with normal glucose levels. Participants with types 1 or 2 diabetes, and those with concurrent systemic diseases, were excluded from the study.
Study measures
Participants who met the selection criteria were provided with information on the study framework and objectives. Written informed consent was obtained from each participant to ensure their voluntary participation. A questionnaire was administered to collect demographic and medical history information. During the OGTT, 3 ml of blood was drawn from each participant, and separated serum samples were frozen at − 80 °C for subsequent laboratory analyses.
Serum LPO levels were assayed using the thiobarbituric acid reactive substances (TBARS) assay [15]. Briefly, serum samples were mixed with 8.1% (w/v) SDS, 20% (v/v) acetic acid, 0.8% (w/v) thiobarbituric acid (pH = 4), and distilled water. The mixture was incubated at 95 °C for 1 h. After cooling, butanol:pyridine mixture (15:1 v/v) was added to each sample, and the absorbance of the organic phase was measured at 532 nm. A standard curve was generated using 5–20 µM tetramethoxypropane.
The concentration of serum nitric oxide (NO) was determined using the Griess assay, which detects nitrite/nitrate (NOx), stable byproducts of NO [16]. Briefly, serum samples were deproteinized by adding zinc sulfate (15 mg/ml), and vanadium (III) chloride was used to reduce nitrate to nitrite. After adding the Griess reagent, absorbance was measured at 540 nm. A standard curve was established using sodium nitrite ranging from 10–60 µM.
The ABTS radical cation decolorization assay was performed to determine serum TAC [17]. Briefly, a mixture of 7 mM ABTS and 7 mM potassium persulfate was incubated for 16 h in the dark at room temperature to generate ABTS• + cations. ABTS• + working solution was diluted with PBS to an absorbance of 0.7 (+ 0.02) at 734 nm. Deproteinized serum samples were added with ABTS• + solution and incubated for 10 min at room temperature. The percentage inhibition was calculated at 655 nm, and the standard curve was plotted using 10–60 µg/ml ascorbic acid.
Measuring the CAT activity is based on the reaction of undecomposed hydrogen peroxide with ammonium molybdate to produce a yellowish complex [18]. To ensure compatibility with a 96-well microtiter plate, the protocol was modified accordingly. Serum samples (10 µl) were mixed with 20 mM H2O2 in sodium and potassium phosphate buffer and incubated at 37 °C for 3 min. Next, 400 µl of 32.4 mmol//l of ammonium molybdate was added, and the absorbance at 374 nm was recorded. A correction factor, standard, and reagent blank were prepared as references.
Oxidative stress was determined using the calculation of the pro-oxidant:antioxidant (P:A) ratio, which serves as an indicator of the presence of oxidative stress; thus, LPO level:TAC [19] and LPO level:CAT activity [20] were used.
CombiROC analysis [21] evaluated the diagnostic accuracy of the four analytes tested and the body mass index (BMI). Based on the area under the curve, sensitivity, and specificity, this method combines ROC curves to identify the optimal combinations of biomarkers that can differentiate between health and disease conditions. The analysis was performed on the freely available web application http://CombiROC.eu.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 26.0. Data are presented as the mean value ± standard deviation (SD). The Shapiro–Wilk test assessed the normality of the data. Student’s t-test or the Mann–Whitney U test were used as appropriate to compare parameters between cases and controls. p < 0.05 was considered statistically significant.
Results
Demographic and anthropometric data of women with GDM and healthy pregnant women are summarized in Table 1. There were no significant differences in the mean age, gestational age, height, and parity between the two groups (p > 0.05). Conversely, compared to the controls, the mean body weight and mean body mass index (BMI) during pregnancy were significantly higher in GDM cases (p < 0.05).
