Abstract
Background
An ideal glucose-lowering drug is expected to not only improve glycemic control, but also have positive effects on weight, blood pressure, dyslipidemia, and also cardiovascular and renal outcomes.
Objective
To investigate and compare the impact of Sodium-glucose transport protein 2 (SGLT2) inhibitors on glycemic and extraglycemic laboratory parameters and the parameters which affect this impact.
Methods
This retrospective study was conducted between January 2022 and December 2022. A total of 250 patients diagnosed with type 2 diabetes mellitus (T2DM) using SGLT2i were included in the study.
Results
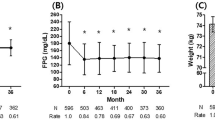
Patients had a mean age of 55.4 ± 9.6, and 53.6% (n = 134) were male. Among the patients, 19.6% (n = 49) used dapagliflozin and 80.4% (n = 201) used empagliflozin. Glucose, HbA1c, and triglyceride levels at 3 and 6 months showed significant reductions compared to baseline, while serum sodium and HDL-C levels showed significant increases (p < 0.001). Additionally, creatinine and serum potassium levels at 6 months were significantly higher than baseline, while LDL-C and urine albumin-to-creatinine ratio levels were significantly lower. Empagliflozin users exhibited significantly higher creatinine levels only at 3. months, higher serum sodium levels only at 6. months, and lower HbA1c levels only at 6. months compared to dapagliflozin users.
Conclusion
While SGLT2i seem to provide positive effects on the lipid profile, as well as their well-recognized effects on glycemic parameters, there may be value in further evaluating renal safety and the long-term alterations in lipid profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a complex metabolic disease associated with conditions, such as obesity, cardiovascular diseases (CVDs), dyslipidemia and nephropathy [1]. Diabetes has also been shown to increase risks for CVDs and diabetic nephropathy [2, 3]. Therefore, an ideal glucose-lowering drug is expected to not only improve glycemic control, but also have positive effects on weight, blood pressure, dyslipidemia, and also cardiovascular and renal outcomes [4].
Sodium-glucose transport protein 2 (SGLT2) inhibitors (SGLT2i) are the most recent glucose-lowering drugs gaining widespread use in T2DM [5]. They block SGLT2 channels in the proximal renal tubule, thereby preventing reabsorption of glucose [6] and increasing urinary glucose excretion –which reduces plasma glucose regardless of the effects of insulin [4]. Studies have shown that SGLT2i use is associated with greater improvement in glycated hemoglobin (HbA1c) compared to placebo and oral antidiabetics [7]. Glycosuria also causes considerable calorie loss, potential weight loss, and a decrease in blood pressure [4]. Randomized controlled trials have shown that SGLT2i can reduce risks for major adverse cardiovascular events, heart failure, and poor renal outcomes [8,9,10]. It has even been reported that these effects may be partially independent of glucose-lowering activity [11, 12]. Based on these results, current guidelines recommend administration of SGLT2i in patients with T2DM and certain cardiovascular/renal comorbidities (or high risks for these) [2, 3, 13]. However, the mechanisms of cardiorenal protection conferred by SGLT2i still need to be clarified [14]. SGLT2i have also been associated with various adverse effects, including cardiovascular, renal and metabolic adverse consequences [5, 7, 15, 16]. The positive and negative consequences of these agents and their relationships with other factors, including concomitant medications, diabetes duration and timing of SGLT2i initiation, have not been adequately investigated.
The metabolic effects of SGLT2i may change over time and could be affected by other concomitant antidiabetic regimens, duration of diabetes, and SGLT2i initiation time. In this context, our aim was to investigate and compare the two SGLT2i medications marketed in our country (dapagliflozin and empagliflozin) by examining longitudinal changes (3 and 6 months) in glucose metabolism, lipid profile, renal functions and serum electrolyte levels. The relationship of these changes with concomitant antidiabetic regimens was also examined.
