Abstract
Background
Elevated serum uric acid (SUA) is increasingly recognized as a risk factor for diabetic kidney disease (DKD). However, the roles of SUA in the declined renal function of non-albuminuric DKD, the prevailing phenotype, are unclear.
Methods
A total of 5285 Chinese inpatients with type 2 diabetes were enrolled in this study. Based on albuminuria and reduced estimated glomerular filtration rate (eGFR), the participants were classified into four DKD phenotypes to assess and compare the influence of SUA levels on renal function. In non-albuminuric DKD, exploratory factor analysis of SUA and other metabolism parameters was performed, and linear regression was used to evaluate the associations between eGFR and SUA, individually and in combination with other covariates.
Results
In non-albuminuric DKD, SUA explained 16.0% (β = − 0.443; p < 0.0001) of the eGFR variance, which was significantly higher than in the other three DKD phenotypes. The values were 3.1%, 6.1%, and 4.6% in no-DKD, albuminuric DKD with preserved eGFR, and albuminuric DKD with reduced eGFR, respectively. In non-albuminuric DKD, SUA was independently and most strongly associated with eGFR (R2 = 18.2%; β = − 0.426; p < 0.0001), followed by triglyceride (R2 = 1.4%). In the combination of metabolism parameters, SUA was most strongly associated with eGFR (R2 = 19.3%; β = − 0.442; p < 0.0001). Analysis adjusted for covariates provided similar results, and SUA remained most strongly associated with eGFR (R2 = 16.3%; β = − 0.425; p < 0.0001).
Conclusions
The management of hyperuricemia may become an important strategy to safeguard renal function in the patients with DKD, especially in non-albuminuric DKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic kidney disease (DKD) is the leading cause of end-stage renal disease (ESRD) worldwide [1], and non-albuminuric renal impairment has been demonstrated to be the prevailing DKD phenotype in type 1 and type 2 diabetes individuals [2,3,4,5,6]. Non-albuminuric rather than albuminuric nephropathies are responsible for the largest share of ESRD burden worldwide [6, 7]. Moreover, despite improvement in glycemic and blood pressure control and the use of renin-angiotensin system blocking (RASB) drugs, the number of persons who develop diabetes-related ESRD is steadily increasing each year [8,9,10], in parallel with the worldwide epidemic of diabetes [11]. Patients with non-albuminuric DKD were reported to have better controlled glucose level, blood pressure, and lipid profiles, compared with albuminuric DKD [12]. Beside, typical glomerular changes were mainly observed in patients with elevated albuminuria [13]. In non-albuminuric DKD patients, renal biopsy findings indicated that predominant interstitial and vascular changes were more frequent, which likely reflect greater contributions from aging, hypertension, and arteriosclerosis [13, 14]. Above all, it is fair to speculate that there are different clinical characteristics and pathophysiologic feature between non-albuminuric and albuminuric nephropathies. While non-albuminuric renal impairment is the prevailing DKD phenotype, risk factors for this phenotype of DKD in type 2 diabetes are a research hotspot which might be one of the keys to reducing the prevalence of DKD [6].
Recently, the incidence of hyperuricemia in China has risen from 1.4% in the early 1980s to 10% in the early twenty-first century [15]. Hyperuricemia is currently considered as an independent risk factor for the occurrence and development of DKD in type 2 diabetic individuals [16, 17]. But at present, there is lack of investigation on the association between non-albuminuric DKD and SUA. A study of 1052 cases revealed that SUA may play an important role in the decrease of eGFR in diabetic patients with normoalbuminuria [18]. Further studies should be conducted on role of SUA in non-albuminuric DKD.
As we all know, SUA level is associated with not only metabolic syndrome [19], but also arteriosclerosis and its risk factors [15], which include high blood pressure, diabetes, dyslipidemia, smoking, and obesity. These metabolism parameters, gender, and age are all involved in the deterioration of renal function [20]. Although individual risk factors can cause renal function decline, whether the contribution of each risk in DKD phenotypes is different and whether risk increases if factors overlap, even if each factor is low?
