Abstract
Background
Insulin degludec/insulin aspart (IDegAsp) is the first dual combination that acts separately (basal and prandial actions). Different insulin regimens can be switched to IDegAsp. Several studies have been conducted evaluating the effectiveness and safety of IDegAsp. The purpose of this real-life study was to determine the effects of IDegAsp on metabolic control and hypoglycemia in patients who were switched from different insulin regimens to IDegAsp therapy.
Methods
We retrospectively analysed the data of 401 patients. Comorbid conditions, metabolic parameters, total insulin dose, injection frequency and hypoglycemia events were recorded. Baseline data were compared with post-IDegAsp data.
Results
Of the 401 patients, 208 were females and 193 were males, with a mean age of 64.8 ± 10.5 years. The number of injections per day and total insulin doses decreased significantly (p < 0.001) at follow-up. Furthermore, a significant improvement in fasting blood glucose and HbA1c was observed 3, 6, 12 and 24 months after IDegAsp. The frequency of minor hypoglycemia decreased after switching to IDegAsp.
Conclusions
IDegAsp reduces the frequency of hypoglycemia with fewer injections and less insulin dose and helps provide metabolic control safely.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) is increasing, and T2DM is now a worldwide public health problem. Moreover, the prevalence of T2DM in Turkey increased to 16.5% in 2010 [1].
Insulin resistance and pancreatic beta-cell dysfunction are involved in the pathogenesis of T2DM. As the duration of diabetes increases, progressive beta-cell loss and insulin deficiency develop in patients [2]. Treatment is usually started with basal insulin, and these patients may need to intensify the treatment with multiple doses over time. The basal-bolus (intensive) insulin regimen helps regulate postprandial and fasting blood glucose levels, and its disadvantage is the risk of multiple injections and hypoglycemia [3, 4]. Multiple injections make it difficult for the patient to adapt. Although the number of injections is less in premixed biphasic insulins, the risk of both nocturnal and daytime hypoglycemia remains [5]. Another side effect of insulin therapy is weight gain.
Insulin degludec/insulin aspart (IDegAsp) is a newly developed dual formulation that contains the ultra-long-acting insulin analogue ‘insulin degludec’ (70%) and the fast-acting insulin analogue ‘insulin aspart’ (30%) in a single injection pen. Insulin degludec meets the basal insulin requirement, while insulin aspart meets premeal insulin [6].
The IDegAsp treatment has been used in our country since September 2017. Until now, few real-life data have been published. Improvements in glycemic control and a decrease in the frequency of hypoglycemic events were observed in these real-life studies [7,8,9].
The aim of this study was to investigate the changes in metabolic parameters, weight and hypoglycemic events of patients after switching to IDegAsp treatment.
Materials and methods
Data were collected from the medical records of T2DM patients who visited the Dokuz Eylül University Faculty of Medicine Endocrinology outpatient clinics between September 2017 and September 2021. The Ethical Committee of the Dokuz Eylül University Faculty of Medicine approved the protocol for this study (2021/14–51/06.05.2021). The medical records of patients who were switched to IDegAsp from various insulin regimens (i.e. basal insulin, intensive insulin and premixed insulin) were evaluated. Patients under basal insulin therapy were using long-acting insulin (i.e. insulin glargine/insulin detemir) once a day. Patients under premixed insulin therapy were using biphasic insulin aspart or biphasic insulin lispro twice daily. Patients under intensive insulin therapy were receiving bolus insulin (i.e. glulisine, lispro or aspart) thrice a day before meals and basal insulin once or twice a day. Those with type 1 diabetes mellitus were excluded from this study. Of the 528 patients examined, 401 patients were eligible for the study, and 127 patients were excluded due to missing data. The reasons for the switch to IDegAsp treatment were frequent hypoglycemic episodes and poor metabolic control. Furthermore, patients who did not comply with multiple daily injections were switched. All oral antidiabetic medications for the patients continued in line with the Turkish Endocrine Metabolism Society guidelines [10].
