Abstract
Objective
Conflicting results on the association of plasminogen activator inhibitor-1 (PAI-1) with prior gestational diabetes mellitus (pGDM) have been observed among studies that imply the need to perform a meta-analysis.
Methods
Literature that determined the levels of PAI-1 in patients with pGDM and non-GDM was retrieved from various database websites. Relevant data were extracted from each study and collated. For the data analysis, Review Manager ver. 5.4 was used. The studies were pooled to compute the standardized mean difference (SMD) and 95% confidence interval (CI) between pGDM and non-GDM groups.
Results
Overall results were heterogeneous, which prompted the identification of the cause using a Galbraith plot. Post-outlier outcomes demonstrate that higher levels of PAI-1 are observed among women with pGDM than those with no history of the disease.
Conclusion
PAI-1 is significantly associated with GDM, especially among patients with pGDM. Further studies should be conducted on the relation of serum PAI-1 with other diabetes-related markers and variables to verify these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pregnancy is associated with numerous physiological changes, which sometimes may lead to the development of pregnancy-related complications. An example is the increasing prevalence of hemostatic disorders in pregnant women. This arises from increased coagulation factors, including plasminogen activator inhibitor-1 (PAI-1) [1], throughout pregnancy [2] and the puerperium [1, 3]. PAI-1 is a serine protease inhibitor produced mainly in the liver [4] and other types of cells like endothelial cells, megakaryocytes, and adipocytes [5]. It inhibits fibrinolysis by inhibiting activators of plasminogen, the inactive form of plasmin.
Increased levels of PAI-1 are associated with numerous metabolic disorders, like cardiometabolic disorders [6], obesity [7], and diabetes [8]. Of intriguing interest here is the association of increased PAI-1 with gestational diabetes mellitus (GDM). GDM is a type of diabetes recognized at the onset of pregnancy, which is seen in approximately 7% of pregnancy complications [9]. Due to hormonal changes in pregnancy, there is an associated increase in adipose tissue deposits [10], which can also produce PAI-1 [7]. As a result, PAI-1 levels are elevated in patients with GDM [11, 12]. Few studies have already been conducted that determined the association of the protein with GDM, especially after delivery [13,14,15,16,17,18,19,20,21,22]. However, results vary with each other, which necessitates a meta-analysis to strengthen the association, if there is one.
Materials and methods
Study design
Articles used for this study were searched in PubMed, ScienceDirect, Google Scholar (title search only), and Cochrane Library using the key search term combination: “gestational diabetes” OR “GDM” AND “plasminogen activator inhibitor-1” and OR “PAI-1” as of June 22, 2023. The resulting studies were first screened by checking their titles and abstracts. After duplicate and inappropriate studies were removed, full texts of the remaining articles were obtained and manually checked for their relevance. Also, cited references from each eligible study were examined.
The following inclusion criteria were used: (a) studies that measured PAI-1 levels in ng/mL; (b) studies that included prior GDM cases; (c) studies that included non-GDM controls; and (d) studies that were written in English. All studies identified were investigated for eligibility by the authors.
Data extraction and conversion
The following data were obtained from all eligible studies: (a) first author’s last name; (b) date of publication; (c) country where the study was conducted; (d) time when blood samples were obtained; (e) number of cases vs. controls; (f) the total number of participants; and (g) concentration of PAI-1 from cases and control groups. This study defined prior GDM (pGDM) when blood collection was done after delivery. Two authors extracted the data (RET and MJD) and agreed on all the items. Disagreements (if any) were resolved by a third person (MC).
For studies that expressed PAI-1 levels in median and interquartile, we converted the measures into their approximate means and standard deviation (SD) using the procedure of Hozo et al. [23]. To avoid discrepancies caused by data conversion, only values in nanograms per milliliter (ng/mL) were used for the study.
Quality assessment of the included studies
The quality of the methodology of all included studies was assessed using the Newcastle–Ottawa Scale (NOS). The resulting studies were each rated based on the respondents’ selection, comparability, and exposure. The scoring system utilized has a maximum rating of nine points. Accumulated scores of ≤ 4 indicate low-quality studies. On the other hand, scores of 5–6 points indicate moderate-quality studies, whereas scores of ≥ 7 points indicate high-quality studies [24].