Materials and methods
Study design
A total of 250 patients diagnosed with type 2 diabetes mellitus, who applied to the diabetes outpatient clinic between January 2022 and December 2022 and had been started on SGLT2i between these dates, were included in the study. Examinations were planned to be performed at 3 and 6 months, and therefore, those using SGLT2i for less than 6 months at the end of the study period were excluded from the study. Additionally, patients using calcium channel blockers, diuretics or statins were excluded. The use of these drugs in baseline or during the 6-month period of the study was considered as exclusion criteria. Whether a statin indication occurred during SGLT2i treatment was evaluated according to the 2019 European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) guidelines [17]. Accordingly, patients with low risk according to the total cardiovascular risk score (SCORE) and low-density lipoprotein-cholesterol (LDL-C) < 116 mg/dL, patients with moderate risk and LDL-C < 100 mg/dL, patients with high risk and LDL-C < 70 mg/dL, and patients with very high risk and LDL-C < 55 mg/dL did not receive antihyperlipidemic therapy.
Data collection
This longitudinal retrospective study was carried out in the Department of Internal Medicine of Dr. Sadi Konuk Training and Research Hospital. All procedures agreed with the ethical standards of the institutional research committee and with the Helsinki declaration and its later amendments. The study plan and procedures were evaluated and approved by the Ethics Committee of Dr. Sadi Konuk Training and Research Hospital (Decision date: 21.02.2022, decision no: 2022–04-14).
Participants’ data included in the study were as follows: age, sex and comorbidities, duration of T2DM, concomitant diabetes medication used with SGLT2i, time between onset of SGLT2i and diagnosis of T2DM, type of SGLT2i (dapagliflozin or empagliflozin), angiotensin converting enzyme inhibitor (ACEi) and angiotensin receptor blocker (ARB) use, laboratory results (detailed below) and were retrieved from the computerized registry of the hospital and patient charts. The information about whether the patients used calcium channel blockers, diuretics or statins at the beginning or during the study was obtained from both the hospital records and the records by the Ministry of Health of the Republic of Turkey.
Laboratory analysis
Laboratory results immediately before SGLT2i initiation (baseline), and 3 months and 6 months after SGLT2i initiation, which were measured in the routine follow-up examination of T2DM patients, including blood fasting glucose, creatinine, urea, serum sodium, potassium and calcium, HbA1c, LDL-C, high density lipoprotein cholesterol (HDL-C), triglyceride levels and urine albumin-to-creatinine ratio (ACR) were examined. All samples were taken in accordance with international standards and measurements were performed in the certified local biochemistry laboratory with calibrated devices (Roche COBAS Integra 800; Roche Diagnostics Corporation, USA) and commercial test kits, according to manufacturer recommendations.
Patients management
Diabetes diagnoses were made and therapeutic decisions (indications and doses of SGLT2i, metformin and insulin) were based on the recommendations of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) [1, 3]. The patients were divided into 2 groups as those using dapagliflozin (n = 49) and empagliflozin (n = 201) and compared in terms of changes in laboratory parameters at baseline, 3 months and 6 months. Based on concomitant therapies, patients were also divided into 4 groups: those not using any additional medication (n = 32), those using metformin (n = 184), those using metformin and basal insulin (n = 16), and those using metformin, basal insulin, and bolus insulin (n = 18) and these four groups were compared in terms of changes in laboratory parameters at baseline, 3 months, and 6 months later.
ACEi or ARB indications of the patients before and after SGLT2 initiation were determined according to the ESC and EAS guidelines [18, 19].
Statistical analysis
Statistical analyses, with significance denoted by p < 0.05 values, were conducted using IBM SPSS, Version 21.0 (IBM, NY, USA). Continuous variables were evaluated for the absence of normal distribution using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Mean ± standard deviation values were used to summarize continuous variables, while frequency (percentage) values were used for categorical variables. Repeated measurements were compared using Wilcoxon test or Friedman's test. Two-group comparisons utilized the Mann–Whitney U test, and comparisons involving more than two groups employed the Kruskal–Wallis test. Post-hoc analysis was adjusted with Bonferroni correction.
