Abstract
Background
Periodontal disease (PD) is a recognized complication of diabetes and is associated with poor glycemic control. Prevalence of PD is high, both in type 2 and type 1 diabetes. However, such information is not available for FCPD, which is a unique subtype of diabetes.
Material and methods
Twenty-five subjects of FCPD were evaluated for detailed dental evaluation and compared with nondiabetic control (N = 25) and T2DM (N = 50). Baseline demographic parameters, HbA1c, were recorded, and periodontal health was evaluated by simplified oral hygiene index (OHIS), gingival index (GI), bleeding on probing (BOP), loss of attachment (LOA), and probing depth (PPD).
Results
PD was significantly higher in FCPD (17/25, 68%) as compared to control (9/25, 36%) (p = 0.04). In T2DM, 31/50 (62%) had PD (p = NS compared to FCPD). In FCPD, it was mild in 20% and moderate in 48%. The duration of diabetes and mean HbA1c was 7.9 ± 2.58 years and 8.10 ± 0.78%, respectively. The mean LOA and PPD were 1.80 ± 0.9 mm and 2.45 ± 0.69 mm, respectively, and OHIS was 2.04 ± 0.59. Bleeding on probing was found in 4 subjects (18%). All parameters in FCPD were significantly worse compared to nondiabetic controls. However, the parameters in FCPD except OHIS were not different from T2DM. There was a positive correlation of HbA1c with GI (r = 0.49, p = 0.04) and the LOA (r = 0.420, p = 0.03) but not with BOP and OHIS in FCPD group.
Conclusion
PD is common in FCPD like other forms of diabetes, and its severity correlates with glycemic control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibrocalculous pancreatic diabetes (FCPD) is a recognized cause of secondary diabetes due to disease of the exocrine pancreas [1]. In subjects with FCPD, in addition to the presence of diabetes (as defined by standard criteria), there is evidence of chronic atrophic pancreatitis with large intraductal pancreatic calculi [2]. Patients frequently have a low body mass and a history of chronic abdominal pain and steatorrhea. In most of the cases, the subjects require insulin treatment for optimal glycemic control.
Periodontal disease is regarded as one of the most common disease known to mankind [3]. This encompasses both gingivitis (e.g., inflammation of the gum tissue) and periodontitis (where inflammation of gum is accompanied by underlying tissue destruction and resorption of alveolar bone) [3]. An intrinsic link between oral health, general health, and quality of life was acknowledged by the WHO [4]. Still, oral health is a neglected area of global health [5]. The relationship between diabetes and oral health has been well documented in T2DM. Data from epidemiological studies suggest that diabetes is a major risk factor for periodontal disease, and the susceptibility is increased by approximately 3-folds in people with diabetes [6]. There is a clear-cut relationship between severity of PD and the degree of hyperglycemia. The American Diabetic Association (ADA) in 2003 also acknowledged that periodontal disease is frequently present in people with diabetes [7]. It is also regarded as the 6th major complication of diabetes [8]. Similar observations has been documented in type 1 diabetes also [9, 10]. In fact, diabetes per se has been shown to be a major risk factor for periodontitis. Conversely, periodontal disease is known to have a negative impact on glycemic control and chronic diabetic complications [3]. In addition, meta-analyses suggest that following effective periodontal therapy, there may be a reduction in HbA1c up to 0.4% [3]. However, similar type of data of periodontal health in FCPD is not currently available.
In this background, we undertook this study to find the prevalence and severity of periodontal disease in this unique subtype of diabetes and compared them with T2DM and control population of same age group and from similar socioeconomic status and evaluated whether there is any relationship of periodontal disease with glycemic control in FCPD.
Materials and methods
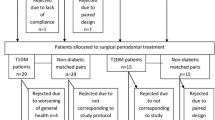
This was a cross-sectional study to compare the prevalence of periodontal disease in FCPD with T2DM and control population. Non-probability purposive sampling was done, and twenty-five consecutive subjects with FCPD attending the diabetes clinic of the institute were included. Twenty-five subjects of same age range and similar socioeconomic status (spouse or close relatives or other person) who did not have diabetes and gave informed consent were also selected as controls. As the prevalence of periodontal health status may vary with socioeconomic status, we selected the control group from the same socioeconomic status. In addition, fifty consecutive subjects with T2DM of same age range attending the diabetes clinic were also included.