Statistical analysis, sensitivity analysis, and publication bias testing
The protocol used for this meta-analysis was based on the procedure from existing studies [25, 26] in treating mean and SD. The standardized mean difference (SMD) and 95% confidence interval (CI) of the levels of PAI-1 between pGDM and non-GDM were calculated from each study and pooled. The pooled SMD estimates were determined either by the fixed- or random-effects model, depending on heterogeneity [27, 28]. Heterogeneity was examined using a chi-based Q test, and its degree was measured using I2 statistics [29, 30]. All p-values (PA) for association were two-sided with a significance threshold set at < 0.05. In contrast, the p-value (PH) for heterogeneity is set at < 0.10 due to the low power of the test [31]. All statistical analysis was performed using Review Manager ver. 5.4. The robustness of the pooled effects was determined using sensitivity analysis. This was done by repeating the overall statistical analysis while omitting one study at a time. This was done to check the influence of the individual study in the pooled SMD. On the other hand, publication bias was no longer assessed due to the low number of studies in the post-outlier analysis [32].
Results
Search results and characteristics of the included studies
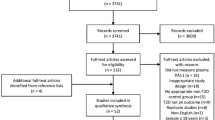
Figure 1 summarizes the selected studies following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [33]. The literature search yielded a total of 219 studies that were manually checked. After the omission of duplicates, animal studies, reviews, and commentaries, studies were screened following the set inclusion criteria, resulting in ten studies being included in the systematic review. However, from these studies, two papers were possible continuations of previous publications. Hence, in the performance of the meta-analysis, the two old duplicate papers [14, 22] were excluded. On the other hand, for the remaining eight studies, two papers [16, 19] contained two sets of data for the GDM cohort. The paper of Farhan et al. in 2006 employed pGDM with impaired insulin sensitivity and those with normal insulin sensitivity. On the other hand, the study of Morimitsu et al. [16] also used two pGDM cohorts, namely, those with impaired glucose tolerance and those with normal glucose tolerance. Hence, for this reason, even if the number of studies included is only eight, the data sets used for analysis are increased to ten because of the multiple pGDM cohorts in the two studies. For the NOS score, we obtained a mean and SD of 5.9 ± 0.9 and a median of 6, indicating that the studies included were of moderate quality.
Table 1 summarizes the characteristics of the studies included. The year of publication ranged from 2005 to 2013. The total sample size included for the meta-analysis is 724 (237 healthy controls and 487 women with pGDM), with a narrow range of total sample sizes across all the studies (34 to 140). Other qualities, such as country of origin, the time of collection of the blood sample, and the subgroup used (if any), are summarized in Table 1.
Overall and post-outlier association
The results for the overall association are shown in Fig. 2. The association model used the random-effects model due to high heterogeneity (I2 = 98%), which prompted us to perform an outlier analysis using a funnel plot (Fig. 3). After removing the outlier data sets, homogeneity was achieved (I2 = 0%) after a repeat analysis (Fig. 4). The change in the I2 and PH values in the association model demonstrates that these studies are responsible for the inconsistency. The post-outlier fixed-effects model analysis showed that the PAI-1 was observed to be elevated (SMD 0.62; OR 0.40, 0.85; PA < 0.00001) among those who previously had GDM.
Sensitivity analysis and publication bias
The outcome from the comparison was robust, indicating the stability of our findings (data not shown). Publication bias analysis was no longer performed due to the limited number of studies per association model.
Discussion
Summary and interpretation of findings
The present meta-analysis summarized the results of 12 studies involving 1226 pregnant women. By pooling the SMDs and 95% CIs from the individual studies, we were able to show that serum PAI-1 is associated with pGDM. Results of the post-outlier outcomes suggest that PAI-1 is significantly higher among participants with a previous history of GDM than those pregnant women without a history of GDM. These significant findings observed in the present study provide strong evidence of the potential effect of GDM on serum PAI-1 levels. This is supported by the homogeneity of the post-outlier results, indicating the combinability of the studies. Moreover, a high degree of significance, consistent precision of effects, and robustness of the post-outlier outcomes enhance the evidence presented in this meta-analysis.