Data regarding demographic characteristics, comorbidities, duration of diabetes, prior treatments, daily insulin dose, the number of daily injections, laboratory data and the frequency of hypoglycemia were recorded. The baseline data were compared to those obtained 3, 6, 12 and 24 months after IDegAsp.
The hypoglycemia was identified as blood glucose level ≤ 70 mg/dL and/or hypoglycemia symptoms according to the American Diabetes Association guidelines. We defined hypoglycemia as ‘mild/minor’ if the patient could treat the hypoglycemic episodes unassisted or ‘severe/major’ if patients needed help or medical intervention from others.
Statistical analysis
In the evaluation of the data, descriptive statistics, frequencies, percentages, means and standard deviations of the patients were calculated. The Shapiro–Wilk and Kolmogorov–Smirnov normality tests were used to determine whether the variables had a normal distribution. The Wilcoxon signed-rank test and paired t test were used to determine the differences between the variables indicated by the measurement according to the normal distribution of the data. The chi-square test and Fisher’s exact test were used to analyse categorical variables. Statistical Package for the Social Sciences (version 24.0) was used in all data analyses. p values of < 0.05 were used to denote statistical significance.
Results
Baseline characteristics
In this study, a total of 401 patients with T2DM, including 208 females and 193 males, with a mean age of 64.8 ± 10.5 years, were enrolled. Of the 401 patients, 54.4% aged > 65 years. The average duration of T2DM was 17.3 years. The three most common comorbidities were hypertension (57.9%), hyperlipidemia (28.4%) and coronary artery disease (22.4%). All patients were using insulin at least once a day, regardless of their oral antidiabetics. The distribution of previous treatments of the patients was as follows: 44.8% were using basal insulin with an oral antidiabetic drug (OAD), 37.4% were using intensive insulin with an OAD, and 17.4% were using premixed insulin with an OAD. The demographic characteristics and baseline treatments of the patients are summarised in Table 1.
The duration of IDegAsp treatment was 25.2 ± 12.4 months. IDegAsp was started once daily in 76.5% of those who previously received basal insulin and twice a day in 23.5%; it was started twice a day in 76.5% of those who received premix insulin and once a day in 23.5%; it was started twice a day in 65.9% of those who received intensive insulin and once a day in 34.1%. In the follow-up, IDegAsp was discontinued in 24 patients (5%). Considering the reasons for the discontinuation of treatment, 10 patients could not comply with the treatment, seven patients had frequent hypoglycemia, three patients had uncontrolled hyperglycemia, and one patient was pregnant. Mortality was observed in three patients during follow-up. Of the three deceased patients, two died of cardiac causes. The data of the other patient could not be accessed.
Metabolic control
FPG and HbA1c values before IDegAsp treatment showed significant improvements 3, 6, 12 and 24 months after the initiation of IDegAsp treatment (initiation vs. 3rd month, p < 0.001; initiation vs. 6th month, p < 0.001; initiation vs. 12th and 24th months, p < 0.001) (Fig. 1a). According to age groups, a significant decrease in HbA1c values was observed in patients < 65 and > 65 years of age. The levels of low-density lipoprotein (LDL), triglycerides (TRG) and microalbumin–creatinine (MAU/Cre) ratio after IDegAsp treatment were compared with the baseline values, and no significant changes were observed (p > 0.05). All changes in the laboratory data of the patients are presented in Table 2. The average body mass index of the patients was 30.13 ± 6.04 kg/m2. After switching to IDegAsp, the average body mass index was 30.36 ± 9.59 kg/m2. No significant weight gain was observed after switching to IDegAsp therapy.