Results
The mean age of the patients was 55.4 ± 9.6, with 53.6% (n = 134) being male. The mean duration of T2DM was 9.12 ± 6.35 years, while the mean time until SGLT2i initiation after T2DM diagnosis was 7.38 ± 6.09 years. Forty-nine (19.6%) patients used dapagliflozin and 201 (80.4%) used empagliflozin. Comorbidities and other drug uses are summarized in Table 1.
Laboratory parameter changes at 3 months and 6 months compared to baseline are summarized in Table 2. Glucose, HbA1c, and triglyceride levels measured 3 and 6 months after treatment were significantly lower than baseline, while serum sodium and HDL-C levels were significantly higher (p < 0.001 for all). Levels of creatinine (p = 0.024) and serum potassium (p = 0.028) measured 6 months after treatment were significantly higher than baseline, while levels of LDL-C (p < 0.001) and urine ACR (p < 0.001) were significantly lower. Glucose, HbA1c, LDL-C, triglyceride and urinary ACR levels measured 6 months after treatment were significantly lower (p < 0.001 for all), while serum potassium levels were significantly higher (p = 0.028) compared to those 3 months after treatment. When compared based on SGLT2i type, patients using empagliflozin had significantly higher creatinine 3 months later (p = 0.048) and serum sodium 6 months later (p = 0.020), and significantly lower HbA1c levels 6 months later (p = 0.024) than dapagliflozin users (Table 3).
Patients using metformin & basal insulin with SGLT2i had significantly higher baseline glucose levels (before metformin & basal insulin treatment) than those using metformin alone with SGLT2i (before metformin treatment) (p = 0.016). Patients using metformin & basal insulin and those using metformin & basal insulin & bolus insulin with SGLT2i had significantly higher HbA1c levels at 6″ months (p = 0.003) compared to those who were only receiving metformin as concomitant medication. HbA1c levels at 6 months were significantly lower in metformin & SGLT2i users compared to those receiving only SGLT2i (p = 0.003). HbA1c values after 6 months decreased significantly in all 4 groups. Except for the Metformin + basal insulin + Bolus insulin group, there were significant decreases in glucose and LDL-C levels of the other 3 groups compared to baseline and 6 months later. A significant increase in creatinine (p = 0.023), sodium (p < 0.001) and potassium (p = 0.043) levels was seen in the metformin group after 6 months. In the only SGLT2i group, urea levels decreased significantly after 6 months (p = 0.032). There was a significant increase in HDL-C levels and a significant decrease in urinary ACR levels after 6 months in those using only SGLT2i (p = 0.043) and in those using metformin in addition to SGLT2i (p = 0.004). There was a significant decrease in triglyceride levels in the metformin (p < 0.001) and metformin + basal (p = 0.006) insulin groups after 6 months (Table 4).
Discussion
The main findings of this study demonstrate that, SGLT2i decreased glucose, HbA1c, LDL-C, triglyceride and urine ACR levels, and significantly increased creatinine, serum sodium and HDL-C levels over time. Compared to dapagliflozin, empagliflozin increased creatine at 3 months and serum sodium at 6 months more, and decreased HbA1c at 6 months more. Other diabetes treatment regimens used concomitantly with SGLT2i had no significant and reasonable differences on laboratory variables other than HbA1c.
Clinical studies have shown that SGLT2i improve both glucose and HbA1c levels in comparison to placebo and other oral antidiabetic drugs [7]. The present study did not include a placebo group or a comparison group (using only other antidiabetics), but it was observed that there was a significant decrease in glucose and HbA1c levels at 6 months after starting SGLT2i. HbA1c levels 6 months after starting empagliflozin were significantly lower compared to dapagliflozin recipients, although there was no difference at baseline. In a meta-analysis of 12 randomized controlled trials, it was demonstrated that SGLT2i use was associated with a greater reduction in HbA1c in comparison to oral antidiabetics. While there was no difference in HbA1c reduction between SGLT2i and metformin, SGLT2i have been shown to provide a greater HbA1c reduction effect compared to sulfonylurea and dipeptidyl peptidase 4 inhibitors. [7]. Although empagliflozin resulted in a significantly greater HbA1c reduction than dapagliflozin in the current study, some limitations should be taken into account, such as the relatively small sample size, retrospective design, significant number differences between groups, and the exclusion of the weight factor, which is an important factor that may affect HbA1c levels.