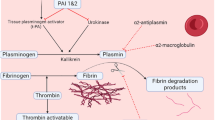
Effect of pregnancy on PAI-1 levels and its association with a thrombotic state
Studies have shown that thromboembolic events are common among pregnant women due to physiological and hormonal changes; a pregnant woman is four- to sixfold at risk for thrombosis compared to a non-pregnant woman [34]. To prevent postpartum bleeding, the pregnant mother adapts its homeostatic system by increasing the production of its coagulation factors like fibrinogen, factor VII, factor VIII, von Willebrand factor, and factor X [35], thereby increasing the ability of the body to form thrombi. In addition, fibrinolytic factor tissue plasminogen activator (tPA) rises throughout pregnancy [11, 36]. However, the same studies also suggest the increase of its antagonist, PAI-1, especially during the third trimester and in the puerperium. PAI-1 is the major serine protease inhibitor compared to PAI-2 and PAI-3, with approximately 60% inhibitory activity in fibrinolysis [34, 37]. This protein regulates the activation of plasminogen into plasmin by inhibiting tPA and urokinase. An increase in PAI-1 could lead to a decrease in clot breakdown, increasing the risk of deep vein thrombosis in pregnant women [38].
Hypofibrinolysis in pregnancy is mainly because of the significantly increased levels of PAI-1 from endothelial cells and PAI-2 from the placenta, which is seldom found in non-pregnant circulation. However, despite hypofibrinolysis, D-dimer increases by two- to fourfold as pregnancy progresses [34]. This is due to excessive thrombin formation brought about by increased clotting factors as manifested through increased prothrombin fragments and fibrinopeptide A, consistent with a mild degree of local intravascular coagulation in some women early in pregnancy [3]. Plasmin inhibitor (alpha 2-antiplasmin) is found to be slightly increased or unchanged during pregnancy [39]. These changes with inhibitors partly explain the higher incidence of thrombotic state during pregnancy.
Increased PAI-1 levels are found in patients with recurrent pregnancy losses (RPL), preeclampsia [40, 41], intrauterine growth restriction (IUGR), endometriosis, polycystic ovary syndrome (PCOS), and GDM [11].
Association of GDM with PAI-1 levels
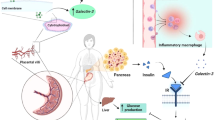
Normally, the self-adaptive pregnancy mechanism of inducing a hypercoagulable state is in place to maintain the balance on hemostasis and prevent too much blood loss during delivery. However, in patients with GDM, this adaptive mechanism may shift to excessive hypercoagulability and hypofibrinolysis [16]. Plasminogen, a serine protease responsible for fibrin degradation when converted to plasmin, is activated by a tissue-plasminogen activator (tPA) and urokinase (uPA). These activators, both tPA and uPA, are inhibited primarily by PAI-1, especially during pregnancy, thus suppressing the plasmin formation and lysis of fibrin clots. Most of these inhibitors in circulation are synthesized by adipose tissue [42] and an increase in adipose tissue deposits is observed among GDM patients. This compensates for the developing fetus’s increasing demand for nutrients, such as glucose [43].
Consequently, pregnant women’s overall body mass index is higher than normal women’s [44]. This increase is usually observed in the second and third trimesters [45], with most adipose tissue being visceral adipose tissue (VAT) in the metabolically active areas of the peritoneum [46]. Subcutaneous adipose tissue (SCAT), on the other hand, is also one of the body compartments where adipose tissue storage occurs. As this increases in size, together with VAT, it also increases the risk of developing diabetes, atherosclerosis, dyslipidemia, and metabolic syndromes in both pregnant and non-pregnant women, the latter being more affected [47]. Although VAT thickness measurement is a more sensitive predictor of GDM [48], a study conducted by Kansu-Celik et al. in 2018 reported that measuring SCAT thickness may also be a useful risk predictor of GDM during early pregnancy [49]. It has been observed that PAI-1 is overexpressed in ectopic fat, especially in macrophages. The macrophage and adipose tissue have been shown to respond to inducers of PAI-1 synthesis, which explains an increase of PAI-1 during obesity [7]. Certain adipokines like PAI-1 have been associated with various diseases, including insulin resistance and cardiovascular disease [50]. Kaji et al. also explained that metabolic conditions like obesity and inflammation lead to adipocyte hypertrophy and impaired regulation of adipokine production.