The diagnosis of FCPD was done according to the following criteria [2]: (a) the presence of recurrent pain abdomen from an early age; (b) the presence of pancreatic calculi suggested on plain X-ray abdomen and subsequently confirmed by ultrasonography; (c) the absence of history of alcoholism, gallstones, or hyperparathyroidism; and (d) diabetes diagnosed by standard criteria. Twenty-five subjects of same age range and similar socioeconomic status (spouse or close relatives or other person) who did not have diabetes and gave informed consent were also selected as controls. As the prevalence of periodontal health status may vary with socioeconomic status, we selected the control group from the same socioeconomic status.
Baseline data on age, sex, body mass index, history of smoking, blood pressure, duration of diabetes, glycemic control, urine for albumin creatinine ratio, creatinine, and treatment details were recorded in all. Digital fundus photography was performed in all FCPD and T2DM subjects to assess diabetic retinopathy. In addition, vibration perception threshold was measured to screen diabetic peripheral neuropathy using a bioesthesiometer and was graded as normal (VPT < 15 mV), mild (15–25 mV), moderate (25–42 mV), and severe (> 42 mV) retinopathy. ECG also is done in all. HbA1c is measured by high-pressure liquid chromatography method using the Bio-Rad D10.
All subjects with FCPD were treated with insulin with a mean requirement of 27.4 units/day (range 16–52 units/day). Only eight patients were also on metformin, and four patients were receiving glimepiride in addition to insulin. The mean duration of diabetes in FCPD and T2DM was 7.9 ± 2.58 years (range 1–15 years) and 9.7 ± 3.59 years (range 2–19 years), respectively. However, none of FCPD subjects was on pancreatic enzyme supplement.
Periodontal assessment
The assessment was done by one trained dental examiner using a University of North Carolina-15 probe (Hu-Friedy) which was calibrated at every millimeter. The periodontal examination was based on the 6 teeth (covering all six sextants) as suggested by Ramfjord et al. [11].
The parameters recorded to evaluate the periodontal status were as follows: gingival index (GI), simplified oral hygiene index (OHIS), bleeding on probing (BOP), and loss of attachment (LOA) and pocket probing depth (PPD). These parameters were assessed on all 4 surfaces for each tooth (e.g., mesial, distal, buccal, and lingual) [12].
The presence of gingival inflammation was objectively documented as gingival index. All four-tooth surfaces were assessed for this purpose, and a score was assigned for each surface. For each surface, it was recorded as follows: scores 0: normal gingiva, 1: mild inflammation/slight change in color or edema, 2: moderate inflammation/redness and edema, and 3: inflammation with marked redness and edema with or without tendency for spontaneous bleeding [12]. The process was completed for all 6 teeth as mentioned above, and the sum of score was then divided by the total number of teeth surfaces (teeth number multiplied by 4) to get GI [12].
BOP was recorded by the gentle stimulation of the bottom of the pocket with a periodontal probe. For each tooth, BOP was scored as follows: 0 = no bleeding or 1 = the presence of bleeding until 30 s after probing the abovementioned tooth surface or pocket [13]. Loss of attachment (LOA) was recorded as the distance from the cementoenamel junction to the bottom of the periodontal pocket and severity of PD was graded as mild (1–2 mm), moderate (3–5 mm), and severe (> 5 mm) [14]. PPD was measured as the distance between the gingival margin and the lowest end of the periodontal pocket (i.e., the penetration depth of the probe) [15]. OHIS was evaluated to assess the oral hygiene, which is the sum of two other indices, i.e., calculus and debris indexes [10]. Calculus index was measured by scoring for each tooth on a scale of 0 to 3 as follows: 0 = no calculus; 1 = mild supragingival calculus extending to the marginal gingiva is present; 2 = moderate supragingival and subgingival calculus or only subgingival calculus is present; and 3 = excessive supragingival and subgingival calculus are present. Sum of the scores for each surface was divided by the total number of teeth surfaces examined to obtain the calculus index. In the same way, debris index was calculated on scoring scale as follows: 0 = no debris; 1 = soft debris over not more than one-third of tooth surface; 2 = soft debris covering more than one-third, but not more than two-thirds of the exposed tooth surface; and 3 = soft debris covering more than two-thirds of the exposed tooth surface.