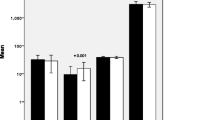
Oxidative and anti-oxidative parameters and oxidative stress ratios of cases and controls are presented in Table 2. Compared to the controls, women with GDM had significantly higher levels of serum NOx and LPO level but manifested significantly lower TAC and CAT activity (p < 0.05). Compared to controls, significantly higher LPO level:TAC and LPO level:CAT activity ratios indicating elevated levels of oxidative stress were observed in women with GDM (p < 0.001) (Fig. 1 and Table 2).
Serum levels of the tested analytes. a NOx concentration. b Lipid peroxidation level. c Total antioxidant capacity. d Catalase activity. e Pro-oxidant:antioxidant ratio. a–d Whiskers extend from the smallest value to the largest value of each data set. e Columns represent mean + SD of 30 subjects per group; *p < 0.05, **p < 0.01, ***p < 0.001 (Mann–Whitney U Test)
Multiple “gold” marker signatures of the four tested analytes and BMI obtained from CombiROC analysis with the maximum area under the curve (AUC), sensitivity (SE), and specificity (SP) are summarized in Table 3 and Fig. 2. Five “gold” combinations of all individual and multiple marker combinations, (NOx-TAC-LPO), (BMI-NOx-TAC-LPO), (BMI-NOx-LPO-CAT), (NOx-TAC-LPO-CAT), and (BMI-NOx-TAC-LPO-CAT), showed maximum values for all metrics including the area under the curve (1.000), sensitivity (1.000), and specificity (1.000). Accordingly, these five combinations can be considered the best diagnostic marker panels for diagnosing GDM. Additionally, 22.4 kg/m2 was obtained as the cut-off for predicting GDM based on the BMI (AUC = 0.698; SE = 0.867; SP = 0.5).
The total of single markers and multiple marker combinations that resulted from CombiROC analysis are presented in Online Resource 1.
Discussion
Multiple studies have highlighted the importance of maintaining an equilibrium between pro- and antioxidants, particularly in complicated situations such as diabetic pregnancy [10]. Studying oxidative stress parameters achieves a deeper understanding of mechanisms underlying the development of GDM [3].
The measurement of free radicals poses several challenges owing to their instability. The presence of numerous bonds in polyunsaturated fatty acids within the cellular membrane renders them highly vulnerable to free radicals. Consequently, the determination of lipid peroxidation products is used to assess free radical activity [8]. In normal pregnancy, increased oxidative stress induces lipid peroxidation products. Nevertheless, antioxidant defenses are sufficient to mitigate lipid peroxidation. In diabetes, elevated glucose levels lead to the overproduction of oxygen radicals causing lipid peroxidation [5]. Previous studies reported that women with GDM have higher levels of lipid peroxidation [5, 9, 10]. In agreement with these findings, the current study also observed a significant increase in lipid peroxidation in women with GDM compared to normal pregnancy. Conversely, outcomes of some studies in women with GDM undergoing insulin treatment, metformin therapy, or diet management are not consistent with our results [22].
Nitric oxide (NO) has crucial biological effects, such as the relaxation of blood vessels, regulation of blood pressure, aggregation of platelets, and neurotransmission [3]. Unusual feto-maternal vascular modifications have been reported in women with GDM due to altered nitric oxide levels [23]. The current study evidently indicated a significant increase in nitric oxide levels, as measured by the concentrations of nitrates and nitrites in the GDM group compared to the healthy controls. Previous studies demonstrated that increased expression of endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) genes and proteins augments the production of nitric oxide (NO) during high glucose levels [5]. Our results are comparable with Karagoz et al. [24] who reported higher levels of NO in GDM mothers. Additionally, elevated nitric oxide in the amniotic fluid of GDM women was reported by von Mandach et al. [25]. In addition, this group of researchers had suggested that decreased levels of nitric oxide in diabetes are due to further oxidation of nitric oxide forming a potent oxidant, peroxynitrite [3]. However, these disparities may be attributed to various factors including the geographical location, genetic background of the population, time required to develop the disease conditions, and changes in patient metabolic controls.