According to the study conducted by Mehmood et al. in 2018, values of PAI-1 in patients with GDM progressively increased at more than 2-year intervals postpartum. Mothers who previously developed GDM have higher PAI-1 than those with current conditions. Another data from Morimitsu et al. [16] showed that elevated PAI-1 concentrations in the circulation are associated with women with a GDM history, especially those with impaired insulin sensitivity. They concluded that the increased concentration of this inhibitor begins between 16 and 24 weeks after delivery. Furthermore, the elevation of PAI-1 in patients with GDM also depends on abdominal obesity. They also concluded that PAI-1 levels do not decrease in women who developed type 2 diabetes mellitus after pregnancy, even if the BMI of patients has improved over time [51]. These conclusions are consistent with the results of this study, where PAI-1 is increased in patients who developed GDM earlier in time than those who have presently elapsing GDM.
Role of PAI-1 in the development of cardiometabolic complications
The risk of cardiovascular diseases (CVDs) is higher in cases of hypercoagulable states. Although usually asymptomatic, atherosclerosis-induced vascular injury could trigger platelet activation and adhesion. The formation of thrombi around damaged vessel walls is called atherothrombosis [6]. This is the leading cause of mortality among CVDs, accounting for an estimated 20% of complications from stroke [6]. As the integrity of vascular walls deteriorates over time due to various metabolic factors, these walls, which form plaques, will likely rupture [52]. Upon rupturing, tissue factor is released from these plaques and triggers the coagulation cascade, activating the extrinsic pathway [53]. These thrombi prevent sufficient blood flow, causing ischemic episodes [54]. Due to PAI-1 being an antifibrinolytic protein, numerous studies have investigated its association with CVDs. It has been shown that an increase in circulating PAI-1 also correlates with an increased risk for myocardial infarction [55], thus being a good predictor for the latter’s development [56]. Because of the relation between diabetes and CVDs, an increase of PAI-1 in circulation is seen as a novel risk factor for both [57, 58] and as a potential target for therapeutic drug development [59] using PAI-1 inhibitors [60].
Limitations of the study
Interpreting these results warrants awareness of the study’s limitations, such as (i) inconsistent sources of cases and controls due to the difference in the inclusion criteria used as well as the time of sample collection; (ii) failure to note the method used for serum PAI-1 analysis; (iii) failure to include other risk factors that could influence serum PAI-1 levels; and (iv) failure to include non-pregnant controls.
Conclusion
To our knowledge, this is the first meta-analysis that explored the association of serum PAI-1 with pGDM. Overall, our findings suggest that serum PAI-1 is significantly associated with pGDM. However, given the limitations of the present study, findings should be treated with caution, mainly when applied to clinical practice. Further studies regarding the relation of serum PAI-1 with other diabetes-related markers and variables should be done to understand its role in GDM. Also, population-based studies can be done to assess population variability of the results.
References
Ramsay M. Normal hematological changes during pregnancy and the puerperium. In: The Obstetric Hematology Manual. 2011. pp. 3–12. https://doi.org/10.1017/cbo9780511676451.002.
Stirling Y, Woolf L, North WRS, Seghatchian MJ, Meade TW. Haemostasis in normal pregnancy. Thromb Haemost. 1984;52(02):176–82. https://doi.org/10.1055/s-0038-1661167.
Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost. 2003;29(2):125–30. https://doi.org/10.1055/s-2003-38897.
Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. 2010;28(5):72–91. https://doi.org/10.1111/j.1755-5922.2010.00171.x.
Zorio E, Gilabert-Estelles J, Espana F, Ramon L, Cosin R, Estelles A. Fibrinolysis: the key to new pathogenetic mechanisms. Curr Med Chem. 2008;15(9):923–9. https://doi.org/10.2174/092986708783955455.
Leys D. Atherothrombosis: a major health burden. Cerebrovasc Dis. 2001;11(Suppl. 2):1–4. https://doi.org/10.1159/000049137.
Alessi MC, Poggi M, Juhan-Vague I. Plasminogen activator inhibitor-1, adipose tissue and insulin resistance. Curr Opin Lipidol. 2007;18(3):240–5. https://doi.org/10.1097/MOL.0b013e32814e6d29.
Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006;26(10):2200–7. https://doi.org/10.1161/01.ATV.0000242905.41404.68.
Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. https://doi.org/10.1016/j.diabres.2018.02.023.
Barr S, Walker B, Morton N, Norman J. Adipose tissue metabolism in obese pregnant women. Arch Dis Child Fetal Neonatal Ed. 2010;95(Supplement 1):Fa33. https://doi.org/10.1136/adc.2010.189753.2.
Ye Y, Vattai A, Zhang X, et al. Role of plasminogen activator inhibitor type 1 in pathologies of female reproductive diseases. Int J Mol Sci. 2017;18(8):1651. https://doi.org/10.3390/ijms18081651.
Lowe LP, Metzger BE, Lowe WL, Dyer AR, McDade TW, McIntyre HD. Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. J Clin Endocrinol Metab. 2010;95(12):5427–34. https://doi.org/10.1210/jc.2010-1662.
Winzer C, Wagner O, Festa A, Schneider B, Roden M, Bancher-Todesca D, Pacini G, Funahashi T, Kautzky-Willer A. Plasma adiponectin, insulin sensitivity, and subclinical inflammation in women with prior gestational diabetes mellitus. Diabetes Care. 2004;27(7):1721–7. https://doi.org/10.2337/diacare.27.7.1721.
Sokup A, Góralczyk B, Góralczyk K, Rość D. Triglycerides as an early pathophysiological marker of endothelial dysfunction in nondiabetic women with a previous history of gestational diabetes. Acta Obstet Gynecol Scand. 2012;91(2):182–8. https://doi.org/10.1111/j.1600-0412.2011.01289.x.
Sokup A, Ruszkowska B, Góralczyk B, et al. Elevation of sE-selectin levels 2–24 months following gestational diabetes is associated with early cardiometabolic risk in nondiabetic women. Int J Endocrinol. 2012;2012:1–6. https://doi.org/10.1155/2012/278050.
Morimitsu LK, Fusaro AS, Sanchez VH, Hagemann CCF, Bertini AM, Dib SA. Fibrinolytic dysfunction after gestation is associated to components of insulin resistance and early type 2 diabetes in Latino women with previous gestational diabetes. Diabetes Res Clin Pract. 2007;78(3):340–8. https://doi.org/10.1016/j.diabres.2007.04.013.
Heitritter SM, Solomon CG, Mitchell GF, Skali-Ounis N, Seely EW. Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab. 2005;90(7):3983–8. https://doi.org/10.1210/jc.2004-2494.
Göbl CS, Bozkurt L, Prikoszovich T, Tura A, Pacini G, Kautzky-Willer A. Estimating the risk after gestational diabetes mellitus: can we improve the information from the postpartum OGTT? Am J Physiol Endocrinol Metab. 2013;304. https://doi.org/10.1152/ajpendo.00461.2012.-Risk.
Farhan S, Winzer C, Tura A, Quehenberger P, Bieglmaier C, Wagner OF, Huber K, Waldhäusl W, Pacini G, Kautzky-Willer A. Fibrinolytic dysfunction in insulin-resistant women with previous gestational diabetes. Eur J Clin Invest. 2006;36(5):345–52. https://doi.org/10.1111/j.1365-2362.2006.01630.x.
Bayraktar F, Akinci B, Celtik A, et al. Insulin need in gestational diabetes is associated with a worse cardiovascular risk profile after pregnancy. Intern Med. 2012;51(8):839–43. https://doi.org/10.2169/internalmedicine.51.5846.
Akinci B, Celtik A, Yener S, et al. Plasma thrombin-activatable fibrinolysis inhibitor levels are not associated with glucose intolerance and subclinical atherosclerosis in women with previous gestational diabetes. Clin Appl Thromb/Hemost. 2011;17(6):E224–30. https://doi.org/10.1177/1076029610397753.
Akinci B, Demir T, Celtik A, et al. Serum osteoprotegerin is associated with carotid intima media thickness in women with previous gestational diabetes. Diabetes Res Clin Pract. 2008;82(2):172–8. https://doi.org/10.1016/j.diabres.2008.07.014.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5. https://doi.org/10.1186/1471-2288-5-13.
Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute. 2013;3:1–4. https://doi.org/10.2307/632432.
Pabalan N, Singian E, Tabangay L, Jarjanazi H, Boivin MJ, Ezeamama AE. Soil-transmitted helminth infection, loss of education and cognitive impairment in school-aged children: a systematic review and meta-analysis. Budke CM, ed. PLoS Negl Trop Dis. 2018;12(1):e0005523. https://doi.org/10.1371/journal.pntd.0005523.
Ezeamama AE, Bustinduy AL, Nkwata AK, et al. Cognitive deficits and educational loss in children with schistosome infection—a systematic review and meta-analysis. Garba A, ed. PLoS Negl Trop Dis. 2018;12(1):e0005524. https://doi.org/10.1371/journal.pntd.0005524.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48. https://doi.org/10.1093/jnci/22.4.719.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. https://doi.org/10.1016/0197-2456(86)90046-2.
Lau J, Ioannidis JPA, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–6. https://doi.org/10.7326/0003-4819-127-9-199711010-00008.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ: Br Med J. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186.
Ioannidis JPA, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007;176(8):1091–6. https://doi.org/10.1503/cmaj.060410.
Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/10.1186/2046-4053-4-1.
Moiz B. A review of hemostasis in normal pregnancy and puerperium. Natl J Health Sci. 2017;2(3):123–7. https://doi.org/10.21089/njhs.23.0123.
Battinelli EM, Marshall A, Connors JM. The role of thrombophilia in pregnancy. Thrombosis. 2013;2013:1–9. https://doi.org/10.1155/2013/516420.
Cerneca F, Ricci G, Simeone R, Malisano M, Alberico S, Guaschino S. Coagulation and fibrinolysis changes in normal pregnancy. Increased levels of procoagulants and reduced levels of inhibitors during pregnancy induce a hypercoagulable state, combined with a reactive fibrinolysis. Eur J Obstet Gynecol Reprod Biol. 1997;73(1):31–36. https://doi.org/10.1016/S0301-2115(97)02734-6.
Kluft C, Jie AFH, Sprengers ED, Verheijen JH. Identification of a reversible inhibitor of plasminogen activators in blood plasma. FEBS Lett. 1985;190(2):315–8. https://doi.org/10.1016/0014-5793(85)81309-0.
Rosenberg VA, Lockwood CJ. Thromboembolism in pregnancy. Obstet Gynecol Clin North Am. 2007;34(3):481–500. https://doi.org/10.1016/j.ogc.2007.06.006.
Hellgren M, Blombäck M. Studies on blood coagulation and fibrinolysis in pregnancy, during delivery and in the puerperium. Gynecol Obstet Invest. 1981;12(3):141–5.
Lucena FC, Lage EM, Teixeira PG, et al. Longitudinal assessment of D-dimer and plasminogen activator inhibitor type-1 plasma levels in pregnant women with risk factors for preeclampsia. Hypertens Pregnancy. 2019;38(1):58–63. https://doi.org/10.1080/10641955.2019.1577435.
Udenze IC, Arikawe AP, Makwe CC. Early pregnancy plasminogen activator inhibitor-1 levels in Nigerian women and its relationship with preeclampsia. Niger J Clin Pract. 2017;20(5):517–22. https://doi.org/10.4103/1119-3077.183256.
Yarmolinsky J, Bordin Barbieri N, Weinmann T, Ziegelmann PK, Duncan BB, Inês Schmidt M. Plasminogen activator inhibitor-1 and type 2 diabetes: a systematic review and meta-analysis of observational studies. Sci Rep. Published online 2016. https://doi.org/10.1038/srep17714
Valsamakis G, Kumar S, Creatsas G, Mastorakos G. The effects of adipose tissue and adipocytokines in human pregnancy. Ann N Y Acad Sci. 2010;1205(1):76–81. https://doi.org/10.1111/j.1749-6632.2010.05667.x.
Barisic T, Mandic V, Barac I. Associations of body mass index and gestational weight gain with term pregnancy outcomes. Mater Socio Med. 2017;29(1):52. https://doi.org/10.5455/msm.2017.29.52-57.
Sidebottom AC, Brown JE, Jacobs DR. Pregnancy-related changes in body fat. Eur J Obstet Gynecol Reprod Biol. 2001;94(2):216–23. https://doi.org/10.1016/S0301-2115(00)00329-8.