Diabetic nephropathy, a leading cause of end-stage renal disease, is associated with increased morbidity and mortality in diabetes and independently elevates the risk of adverse cardiac outcomes [20]. The basic pathophysiology of diabetic nephropathy includes inflammation and fibrosis caused by glomerular hyperfiltration [21, 22]. The CREDENCE (“The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation”) trial included approximately 4500 patients with T2DM and chronic renal failure with proteinuria and was the first renal outcome trial of SGLT2i. It demonstrated renoprotection (30% reduction in adverse renal outcomes) and cardiovascular protection [8]. The superiority of SGLT2i over placebo in preventing renal deterioration was also demonstrated in the EMPA-REG OUTCOME (“Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients”) study [23]. This study also demonstrated that empagliflozin reduced the risk of composite renal outcomes including progression to macroalbuminuria, doubling of serum creatinine, initiation of renal therapy, and death from kidney disease, by 39% [23]. The DECLARE-TIMI 58 (“Dapagliflozin Effect on CardiovascuLAR Events – Thrombolysis in Myocardial Infarction 58”) trial reported lower rates of renal combined outcome by treatment with dapagliflozin compared to placebo [24]. Similar results were reported for canagliflozin in the CANVAS (“Canagliflozin Cardiovascular Assessment Study”) [8, 10]. The DAPA-CKD (“The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease”) study investigating a larger population also demonstrated the renoprotective effects of dapagliflozin in chronic kidney disease patients with or without T2DM [25].
Some researchers have claimed that intensive glucose-lowering therapies can cause harm, resulting in questions surrounding the safety of SGLT2i [16, 26, 27]. Although the results of above mentioned trials do not show increased risks for acute kidney injury [8, 23, 24, 28] with SGLT2i use, there are few studies that have emphasized acute kidney injury [16], thereby demonstrating the need for further studies. Nonetheless, the long-term renoprotective effects of SGLT2i have been associated with reduced transglomerular pressure, similar to agents that block the renin–angiotensin–aldosterone axis [5, 16]. However, SGLT2i can sometimes cause a decline in kidney function and acute renal injury. In the present study, SGLT2i significantly increased serum sodium levels 3 months after baseline, and significantly decreased urinary ACR levels, as well as increased creatinine, serum sodium and serum potassium levels at 6 months relative to baseline. SGLT2i also significantly decreased urinary ACR levels and significantly increased serum potassium levels at 6 months compared to 3 months. The effect of empagliflozin on increasing creatinine at 3 months and serum sodium at 6 months was significantly higher than dapagliflozin. Based on the results of a systemic review, STLG2i were associated with a 0.60 µmol/L increase in creatinine compared to placebo [7]. In terms of creatinine change, similar results were found for SGLT2i and metformin or dipeptidyl peptidase 4 inhibitors [7]. The present study investigated the effect of SGLT2i on serum creatinine and electrolyte levels and how these effects changed over 3-month intervals, contributing to a lack of literature in this regard. We recommend checking serum creatinine, sodium and potassium levels at least every 3 months in patients using SGLT2i. It should be remembered that increases in creatinine and potassium levels may occur after 6 months of use.
Recently, the United States Food and Drug Administration reported cases of acute kidney injury in 101 patients treated with SGLT2i –all receiving dapagliflozin or canagliflozin. The fact that many of these patients required hospitalization and renal replacement therapy has raised concerns [23]. In another meta-analysis, both dapagliflozin and canagliflozin caused increased risk of compound renal events compared to the control group, whereas renoprotective outcomes were reported for empagliflozin. Both canagliflozin and dapagliflozin were associated with a tendency to increase the risk of acute renal failure compared to controls, but these results were not significant. Conversely, empagliflozin has also been associated with a significant reduction in the risk of acute renal failure [29]. Szalat et al. reported several cases of acute kidney injury that may be associated with initiation of SGLT2i therapy, including empagliflozin [16]. According to Szalat et al. [16], there were three possible causes of this damage: (i) effective volume depletion due to excessive diuresis; (ii) loss of trans-glomerular pressure in patients receiving renin–angiotensin–aldosterone blockade; (iii) renal medullary hypoxic injury. However, the role of these mechanisms has not been adequately explored due to the conflicting results of studies on acute kidney injury potentially caused by SGLT2i treatment. According to the results of the present study, the detrimental effect of SGLT2i on kidney and blood electrolytes seems to increase with time, and these effects seem to be more pronounced in empagliflozin. Also, these effects do not seem to be affected by other concomitant antidiabetic treatment regimens, duration of diabetes mellitus and SGLT2i initiation time after diagnosis. However, available data regarding the kidney safety of SGLT2i are conflicting and limited. Further information on the renal damage or protective properties of SGLT2i will be provided by clinical trials designed to evaluate outcomes in large populations.