This cross-sectional study in type 2 diabetes patients aimed to identify the metabolism parameters, which individually and in combination are most strongly associated with estimated glomerular filtration rate (eGFR) decline in non-albuminuric DKD. First, we discussed the different contributions of SUA to renal function decline in each phenotype of DKD. Second, we examined if and how individual metabolism parameters are associated and cluster together, in order to discern whether the various metabolism risk factors represent different underlying metabolism characteristics. Finally, we examined if SUA or other metabolism factors explain most of the variance in eGFR, individually and in concert, in non-albuminuric DKD.
Materials and methods
Study design and participants
A retrospective cross-sectional study was designed and conducted in type 2 diabetic patients who were hospitalized at the Zhongda Hospital affiliated to Southeast University between July 2013 and December 2018. For patients who met all selection criteria and had multiple hospitalization records, only the first hospitalization record was entered.
The inclusion criteria were as follows: (1) type 2 diabetic patients meeting the 1999 World Health Organization’s diagnostic criteria for diabetes and (2) have at least one previous inpatient medical record for diabetes.
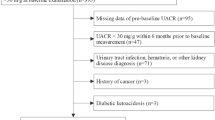
The exclusion criteria were (1) patients with type 1 diabetes mellitus or other special types of diabetes; (2) patients who had been re-hospitalized; (3) patients with missing data on all key variables; (4) patients with other types of nephropathy such as primary nephrotic syndrome and hypertensive nephropathy and patients with acute kidney injury at admission; (5) patients less than 20 years of age; and (6) pregnant women. A total of 5285 patients were finally enrolled in this study (Supplementary Fig. 1).
Outcome definition
Diagnosis codes from the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, were used to extract cases of type 2 diabetes mellitus, glomerulonephritis, and other associated diagnoses.
DKD was defined as albuminuria, reduced eGFR, or both. The eGFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI). The albumin-to-creatinine (ACR) values of < 30 and ≥ 30 mg/g SCr were considered as normoalbuminuria and albuminuria, respectively. On the basis of albuminuria (ACR < 30 or ≥ 30 mg/g SCr) and eGFR (≥ 60 or < 60 ml/min/1.73m2), individuals were classified into the following four DKD phenotypes: no-DKD, albuminuria alone (albuminuric DKD with preserved eGFR), reduced eGFR alone (non-albuminuric DKD), or albuminuria and reduced eGFR (albuminuric DKD with reduced eGFR).
Measurements
The medical records of all patients were collected, including demographic variables, such as gender, age, ethnicity, height, weight, drinking history, and smoking history, as well as medication information. Body mass index (BMI) was calculated as the body weight divided by the square of the height (kg/m2). Blood samples, collected in the clinic from the subjects the next morning after their admission to the hospital, were used to determine fasting blood glucose (FBG), glycated hemoglobin A1c (HbA1c), triglyceride (TG), total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), serum creatinine (SCr), and SUA. The measurement of urine ACR was performed on spot urine samples. Internal and external quality control of the Laboratory Center of the Affiliated Zhongda Hospital of Southeast University was used in accordance with the Chinese Laboratory Quality Control.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Science (SPSS) version 25.0. Missing data were not imputed.
First, the results are presented as mean (standard deviation) for numerical variables with Gaussian distribution and median (interquartile range) for numerical variables with non-parametric distribution, respectively, as percentage for nominal variables. Comparisons of continuous variables among the four DKD phenotypes were performed by one-way ANOVA. Comparisons between frequencies in the study groups were made by X2 tests.
Second, the linear regression models of four DKD phenotypes were conducted, respectively, to investigate the relationships between eGFR and SUA, when all other metabolism parameters and confounding or potential covariates were controlled. Results were reported in standardized β coefficients with 95% CIs, p value, and explained variance (R2 [%]; [explained variance/total variance] × 100), for comparing SUA among the four phenotypes.