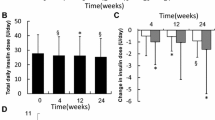
Changes in HbA1c, daily insulin dose, number of daily injections and number of hypoglycemic events. a Change in HbA1c values after treatment switch. HbA1c values before IDegAsp treatment showed significant improvements 3, 12 and 24 months after the initiation of IDegAsp treatment (initiation vs. 3rd month, p < 0.001; initiation vs. 12th and 24th months, p < 0.001). b Pre-switch daily insulin dose (unit/day) decreased significantly (p < 0.001). c The pre-switch number of daily injections decreased significantly (p < 0.001). d Number of minor hypoglycemic events per month showed significant improvement (p = 0.001)
When we consider patients with coronary artery disease (n = 90), HbA1c values before IDegAsp treatment showed significant improvements 3, 6, 12 and 24 months after the initiation of IDegAsp treatment (initiation vs. 3rd month, p = 0.001; initiation vs. 6th month, p < 0.001; initiation vs. 12th month, p = 0.047; and initiation vs. 24th month, p = 0.020).
Sub-analysis based on the baseline regimen (basal/premixed/intensive) revealed that in the first 3 months, patients who were switched from intensive treatment to IDegAsp had the most significant decrease in HbA1c values. However, the previous treatments of the patients had no significant impact on HbA1c values in the following months.
Insulin dose, number of injections and number of OADs
IDegAsp was started as a single injection in 51.7% of the patients and two injections in 48.3%. In 90 patients, one or two bolus injections were added to IDegAsp per day according to their needs. The oral antidiabetics taken by the patients were not changed at the beginning; however, the number of OADs decreased significantly in the follow-up (p < 0.001). The mean number of daily injections decreased from 2.2 ± 1.3 to 1.81 ± 0.7 (p < 0.001). The mean total daily insulin dose decreased from 38.2 ± 28.6 to 33.5 ± 20.1 units/day (p < 0.001). Moreover, the total daily insulin dose reduced by approximately 12%. The mean total daily insulin dose and the number of daily injections were statistically different from the baseline (Fig. 1b and c). Additionally, the insulin dose per body weight before the initiation of IDegAsp treatment significantly decreased after the therapy switch. All changes in the treatments of the patients are summarised in Table 3.
Hypoglycemia
The frequency of mild hypoglycemic events decreased from 1.19 ± 3.1/month to 0.82 ± 1.97/month (p = 0.001) after the initiation of IDegAsp treatment (Fig. 1d). A non-statistically significant decline in the frequency of serious hypoglycemic events was also observed (from 0.12 ± 0.7/month to 0.07 ± 0.4/month).
In patients with established cardiovascular diseases (n = 90), the improvement of minor hypoglycemic episodes was significant (the number of hypoglycemic events decreased from 0.96 ± 1.70/month to 0.70 ± 1.50/ month (p = 0.01)).
Discussion
IDegAsp has been on the market in our country for over 4 years (since September 2017). In this retrospective study, we analysed patients, in a real-life setting, whose treatment had been switched from different insulin regimens (i.e. basal insulin, premixed insulin or basal-bolus insulin) to IDegAsp therapy in the last 4 years. The diabetes duration of our patient group was quite long (average 17 years), and their basal metabolic status was poor. A significant improvement was found in HbA1c values, daily insulin dose, the number of daily injections and the frequency of hypoglycemic events (Fig. 1a–d). Additionally, the number of OADs used by the patients decreased significantly (Fig. 2a and b).
When the studies regarding IDegAsp were reviewed, some real-life studies involved fewer patients and had shorter study duration [7,8,9, 11]. This study has the advantages of much more patients and a more extended follow-up period (24 months) than these studies. In studies conducted so far, a significant improvement in HbA1c values was observed when the baseline data were compared with the 3rd and 6th month data [7,8,9]. We also obtained the 12th and 24th month results. Although a significant decrease was observed from the baseline to all time points, the most significant decrease was observed in the first 3 months. Afterwards, we observed that HbA1c remained relatively constant until the 24th month.
Our results indicated that the number of hypoglycemic events, the number of daily injections and the total daily insulin dose decreased significantly after therapy switch, as shown in previous studies [7, 9]. Reducing the number of daily injections is an essential advantage in patients with low adherence to treatment. In a study, new OADs were added to the initial therapy in 28 patients [8]. In contrast, the number of OADs decreased in our patients. The decrease in the number of OADs was also an indicator of the improvement in glycemic control. Additionally, the decrease in the number of OADs increases the quality of life of patients and ensures their compliance with treatment. Furthermore, treatment costs are reduced.