PD was defined as having a gingival score of 1 or more along with a BOP index of 1 or more or OHIS of 1 or more or with a loss of attachment of 1 mm or more.
Statistical analysis
All analyses were performed by using Microsoft Excel 2007 (Microsoft Inc.) and SPSS (Version 21.0; SPSS Inc., Chicago, IL, USA). The data were tested for normality using the Kolmogorov–Smirnov test. Continuous data are presented as mean ± standard deviation, and categorical data are presented as percentage. Welch’s t-test was done to compare the continuous variables, and chi-square test was done to compare proportions. Pearson’s correlation analyses were done to find correlation between continuous variables.
Results
Twenty-five consecutive subjects with diagnosed FCPD attending the specialty clinic were included, of which 13 were males and 12 were females. In the nondiabetic control group, there were 11 males and 14 females, and in T2DM group, there were 27 males and 23 females. The mean (± SD) age of FCPD, nondiabetic control, and T2DM subjects was 33.6 ± 10.34 years (range 20–46 years) and 30.7 ± 8.13 years (range 20–43 years) and 34.3 ± 8.17 years, respectively. The mean BMI in FCPD, nondiabetic control, and T2DM group was 18.11 ± 0.97 kg/m2 versus 23.62 ± 2.11 kg/m2 versus 23.91 ± 2.97 kg/m2, respectively. The mean duration of diabetes in FCPD and T2DM was 7.9 ± 2.58 years (range 1–15 years) and 9.7 ± 3.59 years (range 2–19 years), respectively. The mean HbA1c of FCPD, nondiabetic control, and T2DM subjects was 8.10 ± 0.78% (65 mmol/mol) and 5.10 ± 0.59% (32 mmol/mol) and 7.99 ± 1.77% (64 mmol/mol), respectively. The smoking history was present in 4 subjects in FCPD and 5 subjects in the control group and 11 subjects in T2DM. Incidentally, all smokers were male in all the groups. The baseline parameters have been described in Table 1.
Among FCPD patients, 17 (68%) were diagnosed to have periodontal disease as compared to 9 (36%) subjects in the nondiabetic control group (p = 0.04). In T2DM, 31 (62%) subjects had periodontal disease (p = NS when compared to FCPD).
In FCPD group, the key findings were as follows: the mean GI, LOA, and periodontal pocket depth was 2.16 ± 0.8, 1.80 ± 0.9 mm, and 2.45 ± 0.69 mm, respectively. The measured LOA was mild in (1–3 mm) 5 (20%) subjects and moderate (4–5 mm) in 12 subjects (48%), but none of them had severe (> 5 mm) LOA. There was a positive correlation of HbA1c with GI (r = 0.49, p = 0.04) and the LOA (r = 0.420, p = 0.03). The mean OHIS was 2.04 ± 0.59. Bleeding on probing was found in 4 subjects (16%). There was no significant correlation between HbA1c and OHIS and BOP. Peripheral sensory neuropathy and diabetic retinopathy were diagnosed in 7 (28%) and 3 (12%) subjects with FCPD respectively. There was no significant association of PD with either peripheral sensory neuropathy or diabetic retinopathy in subjects with FCPD. No significant correlation was seen between the duration of diabetes and the examined parameters.
All parameters in FCPD were significantly worse compared to nondiabetic controls. However, the parameters in FCPD except OHIS were not different from T2DM. The summary of the dental parameters has been described in Table 2.
Discussion
Though periodontitis is an important complication of diabetes and it is established to have a two-way relationship with diabetes [16], neither the prevalence of PD nor its relationship with glycemic state has been studied in FCPD so far. In India, the prevalence of some form PD even in general population without diabetes is also substantial and is reported to be as high as 97% [17].