Third, in the non-albuminuric DKD group, we performed an exploratory factor analysis to evaluate the association between the different individual metabolism parameters and potential underlying metabolism characteristics (latent variables or constructs; explanatory metabolism variables that are not directly observable). Factor matrixes were extracted using the maximum likelihood method and varimax orthogonal rotations with Kaiser normalization. Scree plot analysis (cutoff of 0.85) was used to determine the appropriate number of factors to retain. The underlying metabolism characteristics were derived from the rotated factor matrix; a factor-loading cutoff of 0.4 was used to discern factor characteristics. Factor analysis was acceptable in this dataset, as indicated by the Kaiser–Meyer–Olkin measure of sampling adequacy (0.474) and Bartlett test of sphericity (X2 = 356.899; df 15; p < 0.001).
Finally, in order to assess the metabolism parameters that were most strongly associated with eGFR, we performed linear regression analyses. Results were reported in standardized β coefficients with 95% CIs, p value, and explained variance (R2 [%]; [explained variance/total variance] × 100), for comparability of model parameters among the individual metabolism factors. Backward stepwise regression analysis was performed in order to assess the combined metabolism factors that were most strongly associated with eGFR. The backward elimination approach involves starting with all candidate metabolism factors, deleting the parameter for which loss gives the least deterioration of the model fit, and repeating the process until no further metabolism parameters can be deleted without a significant loss of fit (i.e., defined as p < 0.1 for the individual metabolism factors). Thereafter, we assessed the effects of confounding and potential explanatory covariates (Model 2).
Results
Characteristics of the participants with type 2 diabetes
In this study, the prevalence of DKD phenotypes was 57.0% for no-DKD, 21.9% for albuminuric DKD with preserved eGFR, 6.6% for non-albuminuric DKD, and 14.4% for albuminuric DKD with reduced eGFR (Table 1).
Among the four DKD phenotypes, patients with non-albuminuric DKD were more frequently never smokers, older, and had lower levels of TG and higher prevalence of CVD than patients with no-DKD and albuminuric DKD phenotypes. In addition, patients with non-albuminuric DKD were more frequently female and had longer duration of diabetes and lower levels of HbA1c, HDL, and LDL but higher level of SUA than those with no-DKD or albuminuric DKD with preserved eGFR but similar to those with albuminuric DKD with reduced eGFR (Table 1).
Relationship between eGFR and SUA in four DKD phenotypes
A strong negative association was established between SUA and eGFR in all DKD phenotypes. In the analysis adjusted by variables used for Model 3, SUA explained 16.0% (β = − 0.443, p < 0.0001) of the eGFR variance in non-albuminuric DKD, while only 3.1% (β = − 0.203, p < 0.0001), 6.1% (β = − 0.298, p < 0.0001), and 4.6% (β = − 0.239, p < 0.0001) were explained in no-DKD, albuminuric DKD with preserved eGFR, and albuminuric DKD with reduced eGFR, respectively. Therefore, the SUA level was most strongly associated with eGFR in the non-albuminuric DKD group (Table 2).
SUA and risk of non-albuminuric DKD
Distinct characteristics of metabolism parameters
Associations among individual metabolism parameters ranged from r = 0.002 (p = 0.970) to r = 0.719 (p < 0.001) (Supplementary Table 1). Factor analysis suggested four underlying metabolic characteristics: factor 1 linking the glucose concentrations (FBG, HbA1c), factor 2 linking the lipid concentrations (TG, CHOL), factor 3 linking lipid ratio (HDL-C/LDL-C), and factor 4 linking SUA concentration (Table 3). These results suggested that SUA is an independent metabolism characteristic.
SUA was proven to be more strongly associated with eGFR than other metabolism parameters in non-albuminuric DKD
SUA and TG were significantly associated with eGFR, while CHOL, FBG, HbA1c, and HDL/LDL were not (Table 4). In unadjusted analysis of individual metabolism factors that were modeled separately, SUA explained most of the variance in eGFR (β = − 0.426; p < 0.0001; R2 = 18.2%) (Table 4), followed by TG (β = − 0.118; p = 0.028; R2 = 1.4%) (Table 4).