As the first real-life study worldwide, when we reviewed the 52-week, 48-patient study conducted in India, an improvement in HbA1c values and a decrease in the daily insulin dose were observed. However, weight gain was detected in patients compared with baseline [12]. In our larger patient population, we did not observe weight gain, a feared side effect of insulin therapy. Patients remained neutral in terms of weight changes. In a retrospective database study conducted in Japan, the medical records of 10,798 patients who were previously on insulin therapy were reviewed. Switching to IDegAsp was associated with a significant improvement in glycemic control and a reduction in hypoglycemic events in elderly patients [13]. The results of this database study are consistent with our findings.
In this study, the mean age of the patients was 64.8 ± 10.5 years; therefore, the elderly patient population had long diabetes duration and multiple comorbidities. With treatment switch, glycemic control was better, and a significant decrease in the frequency of hypoglycemic episodes was observed. Hypoglycemia increases morbidity and mortality in elderly patients with multiple comorbidities.
Another strong aspect of this study is that in the patient subgroup with coronary artery diseases, an effective HbA1c reduction was achieved, and the frequency of mild hypoglycemic events was reduced. When previous real-life studies were reviewed, our study is the first to demonstrate the effectiveness and safety of IDegAsp in patients with coronary artery diseases in a real-life setting.
In this study, we did not compare the effect of one or two injections of the IDegAsp regimen on metabolic control and hypoglycemia because bolus insulin was added to some patients during the follow-up process as needed and some OADs were discontinued. Therefore, the interpretation of the data may not reflect reality.
There were some limitations in this study due to the retrospective design. Since the data were accessed through the hospital registry system, body mass index information and hypoglycemia records of some patients could not be accessed. Furthermore, since the last 2 years of the study passed during the pandemic period, many patients with diabetes delayed their regular control due to restrictions. Therefore, laboratory data of some patients were also missing in the statistical analysis.
Finally, we found that variables such as age, sex, diabetes duration, and previous insulin regimen did not affect HbA1c reduction. The decrease in HbA1c levels was independent of these factors.
IDegAsp reduces the frequency of hypoglycemic events with fewer injections and less insulin dose and helps provide glycemic control safely in elderly patients. Reducing the number of injections may increase treatment compliance, particularly in patients with poor metabolic control, advanced age and multiple comorbidities.
Data availability
The datasets used and/or analysed in this study are available upon reasonable request from the corresponding author.
References
Satman I, Omer B, Tutuncu Y, Kalaca S, Gedik S, Dinccag N, Karsidag K, Genc S, Telci A, Canbaz B, Turker F. Twelve-year trends in the prevalence and risk factors of diabetes and prediabetes in Turkish adults. Eur J Epidemiol. 2013;28:169–80. https://doi.org/10.1007/s10654-013-9771-5.
Jang HN, Yang YS, Lee SO, Oh TJ, Koo BK, Jung HS. Favorable glycemic control with once-daily insulin degludec/insulin aspart after changing from basal insulin in adults with type 2 diabetes. Endocrinol Metab (Seoul). 2019;34:382–9. https://doi.org/10.3803/EnM.2019.34.4.382.
Lamos EM, Younk LM, Tate DB, Davis SN. Pharmacokinetics and pharmacodynamics of insulin glargine-insulin glulisine basal-bolus and twice-daily premixed analog insulin in type 1 diabetes mellitus patients during three standardized meals. J Clin Transl Endocrinol. 2016;3:14–20. https://doi.org/10.1016/j.jcte.2015.12.002.
Sarbacker GB, Urteaga EM. Adherence to insulin therapy. Diabetes Spectr. 2016;29:166–70. https://doi.org/10.2337/diaspect.29.3.166.