Antioxidants have the capacity to stabilize free radicals and act as the primary defense mechanism against free radical damage. The combined action of antioxidants in the body offer enhanced defense against the harmful effects of free radicals, rather than relying on individual antioxidants. Therefore, total antioxidant capacity (TAC) indicates the oxygen radical absorbance capacity of body fluids instead of measuring a specific antioxidant activity [4, 26]. We found significantly lower total antioxidant capacity in GDM women compared to healthy pregnant women. Our results agree with the findings of several previous reports [7, 8]. However, disparities between our study and other research on total antioxidant capacity (TAC) in the saliva of women with GDM [7] may be generally attributed to variations in sampling methods, and laboratory techniques used.
To evaluate enzymatic antioxidant activities, catalase (CAT) activity measures the ability to trap free radicals that are produced in pathological conditions [26]. The significantly lower catalase activity we observed in the GDM group compared to the control group was consistent with previous studies [9]. A probable hypothesis for the observed decrease in catalase activity during hyperglycemia is that protein glycation causes structural alterations in the catalase enzyme, affecting its secondary and tertiary structure. These modifications lead to the loss of enzyme function thereby diminishing the enzyme’s capacity to counteract free oxygen radicals [27]. Conversely, some researchers who used different diagnostic criteria have reported no changes in CAT activity between GDM and non-GDM women [4].
The body’s compensatory mechanisms increase antioxidant levels in vivo to prevent oxidative stress and maintain a normal oxidant-antioxidant ratio. However, the present study showed that GDM can disregulate this balance, and can cause an increase in the oxidant-antioxidant ratio in affected women.
No previous studies had identified combinations of biomarkers for the diagnosis of GDM. A recently developed Web application CombiROC was used to identify and evaluate optimal diagnostic markers for GDM. This technique facilitates the identification of best performing combinations of markers that can be used to differentiate between health and disease condition [20]. Several combinations of marker panels were identified as having high potential to diagnose GDM. These panels consisted of four biomarkers (NOx, LPO level, TAC, and CAT activity) and BMI. The current study revealed that 22.4 kg/m2 is the most optimal cut-off value for predicting GDM during pregnancy. It may be an important step forward as a diagnostic marker because of its non-invasiveness. Moreover, these findings are consistent with substantial evidence from previous studies showing a significantly increased risk of GDM in individuals with higher BMI [28].
Conclusion
In conclusion, this pilot study emphasized that a cohort of Sri Lankan women with GDM had higher levels of oxidative stress and lower levels of antioxidant defenses. Therefore, administering antioxidant treatment to women with GDM may be advantageous. Further research is warranted to determine the potential benefits of an antioxidant-rich diet for GDM women. Additionally, the current study highlighted the importance of maintaining a healthy BMI during pregnancy. The identification of potential marker panels for the diagnosis of GDM is a promising advancement. However, it is crucial to validate these findings with larger study cohorts to validate the potential diagnostic markers identified for GDM.
Data availability
Primary data of the study is available as supplementary Information.
Abbreviations
- ABTS + :
-
2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt
- BMI:
-
Body mass index
- CAT:
-
Catalase
- eNOS:
-
Endothelial nitric oxide synthase
- ERC:
-
Ethics Review Committee
- GDM:
-
Gestational diabetes mellitus
- IADPSG:
-
International Association of Diabetes and Pregnancy Study Groups
- iNOS:
-
Inducible nitric oxide synthase
- LPO:
-
Lipid peroxidation
- NO:
-
Nitric Oxide
- NOx:
-
Nitric oxide derivatives
- OGTT:
-
Oral glucose tolerance test
- SPSS:
-
Statistical Package for the Social Science
- TBA:
-
Thiobarbituric acid
- TBARS:
-
Thiobarbituric acid reactive substances
References
Metzger BE, Coustan DR. Organizing Committee. Summary and recommendations of the fourth international workshop-conference on gestational diabetes mellitus. Diabetes care. 1998;21:B161.