The cardioprotective effects of SGLT2i have been demonstrated in many randomized controlled trials. In the EMPA-REG OUTCOME study of 7020 patients with T2DM and CVD, Zinman et al. showed that the composite incidence of major adverse cardiovascular events was lower in patients using empagliflozin compared to placebo [9]. The CANVAS trial compared the results of canagliflozin and placebo in 10,142 patients with T2DM and high cardiovascular risk. Patients treated with canagliflozin had a lower risk of cardiovascular events than those treated with placebo [10]. On the other hand, other meta-analyses showing no effects on cardiovascular events, death, and major safety outcomes have been published [7, 30]. These conflicting results may be due to the uncertain effects of SGLTi on lipid profile. In this study, we also investigated the temporal changes of SGLTi on lipid parameters. SGLT2i significantly decreased triglyceride levels and significantly increased HDL-C levels 3 months after baseline, and significantly decreased LDL-C and triglyceride levels and significantly increased HDL-C levels at 6 months. In a meta-analysis, SGLT2i were associated with increased HDL-C and LDL-C and decreased triglyceride compared with placebo. SGLT2i increased HDL-C and LDL-C compared to sulfonylurea treatment and dipeptidyl peptidase 4 inhibitors, but did not cause lower levels of triglycerides [7]. Other studies have also shown that SGLT2i increase HDL-C and LDL-C levels [9, 31, 32]. In a randomized placebo-controlled trial, dapagliflozin was not found to cause any alterations in lipid-related parameters, including HDL subfractions, cholesterol efflux, enzymes mediating the antioxidant role of HDL (PON1 and ARE), when compared to placebo [33]. In an experimental study using a mouse model to examine the effects of SGLT2 inhibition on plasma lipoprotein metabolism, SGLT2 inhibition was demonstrated to increase circulating LDL-C and reduce plasma triglycerides. Also, SGLT2 inhibition was shown to delay LDL turnover [15]. According to the current findings, SGLT2i seem to have increasing positive effects on lipid profile over time, and these effects seem to be independent of SGLT2i type, other medications, time with disease, and SGLT2i initiation time. Comprehensive studies are needed to demonstrate the precise effects of SGLT2i on lipid profile and to assess long-term effects.
Limitations
This was a single-center study and has relatively few participants compared to previously published comprehensive randomized controlled trials. This limits the generalizability of the results. As it is a retrospective study, the effect of SGLT2i on long-term outcomes and other laboratory parameters could not be investigated. In addition, due to the retrospective design, the weight factor, which is an important factor that may affect HbA1c levels, was not included in the study. A placebo control group or a comparison group using only other antidiabetics could not be created and included in the study. Differences in numbers between patients using dapagliflozin and empagliflozin may have affected the statistical results. The differences in these numbers were a natural consequence of following the recommendations of the ADA and the European Association for the Study of Diabetes. The sample size was not computed to compare the two types of inhibitors in the study, and the sample sizes in the two groups are unequal, caution should be exercised while drawing inferences from this comparison. Furthermore, as the study already has a retrospective nature, the probability of selection bias risk is very low. Finally, since canagliflozin was not marketed in Turkey at the time of the study, the results of this agent could not be included.