Anyanwagu U, Mamza J, Gordon J, Donnelly R, Idris I. Premixed vs basal-bolus insulin regimen in type 2 diabetes: comparison of clinical outcomes from randomized controlled trials and real-world data. Diabet Med. 2017;34:1728–36. https://doi.org/10.1111/dme.13518.
Haluzík M, Fulcher G, Pieber TR, Bardtrum L, Tutkunkardas D, Rodbard HW. The co-formulation of insulin degludec and insulin aspart lowers fasting plasma glucose and rates of confirmed and nocturnal hypoglycemia, independent of baseline glycated haemoglobin levels, disease duration or body mass index: a pooled meta-analysis of phase III studies in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:1585–92. https://doi.org/10.1111/dom.13261.
Haliloglu O, Korkmaz M, Korkmaz OP, Sahin S, Durcan E, Siva ZO. Retrospective evaluation of insulin degludec/insulin aspart co-formulation therapy in patients with type 2 diabetes mellitus: a single-center experience. Ann Med Res. 2020;27:1961–5.
Onder C, Kuşkonmaz S, Koc G, Firat S, Omma T, Taskaldiran I, Gokbulut P, Culha C. Factors that affect the glycemic control achieved by switching to insulin degludec/aspart in insulin-treated patients with type 1 and type 2 diabetes in a real-world setting: a non-interventional, retrospective cohort study. Acta Endocrinol (Bucharest). 2020;16:443–8.
Özçelik S, Çelik M, Vural A, Aydın B, Özçelik M, Gozu H. Outcomes of transition from premixed and intensive insulin therapies to insulin aspart/degludec co-formulation in type 2 diabetes mellitus: a real-world experience. Arch Med Sci. 2021;17:1–8. https://doi.org/10.5114/aoms.2020.93264.
Salman S, Yılmaz C, İmamoğlu Ş, et al. Diagnosis, treatment and follow-up guide of diabetes mellitus and its complications 2020. 14 ed. 2020. pp. 81–129. https://temd.org.tr/yayinlar/kilavuzlar. Accessed 5 Jan 2022.
Kisioglu SV, Demir AS, Tufekci D, Emur Gunay Y, Coskun H, Ucuncu O, Nuhoglu I, Kocak M, Karakullukcu S, Ersoz HO. Clinical research of insulin glargine U300 basal-bolus therapy and insulin degludec/aspart co-formulation in type 2 diabetes mellitus: a real world experience. Int J Clin Pract. 2021;75: e14377. https://doi.org/10.1111/ijcp.14377.
Kalra S, Baruah MP. Insulin degludec aspart: one-year real world experience. Indian J Endocrinol Metab. 2016;20:369–71. https://doi.org/10.4103/2230-8210.177416.
Kaneko S, da Rocha Fernandes JD, Yamamoto Y, Langer J, Faurby M. A Japanese study assessing glycemic control with use of IDegAsp co-formulation in patients with type 2 diabetes in clinical practice: the JAGUAR study. Adv Ther. 2021;38:1638–49. https://doi.org/10.1007/s12325-021-01623-y.
Acknowledgments
Preparation for publication of this article is supported by the Society of Endocrinology and Metabolism of Turkey.
Author information
Authors and Affiliations
Contributions
GGS, IS, SY, AC and TD, conceptualisation, methodology, software, writing—original draft preparation, writing—reviewing and editing, and critical review. MEA, statistical analysis, conceptualisation, methodology, software, writing—original draft preparation, writing—reviewing and editing, and critical review. The final manuscript was reviewed and approved by all authors.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval of the study was obtained from the Ethics Committee of Dokuz Eylul University Faculty of Medicine (2021/14–51/06.05.2021).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Semiz, G.G., Selimoğlu, İ., Arayici, M.E. et al. Evaluation of the efficiency of insulin degludec/insulin aspart therapy in controlling hyperglycemia and hypoglycemia in patients with type 2 diabetes mellitus: a real-life experience. Int J Diabetes Dev Ctries 43, 544–550 (2023). https://doi.org/10.1007/s13410-022-01140-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-022-01140-w