Behboudi-Gandevani S, Amiri M, Bidhendi Yarandi R, Ramezani TF. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta-analysis. Diabetol metab. 2019;11:1–18. https://doi.org/10.1186/s13098-019-0406-1.
Lappas M, Hiden U, Desoye G, Froehlich J, Mouzon SHD, Jawerbaum A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal. 2011;15(12):3061–100. https://doi.org/10.1089/ars.2010.3765.
Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol. 2002;57(5):609–13. https://doi.org/10.1046/j.1365-2265.2002.01638.x.
Chaudhary L, Tandon OP, Vaney N, Agarwal N. Lipid peroxidation and antioxidant enzymes in gestational diabetics. Indian J Physiol Pharmacol. 2003;47:441–6.
Adela R, Nethi SK, Bagul PK, Barui AK, Mattapally S, Kuncha M, et al. Hyperglycaemia enhances nitric oxide production in diabetes: a study from South Indian patients. PLoS ONE. 2015;10(4):e0125270. https://doi.org/10.1371/journal.pone.0125270.
Zamani-Ahari U, Zamani-Ahari S, Fardi-Azar Z, Falsafi P, Ghanizadeh M. Comparison of total antioxidant capacity of saliva in women with gestational diabetes mellitus and non-diabetic pregnant women. J Clin Exp Dent. 2017;9(11):e1282. https://doi.org/10.4317/2Fjced.53845.
Parast VM, Paknahad Z. Antioxidant status and risk of gestational diabetes mellitus: a case-control study. Clin Nutr Res. 2017;6(2):81. https://doi.org/10.7762/2Fcnr.2017.6.2.81.
Grissa O, Atègbo JM, Yessoufou A, Tabka Z, Miled A, Jerbi M, et al. Antioxidant status and circulating lipids are altered in human gestational diabetes and macrosomia. Transl Res. 2007;150(3):164–71. https://doi.org/10.1016/j.trsl.2007.03.007.
Djordjevic A, Spasic S, Jovanovic-Galovic A, Djordjevic R, Grubor-Lajsic G. Oxidative stress in diabetic pregnancy: SOD, CAT and GSH-Px activity and lipid peroxidation products. J Matern Fetal Neonatal Med. 2004;16(6):367–72. https://doi.org/10.1080/jmf.16.6.367.372.
Atiba AS, Olofinbiyi BA, Aduloju OP, Akindele RA, Tolorunju K, Adekanle DA. Product of free radical injury and antioxidant status in patients with gestational diabetes mellitus (GDM). IJHSR. 2018;8(32):32–7.
Mahmoud F, Dashti A, Abul H, Jumath OA. Antioxidant enzymes in gestational diabetes: a study on a Kuwaiti population. Bioenerg. 2014;3(117):62–7. https://doi.org/10.4172/2167-7662.1000117.
Maejima K, Nakano S, Himeno M, Tsuda SI, Makiishi H, Ito T, et al. Increased basal levels of plasma nitric oxide in type 2 diabetic subjects: relationship to microvascular complications. J Diabetes Complications. 2001;15(3):135–43. https://doi.org/10.1016/S1056-8727(01)00144-1.
Tessari P, Cecchet D, Cosma A, Vettore M, Coracina A, Millioni R, et al. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes. 2010;59(9):2152–9. https://doi.org/10.2337/db09-1772.
De Leon JAD, Borges CR. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. JoVE. 2020;159:e61122. https://doi.org/10.3791/61122.
Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. https://doi.org/10.1006/niox.2000.0319.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–7. https://doi.org/10.1016/S0891-5849(98)00315-3.
Hadwan MH, Abed HN. Data supporting the spectrophotometric method for the estimation of catalase activity. Data Brief. 2016;6:194–9. https://doi.org/10.1016/j.dib.2015.12.012.
Mahmood HG, Ali ZA, Abdulhameed RA, Hussein IM. Oxidative stress and total antioxidant capacity in rheumatoid arthritis. J Facul Med Baghdad. 2014;56(3):329–33. https://doi.org/10.32007/jfacmedbagdad.563528.