Straughen JK, Trudeau S, Misra VK. Changes in adipose tissue distribution during pregnancy in overweight and obese compared with normal weight women. Nutr Diabetes. 2013;3(8):e84–e84. https://doi.org/10.1038/nutd.2013.25.
Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11–8. https://doi.org/10.1111/j.1467-789X.2009.00623.x.
Gur EB, Ince O, Turan GA, et al. Ultrasonographic visceral fat thickness in the first trimester can predict metabolic syndrome and gestational diabetes mellitus. Endocrine. 2014;47(2):478–84. https://doi.org/10.1007/s12020-013-0154-1.
Kansu-Celik H, Karakaya BK, Tasci Y, et al. Relationship maternal subcutaneous adipose tissue thickness and development of gestational diabetes mellitus. Interv Med Appl Sci. 2018;10(1):13–8. https://doi.org/10.1556/1646.10.2018.01.
Kaji H. Adipose tissue-derived plasminogen activator inhibitor-1 function and regulation. In: Comprehensive Physiology. vol 6. John Wiley & Sons, Inc.; 2016. pp. 1873–1896. https://doi.org/10.1002/cphy.c160004.
Mehmood S, Ye C, Connelly PW, Hanley AJ, Zinman B, Retnakaran R. Rising plasminogen activator inhibitor-1 and hypoadiponectinemia characterize the cardiometabolic biomarker profile of women with recent gestational diabetes. Cardiovasc Diabetol. 2018;17(1):1–9. https://doi.org/10.1186/s12933-018-0776-y.
Viles-Gonzalez JF, Fuster V, Badimon JJ. Atherothrombosis: a widespread disease with unpredictable and life-threatening consequences. Eur Heart J. 2004;25(14):1197–207. https://doi.org/10.1016/j.ehj.2004.03.011.
MacKman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg. 2009;108(5):1447–52. https://doi.org/10.1213/ane.0b013e31819bceb1.
Iacoviello L, Agnoli C, De Curtis A, et al. Type 1 plasminogen activator inhibitor as a common risk factor for cancer and ischaemic vascular disease: the EPICOR study. BMJ Open. 2013;3(11): e003725. https://doi.org/10.1136/bmjopen-2013-003725.
Hamsten A, Walldius G, Szamosi A, et al. Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. The Lancet. 1987;330(8549):3–9. https://doi.org/10.1016/S0140-6736(87)93050-9.
Collet JP, Montalescot G, Vicaut E, et al. Acute release of plasminogen activator inhibitor-1 in ST-segment elevation myocardial infarction predicts mortality. Circulation. 2003;108(4):391–4. https://doi.org/10.1161/01.CIR.0000083471.33820.3C.
Song C, Burgess S, Eicher JD, et al. Causal effect of plasminogen activator inhibitor type 1 on coronary heart disease. J Am Heart Assoc. 2017;6(6). https://doi.org/10.1161/JAHA.116.004918.
Trost S, Pratley RE, Sobel BE. Impaired fibrinolysis and risk for cardiovascular disease in the metabolic syndrome and type 2 diabetes. Curr Diab Rep. 2006;6(1):47–54. https://doi.org/10.1007/s11892-006-0052-5.
Tofler GH, Massaro J, O’Donnell CJ, et al. Plasminogen activator inhibitor and the risk of cardiovascular disease: the Framingham Heart Study. Thromb Res. 2016;140:30–5. https://doi.org/10.1016/j.thromres.2016.02.002.
Brown NJ. Therapeutic potential of plasminogen activator inhibitor-1 inhibitors. Ther Adv Cardiovasc Dis. 2010;4(5):315–24. https://doi.org/10.1177/1753944710379126.
Author information
Authors and Affiliations
Contributions
All authors have contributed substantially to collecting and analyzing the data and writing and critically revising the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human or animal subjects.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tiongco, R.E., Dizon, G., Catacata, M. et al. Plasminogen activator inhibitor-1 levels in prior gestational diabetes mellitus: A systematic review and meta-analysis. Int J Diabetes Dev Ctries (2024). https://doi.org/10.1007/s13410-024-01352-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13410-024-01352-2