Stepwise regression, in which all metabolism parameters were entered in a single model, suggested that SUA was most strongly associated with eGFR (β = − 0.442, p < 0.0001), and SUA explained 19.3% of the variance in eGFR (Table 5).
The effects of confounding and potential explanatory factors in non-albuminuric DKD
The following variables were identified as confounding or potential explanatory factors: age, gender, BMI, diabetes duration, hypertension, smoking, drinking, antiplatelet use, statins use, insulin use, metformin use, ACE (angiotensin-converting enzyme), ARBs (angiotensin receptor blockers inhibitors), other antihypertensive drugs, and urate-lowering drugs. When we adjusted for these covariates, associations between individual metabolism parameters and eGFR weakened slightly, suggesting that SUA, as the metabolism parameter, primarily accounted for the variance in eGFR (Model 2, β = − 0.420, p < 0.0001, R2 = 16.4%; Model 3, β = − 0.394, p < 0.0001, R2 = 18.0%) (Table 4).
Stepwise regression analysis, in which all metabolism parameters and confounding or potential covariates were entered in a single model, identified antiplatelet drugs and other antihypertensive drugs as covariates that were associated with eGFR (β = − 0.196, p = 0.027; β = − 0.183, p = 0.039, respectively) and explained 5.5% of the variance in eGFR. SUA was most strongly associated with eGFR (β = − 0.425, p < 0.0001) and explained 16.3% of the variance in eGFR (Table 5). Obviously, variability of SUA remained more strongly associated with eGFR than other metabolism parameters in non-albuminuric DKD when these covariates were adjusted.
Discussion
Non-albuminuric DKD has become the prevailing DKD phenotype [8, 10]. In this study, non-albuminuric DKD accounted for 6.6% of all the type 2 diabetes cases, which was comparable to other Chinese studies of inpatients [21]. Similar to clinical features in previous studies [22], patients with this phenotype of DKD were older and predominantly women.
Moreover, hyperuricemia was associated with reduced eGFR in all DKD phenotypes. While in non-albuminuric DKD, SUA explained 14.7–18.2% of the variance of eGFR in unadjusted or adjusted models, which was stronger than other groups. We also investigated which metabolism parameters were most strongly associated with eGFR in patients with non-albuminuric DKD of type 2 diabetes. Metabolism parameters included HbA1c, FBG, CHOL, TG, HDL-C/LDL-C, and SUA. Four underlying metabolism factors were identified: glucose concentration, lipid concentration, lipid ratio, and SUA concentration. SUA, which appeared to be an independent metabolism characteristic, was individually most strongly associated with eGFR, explaining 18.2% of the variance in eGFR. SUA was followed by TG (1.4%), which reflected partial lipid concentration. CHOL, FBG, HbA1c, and HDL-C/LDL-C were not associated with eGFR. SUA was most strongly associated with eGFR (R2 = 19.3%) when all metabolism parameters were entered in a single model of stepwise regression. Analysis adjusted for these covariates provided similar results, although the strength of associations was generally decreased and showed SUA to be most strongly associated with eGFR, explaining 16.3% of the variance in eGFR. The effects by antiplatelet and antihypertensive drugs also partly explain the variance in eGFR (R2 = 5.5).
These findings supported the idea that SUA is an independent risk factor of eGFR decline in non-albuminuric DKD. Observational studies have shown that high level of SUA is associated with the loss of kidney function not only in DKD [16, 23,24,25,26] but also in non-albuminuric diabetic patients [18]. In our knowledge, this study might be one of the first time to compare the contribution of SUA and other risk factors in different DKD phenotypes, which proved that the relationship between SUA and eGFR in non-albuminuric DKD was stronger than in other phenotypes. Thus, SUA instead of other metabolism parameters might play a significant role in the development of non-albuminuric DKD, which indicated the different underlying pathogeneses and determinant factors from albuminuric DKD.