Loverro G, Greco P, Capuano F, Carone D, Cormio G, Selvaggi L. Lipoperoxidation and antioxidant enzymes activity in pregnancy complicated with hypertension. Eur J Obstet Gynecol Reprod Biol. 1996;70(2):123–7. https://doi.org/10.1016/S0301-2115(95)02561-8.
Mazzara S, Rossi RL, Grifantini R, Donizetti S, Abrignani S, Bombaci M. CombiROC: an interactive web tool for selecting accurate marker combinations of omics data. Sci Rep. 2017;7(1):45477. https://doi.org/10.1038/srep45477.
Dey P, Gupta P, Acharya NK, Rao SN, Ray S, Chakrabarty S, et al. Antioxidants and lipid peroxidation in gestational diabetes-a preliminary study. Indian J Physiol Pharmacol. 2008;52(2):149–56.
Echeverria C, Eltit F, Santibanez JF, Gatica S, Cabello-Verrugio C, Simon F. Endothelial dysfunction in pregnancy metabolic disorders. Biochimica et Biophysica Acta (BBA)-Mol Basis Dis. 2020;1866(2):165414. https://doi.org/10.1016/j.bbadis.2019.02.009.
Karagoz ZK, Aydin S, Ugur K, Tigli A, Deniz R, Baykus Y, et al. Molecular communication between Apelin-13, Apelin-36, Elabela, and nitric oxide in gestational diabetes mellitus. Eur Rev Med Pharmacol Sci. 2022;26(9):3289–300. https://doi.org/10.26355/eurrev_202205_28748.
Von Mandach U, Lauth D, Huch R. Maternal and fetal nitric oxide production in normal and abnormal pregnancy. J Matern Fetal Neonatal Med. 2003;13(1):22–7. https://doi.org/10.1080/jmf.13.1.22.27.
Pieme CA, Tatangmo JA, Simo G, Biapa Nya PC, Ama Moor VJ, Moukette Moukette B, et al. Relationship between hyperglycemia, antioxidant capacity and some enzymatic and non-enzymatic antioxidants in African patients with type 2 diabetes. BMC Res Notes. 2017;10:1–7. https://doi.org/10.1186/s13104-017-2463-6.
Koohshekan B, Divsalar A. In vitro glycation of bovine liver catalase by glucose and fructose and antigycation effects of aspirin: a spectroscopic study. J Biomol Struct Dyn. 2017;35(14):3061–9. https://doi.org/10.1080/07391102.2016.1241189.
Sperling MM, Leonard SA, Blumenfeld YJ, Carmichael SL, Chueh J. Prepregnancy body mass index and gestational diabetes mellitus across Asian and Pacific Islander subgroups in California. AJOG Global Reports. 2023;3(1):100148. https://doi.org/10.1016/j.xagr.2022.100148.
Acknowledgment
We would like to acknowledge the Gestational diabetes patients and the controls for participating in this study.
Funding
This study was funded by the University of Colombo, Sri Lanka, through funds provided for undergraduate special research projects in 2022.
Author information
Authors and Affiliations
Contributions
AK(1) conceptualized, generate data, conducted statistical analysis, and wrote the initial draft of the manuscript. AK(2) and SW provided samples and provided critical insights and revised the manuscript. PU organized and supervised the project and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures were approved by the Ethics Review Committee (ERC) of the Faculty of Graduate Studies, University of Colombo (FGS/ERC/2022/010), and the Ethics Review Committee of the De Soysa Hospital for Women (023). All subjects provided written informed consent prior to their inclusion in the study. Details that disclose the identity of the subjects were omitted.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumarage, A., Kaluarachchi, A., Wijeratne, S. et al. A Sri Lankan pilot case–control study on gestational diabetes mellitus: oxidative stress and a potential diagnostic marker panel. Int J Diabetes Dev Ctries (2024). https://doi.org/10.1007/s13410-024-01379-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13410-024-01379-5