Prevalence of PD in diabetes is also alarmingly high even in the developed countries. NHANES III survey in the USA documented that adults with HbA1c above 9% had a significantly higher prevalence of severe periodontitis as compared to those without diabetes (OR 2.90; 95% CI: 1.40, 6.03) after adjustment for age, sex, smoking, ethnicity, and education [18]. Even in children with diabetes, the proportion of PD was greater than those without diabetes (> 20% vs 8%, respectively) [19]. In a recent meta-analysis of 53 observational studies, the risk of developing periodontitis was 34% higher in type 2 diabetes (p = 0.002) [20]. Malawat et al. reported that the prevalence of PD in type 2 diabetes was reported to be 61.9% [21]. Children with type 1 diabetes are also known to have increased prevalence of PD as compared to their nondiabetic peers, and puberty may have a possible role to play in this regard [10]. A study by Cianciola et al. identified that around 10% of children (< 18 years) with type 1 diabetes mellitus had increased attachment loss and bone loss compared with controls, despite comparable plaque scores [9]. In this study, we found that in FCPD also, the prevalence of PD is significantly higher as compared to the general population, and it was similar to the subjects with T2DM.
Periodontitis is also reported to be more prevalent in obese subjects. Meta-analysis also revealed a significant association between obesity and periodontal disease (OR 1.35; 95% CI 1.23–1.47) [22]. Insulin resistance is proposed to be a mediator for this. Insulin resistance is also known to be present in FCPD [23, 24]. However, in our cohort of FCPD, all subjects were lean as compared to the controls, and still they had a significantly higher prevalence of PD. This might point to the fact that association of diabetes with a high HbA1c per se is a much stronger factor leading to PD.
Emrich et al. had demonstrated a strong correlation between severity of PD with duration and severity of diabetes [25]. Subjects in this study were shown to have an increased risk of destructive periodontitis with an odds ratio of 2.81 (95% CI 1.91–4.13). This was reflected in our study with 48% of having mild LOA (1–2 mm) and 20% had moderate LOA (3–5 mm), although none had severe LOA (> 5 mm). We also found a positive correlation between the HbA1c and LOA. However, we could not find any significant association of PD with either peripheral sensory neuropathy or diabetic retinopathy in subjects with FCPD which could be due to small number of these complications.
Conclusion
We conclude that PD is also very common in FCPD like that of other types of diabetes, and its severity correlates with the glycemic control.
Limitations of the study
This was an open-label study as the subjects with FCPD were aware of their disease. In addition, as the prevalence of FCPD is very low, we used the non-probability purposive sampling. Hence, the sample size was also small. However, considering the dearth of available information on dental parameters in FCPD, this data could have significant importance about dental involvement in FCPD, and consequent attempts might be reinforced to improve dental hygiene in this subgroup which might improve diabetic control complications like that in type 2 diabetes.
References
American Diabetes Association Professional Practice Committee, American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, Green J, Huang E, Isaacs D, Kahan S, Leon J, Lyons SK, Peters AL, Prahalad P, Reusch JEB, Young-Hyman D, Das S. Kosiborod M. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Supplement_1):S17–38. https://doi.org/10.2337/dc22-S002.
Mohan V, Nagalotimath SJ, Yajnik CS, Tripathy BB. Fibrocalculous pancreatic diabetes. Diabetes Metab Rev. 1998;14:153–70.
Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, Taylor R. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55(1):21–31. https://doi.org/10.1007/s00125-011-2342-y.
WHO (2007) Oral health: action plan for promotion and integrated disease prevention (EB120/10). 120th Session, 22–30 January. WHO, Geneva. Int J Dent Hyg. 2009;7(1):71–3. https://doi.org/10.1111/j.1601-5037.2008.00325.x.
Oral health: prevention is key. Lancet. 2009;373(9657):1. https://doi.org/10.1016/S0140-6736(08)61933-9
Mealey BL, Ocampo GL. Diabetes mellitus and periodontaldisease. Periodontol. 2007;2000(44):127–53.
American Diabetes Association Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–20.
Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–34.
Cianciola LJ, Park BH, Bruck E, Mosovich L, Genco RJ. Prevalence of periodontal disease in insulin-dependent diabetes mellitus (juvenile diabetes). J Am Dent Assoc. 1982;104(5):653–60.
Chakraborty P, Mukhopadhyay P, Bhattacharjee K, Chakraborty A, Chowdhury S, Ghosh S. Periodontal disease in type 1 diabetes mellitus: influence of pubertal stage and glycemic control. EndocrPract. 2021;27(8):765–8. https://doi.org/10.1016/j.eprac.2021.01.010.