The presence of low eGFR in diabetic patients with normoalbuminuria is associated with the presence of metabolic syndrome [27]. Risk factors, including hyperglycemia, hypertension, and dyslipidemia, may trigger a progressive decrease of GFR. However, it is reported that patients of non-albuminuric DKD has lower level of blood pressure, LDL-C, and HbA1c than those of albuminuric diabetes with renal insufficiency [12]. And compared to patients with albuminuric DKD, both vascular lesion and tubulointerstitial injury of individuals with non-albuminuric DKD were more advance. Glomerular lesions were found to be less advanced in those with non-albuminuric DKD [28]. Arteriosclerosis reduces the glomerular blood flow and has been found to be a histological predictor for GFR decline in diabetic patients with normoalbuminuria [29].Hyperuricemia has been proved to induce vascular lesion and tubulointerstitial injury of the kidney [30,31,32]. In our study, SUA explains more eGFR decline than other metabolism parameters in non-albuminuric DKD. Thus, hyperuricemia, instead of hyperglycemia, high blood pressure, and dyslipidemia, might explain more part of renal function decline in non-albuminuric DKD.
Nevertheless, the SUA level increases linearly with decreasing GFR also as a result of reduced excretion [33]. Thus, whether elevated SUA level plays a causative role in the progression of kidney disease, is an indirect marker of decreased kidney function, or both, should be investigated in different causes and clinical features of CKD [34]. In type 2 diabetes, SUA is more likely to play a causative role, because hyperuricemia is considered to be a component of metabolic syndrome that is initially involved in the progression of type 2 diabetes [35]. Recently, two pivotal trials have failed to show statistically significant benefit of allopurinol on kidney outcomes [36, 37]. However, type 2 diabetes was not discussed in both trials. Hence, the relationship between SUA and DKD in type 2 diabetes should be further investigated, especially in non-albuminuric DKD.
This study had several limitations. First, we used a cross-sectional study design; therefore, the causal relationship between risk factors and non-albuminuric DKD could not be established. However, a large sample size provided statistical power, which was sufficiently large to identify the significant risk factors for non-albuminuric DKD in type 2 diabetes. Second, assessment of glycemia by HbA1c is hampered by various CKD-associated conditions that can bias the measure either to the low or high range. However, alternative glycemic biomarkers, such as glycated albumin or fructosamine, are even less reliable than HbA1c. Hence, HbA1c remains the preferred glycemic biomarker despite its limitations [38]. Third, serum creatinine and albuminuria were measured only once in each patient. Fourth, this study lacks the generalizability as it focused only one hospital. Hence, multicenter study should be conducted in the future.
In summary, the results indicated that SUA was primarily associated with eGFR decline in non-albuminuric DKD than in other DKD phenotypes. The prevention of hyperuricemia may serve as a primary focus in the management of renal decline of non-albuminuric DKD of type 2 diabetes.
Data availability
Not applicable.
Code availability
Not applicable.
References
Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, Li Y, Yeung RO, Wang J, Matsushita K, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375(9):905–6.
Thomas MC, Macisaac RJ, Jerums G, Weekes A, Moran J, Shaw JE, Atkins RC. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11). Diabetes Care. 2009;32(8):1497–502.
Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20(8):1813–21.
Drury PL, Ting R, Zannino D, Ehnholm C, Flack J, Whiting M, Fassett R, Ansquer JC, Dixon P, Davis TM, et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 2011;54(1):32–43.
Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, Trevisan R, Vedovato M, Gruden G, Cavalot F, et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens. 2011;29(9):1802–9.
Bolignano D, Zoccali C. Non-proteinuric rather than proteinuric renal diseases are the leading cause of end-stage kidney disease. Nephrol Dial Transplant. 2017;32(suppl_2):ii194–9.
Viazzi F, Russo GT, Ceriello A, Fioretto P, Giorda C, De Cosmo S, Pontremoli R. Natural history and risk factors for diabetic kidney disease in patients with T2D: lessons from the AMD-annals. J Nephrol. 2019;32(4):517–25.
de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–9.