Conclusion
In conclusion, SGLT2i significantly decreased glucose and HbA1c over time, and this positive effect was more pronounced in empagliflozin. SGLT2i increased creatinine, serum sodium, and serum potassium over time and decreased urinary ACR levels, and the impact on sodium was more pronounced in empagliflozin recipients. SGLT2i significantly increased HDL-C levels, while significantly decreasing LDL-C and triglyceride levels over time. While SGLT2i seems to provide positive effects on the lipid profile in addition to its positive glycemic effects in patients with T2DM, it would be useful to review it in terms of renal safety and to perform further trials examining their effects on lipid profile. For this, more comprehensive studies are required.
References
American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13-s28.
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
Davies MJ, D’alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61(12):2461–98.
Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398(10296):262–76.
Scheen AJ. An update on the safety of SGLT2 inhibitors. Expert Opin Drug Saf. 2019;18(4):295–311.
Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. 2015;66:255–70.
Storgaard H, Gluud LL, Bennett C, Grøndahl MF, Christensen MB, Knop FK, et al. Benefits and harms of sodium-glucose co-transporter 2 inhibitors in patients with type 2 diabetes: A systematic review and meta-analysis. PLoS ONE. 2016;11(11): e0166125.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: The CVD-REAL study (Comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation. 2017;136(3):249–59.
Raschi E, Poluzzi E, Fadini GP, Marchesini G, De Ponti F. Observational research on sodium glucose co-transporter-2 inhibitors: A real breakthrough? Diabetes Obes Metab. 2018;20(12):2711–23.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S90-s102.
Bonora BM, Avogaro A, Fadini GP. Extraglycemic effects of SGLT2 inhibitors: A review of the evidence. Diabetes Metab Syndr Obes. 2020;13:161–74.
Basu D, Huggins LA, Scerbo D, Obunike J, Mullick AE, Rothenberg PL, et al. Mechanism of increased LDL (Low-Density Lipoprotein) and decreased triglycerides with SGLT2 (Sodium-Glucose Cotransporter 2) inhibition. Arterioscler Thromb Vasc Biol. 2018;38(9):2207–16.
Szalat A, Perlman A, Muszkat M, Khamaisi M, Abassi Z, Heyman SN. Can SGLT2 inhibitors cause acute renal failure? Plausible role for altered glomerular hemodynamics and medullary hypoxia. Drug Saf. 2018;41(3):239–52.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2019;41(1):111–88.
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104.
National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850–86.
Samadi A, Sabuncuoglu S, Samadi M, Isikhan SY, Chirumbolo S, Peana M, et al. A comprehensive review on oxysterols and related diseases. Curr Med Chem. 2021;28(1):110–36.
Thomas MC, Cherney DZI. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia. 2018;61(10):2098–107.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, Von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34.
Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–17.
Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(1):22–31.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
Patel A, Macmahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72.
Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845–54.
Tang H, Li D, Zhang J, Li Y, Wang T, Zhai S, et al. Sodium-glucose co-transporter-2 inhibitors and risk of adverse renal outcomes among patients with type 2 diabetes: A network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19(8):1106–15.
Wu JH, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016;4(5):411–9.
Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Broedl UC, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650–9.
Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16(2):147–58.
Fadini GP, Bonora BM, Zatti G, Vitturi N, Iori E, Marescotti MC, et al. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo-controlled trial. Cardiovasc Diabetol. 2017;16(1):42.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: Ezgi Sahin; Methodology: Ezgi Sahin; Formal analysis and investigation: Elif Ezirmik, Fatma Akyol, Bahar Guler Filiz; Writing—original draft preparation: Deniz Yilmaz; Writing—review and editing: Deniz Yilmaz; Funding acquisition: Deniz Yilmaz; Resources: Ezgi Sahin; Supervision: Deniz Yılmaz.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of Dr. Sadi Konuk Training and Research Hospital (Decision date: 21.02.2022, decision no: 2022–04-14).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yilmaz, D., Sahin, E., Akyol, F. et al. The effects of sodium-glucose cotransporters type 2 inhibitors on glycemic and extraglycemic laboratory parameters. Int J Diabetes Dev Ctries (2024). https://doi.org/10.1007/s13410-023-01307-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13410-023-01307-z