Ramfjord S. Indices for prevalence and incidence of periodontal disease. J Periodontol. 1959;30(1):51e59. https://doi.org/10.1902/jop.1959.30.1.51.
Orbak R, Simsek S, Orbak Z, Kavrut F, Colak M. The influence of type 1 diabetes mellitus on dentition and oral health in children and adolescents. Yonsei Med J. 2008;49(3):357. https://doi.org/10.3349/ymj.2008.49.3.357.
Armitage G. Clinical evaluation of periodontal diseases. Periodontol 2000. 1995;7(1):39e53. https://doi.org/10.1111/j.1600-0757.1995.tb00035.x.
Rheu GB, Ji S, Ryu JJ, Lee JB, Shin C, Lee JY, Huh JB, Shin SW. Risk assessment for clinical attachment loss of periodontal tissue in Korean adults. J Adv Prosthodont. 2011;3(1):25–32. https://doi.org/10.4047/jap.2011.3.1.25.
Yoneyama T, Okamoto H, Lindhe J, Socransky S, Haffajee A. Probing depth, attachment loss and gingival recession. Findings from a clinical examination inUshiku, Japan. J Clin Periodontol. 1988;15(9):581e591. https://doi.org/10.1111/j.1600-051x.1988.tb02133.x.
Taylor GW. Bidirectional interrelationships between diabetesand periodontal diseases: an epidemiologic perspective. Ann Periodontol. 2001;6:99–11.
Kundu D, Mehta R, Rozra S. Periodontal status of a given population of West Bengal: an epidemiological study. J Indian SocPeriodontol. 2011;15(2):126–9.
Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–92.
Lalla E, Cheng B, Lal S, et al. Diabetes mellitus promotesperiodontal destruction in children. J Clin Periodontol. 2007;34:294–8.
Wu CZ, Yuan YH, Liu HH, Li SS, Zhang BW, Chen W, An ZJ, Chen SY, Wu YZ, Han B, Li CJ, Li LJ. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20(1):204. https://doi.org/10.1186/s12903-020-01180-w.
Malawat A, Chakraborty P, Mukhopadhyay P, Bhattacharjee K, Chakraborty A, Ghosh S. Prevalence of periodontal diseases in type 2 diabetes. J Ind Med Assoc. 2019;117(11):30-2&35.
Al-Zahrani MS, Bissada NF, Borawskit EA. Obesityand periodontal disease in young, middle-aged, and older adults. J Periodontol. 2003;74:610–5.
Mohan V, Ramachandran A, Vijay Kumar G, Snehalatha C, Viswanathan M. Insulin resistance in fibrocalculous (tropical) pancreatic diabetes. Horm Metab Res. 1988;20(12):746–8. https://doi.org/10.1055/s-2007-1010937.
Singla MK, Mukhopadhyay P, Pandit K, Chowdhury S. A clinical profile of fibrocalculous pancreatic diabetes patients from eastern India with special reference to body fat percentage and insulin resistance. J Indian Med Assoc. 2009;107(11):762–4.
Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol. 1991;62:123–31. https://doi.org/10.1902/jop.1991.62.2.123.
Funding
RSSDI, West Bengal Chapter, Grant/Award Number: RSSDI/ WB//2016/11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by the Ethics Committee of Institute of Post Graduate Medical Education and Research, Kolkata, India, and written informed consent was obtained from each subject.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Periodontal disease (PD) is a recognised complication of diabetes and is associated with poor glycemic control.
• Prevalence of PD is high, both in type 2 and type 1 diabetes.
• Prevalence of PD in fibrocalculous pancreatic diabetes (FCPD) is unknown.
• In this cross-sectional study, we demonstrated that PD is common in FCPD, and its severity correlates with glycemic control.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
M., B.A., Chakraborty, P., Mukhopadhyay, P. et al. Periodontal disease in fibrocalculous pancreatic diabetes (FCPD): common complication of an uncommon disease. Int J Diabetes Dev Ctries 43, 709–714 (2023). https://doi.org/10.1007/s13410-022-01148-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-022-01148-2