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60.
Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA. 2016;316(6):602–10.
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Deng L, Li W, Xu G. Update on pathogenesis and diagnosis flow of normoalbuminuric diabetes with renal insufficiency. Eur J Med Res. 2021;26(1):144.
Ekinci EI, Jerums G, Skene A, Crammer P, Power D, Cheong KY, Panagiotopoulos S, McNeil K, Baker ST, Fioretto P, et al. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care. 2013;36(11):3620–6.
Klimontov VV, Korbut AI. Albuminuric and non-albuminuric patterns of chronic kidney disease in type 2 diabetes. Diabetes Metab Syndr. 2019;13(1):474–9.
Lai SW, Tan CK, Ng KC. Epidemiology of hyperglycemia in elderly persons. J Gerontol A Biol Sci Med Sci. 2000;55(5):M257-259.
Zoppini G, Targher G, Chonchol M, Ortalda V, Abaterusso C, Pichiri I, Negri C, Bonora E. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care. 2012;35(1):99–104.
Kim WJ, Kim SS, Bae MJ, Yi YS, Jeon YK, Kim BH, Song SH, Kim IJ, Kim YK. High-normal serum uric acid predicts the development of chronic kidney disease in patients with type 2 diabetes mellitus and preserved kidney function. J Diabetes Complications. 2014;28(2):130–4.
Qin Q, Qian Y, Zhu G, Fan W, Niu J, Gu Y. The correlation between serum uric acid and renal function in elderly Chinese diabetes with normoalbuminuria. Int J Endocrinol. 2019;2019:1435875.
Mikolaityte J, Badariene J, Puronaite R, Cypiene A, Rutkauskiene I, Dadoniene J, Laucevicius A: Which clusters of metabolic syndrome are the most associated with serum uric acid? Medicina (Kaunas) 2022, 58(2).
Miao L, Min Y, Qi B, Zhu CM, Chen JH, Deng GX, Wang Y, Li JF, Li RS. Causal effect between total cholesterol and HDL cholesterol as risk factors for chronic kidney disease: a Mendelian randomization study. BMC Nephrol. 2021;22(1):35.
Dai Q, Chen N, Zeng L, Lin XJ, Jiang FX, Zhuang XJ, Lu ZY. Clinical features of and risk factors for normoalbuminuric diabetic kidney disease in hospitalized patients with type 2 diabetes mellitus: a retrospective cross-sectional study. BMC Endocr Disord. 2021;21(1):104.
Yokoyama H, Araki SI, Kawai K, Yamazaki K, Tomonaga O, Shirabe SI, Maegawa H. Declining trends of diabetic nephropathy, retinopathy and neuropathy with improving diabetes care indicators in Japanese patients with type 2 and type 1 diabetes (JDDM 46). BMJ Open Diabetes Res Care. 2018;6(1):e000521.
Chang YH, Lei CC, Lin KC, Chang DM, Hsieh CH, Lee YJ. Serum uric acid level as an indicator for CKD regression and progression in patients with type 2 diabetes mellitus-a 4.6-year cohort study. Diabetes Metab Res Rev. 2016;32(6):557–64.
Gu L, Huang L, Wu H, Lou Q, Bian R. Serum uric acid to creatinine ratio: a predictor of incident chronic kidney disease in type 2 diabetes mellitus patients with preserved kidney function. Diab Vasc Dis Res. 2017;14(3):221–5.
Khadka M, Pantha B, Karki L. Correlation of uric acid with glomerular filtration rate in chronic kidney disease. JNMA J Nepal Med Assoc. 2018;56(212):724–7.
Wang J, Yu Y, Li X, Li D, Xu C, Yuan J, Wei S, Li X, Yang K, Zheng D, et al. Serum uric acid levels and decreased estimated glomerular filtration rate in patients with type 2 diabetes: a cohort study and meta-analysis. Diabetes Metab Res Rev. 2018;34(7):e3046.
Kramer CK, Leitao CB, Pinto LC, Silveiro SP, Gross JL, Canani LH. Clinical and laboratory profile of patients with type 2 diabetes with low glomerular filtration rate and normoalbuminuria. Diabetes Care. 2007;30(8):1998–2000.
Shimizu M, Furuichi K, Toyama T, Kitajima S, Hara A, Kitagawa K, Iwata Y, Sakai N, Takamura T, Yoshimura M, et al. Long-term outcomes of Japanese type 2 diabetic patients with biopsy-proven diabetic nephropathy. Diabetes Care. 2013;36(11):3655–62.
Moriya T, Omura K, Matsubara M, Yoshida Y, Hayama K, Ouchi M. Arteriolar hyalinosis predicts increase in albuminuria and GFR decline in normo- and microalbuminuric Japanese patients with type 2 diabetes. Diabetes Care. 2017;40(10):1373–8.
Kimura Y, Tsukui D, Kono H: Uric acid in inflammation and the pathogenesis of atherosclerosis. Int J Mol Sci 2021, 22(22).
Hayfron-Benjamin CF, van den Born BJ, Amoah AGB. Maitland-van der Zee AH, Meeks KAC, Beune E, Klipstein-Grobusch K, Agyemang C: Associations of serum uric acid levels with macrovascular and renal microvascular dysfunction among individuals from sub-Saharan Africa. JAMA Netw Open. 2021;4(10):e2128985.
Romi MM, Arfian N, Tranggono U, Setyaningsih WAW, Sari DCR. Uric acid causes kidney injury through inducing fibroblast expansion, endothelin-1 expression, and inflammation. BMC Nephrol. 2017;18(1):326.
Jing J, Kielstein JT, Schultheiss UT, Sitter T, Titze SI, Schaeffner ES, McAdams-DeMarco M, Kronenberg F, Eckardt KU, Kottgen A, et al. Prevalence and correlates of gout in a large cohort of patients with chronic kidney disease: the German Chronic Kidney Disease (GCKD) study. Nephrol Dial Transplant. 2015;30(4):613–21.
Valsaraj R, Singh AK, Gangopadhyay KK, Ghoshdastidar B, Goyal G, Batin M, Mukherjee D, Sengupta U, Chatterjee S, Sengupta N. Management of asymptomatic hyperuricemia: Integrated Diabetes & Endocrine Academy (IDEA) consensus statement. Diabetes Metab Syndr. 2020;14(2):93–100.
Khichar S, Choudhary S, Singh VB, Tater P, Arvinda RV, Ujjawal V. Serum uric acid level as a determinant of the metabolic syndrome: a case control study. Diabetes Metab Syndr. 2017;11(1):19–23.
Badve SV, Pascoe EM, Tiku A, Boudville N, Brown FG, Cass A, Clarke P, Dalbeth N, Day RO, de Zoysa JR, et al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med. 2020;382(26):2504–13.
Doria A, Galecki AT, Spino C, Pop-Busui R, Cherney DZ, Lingvay I, Parsa A, Rossing P, Sigal RJ, Afkarian M, et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med. 2020;382(26):2493–503.
Galindo RJ, Beck RW, Scioscia MF, Umpierrez GE, Tuttle KR: Glycemic monitoring and management in advanced chronic kidney disease. Endocr Rev 2020, 41(5).
Acknowledgment
We express our heartfelt gratitude to all participants and staff of the Department of Endocrinology, Affiliated Zhongda Hospital of Southeast University.
Author information
Authors and Affiliations
Contributions
Inspiration and thinking and guidance: Shaohua Wang and Zuolin Xie.
Research design: Shaohua Wang and Xi Huang.
Data collection and statistics analysis: Xi Huang and Chenchen Wang.
Writing manuscript: Xi Huang.
Modify paper and afford guidance: Shaohua Wang.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval was obtained from the Research Ethics Committee of Zhongda Hospital affiliated to Southeast University (Registration number: 2020ZDSYLL028-P01).
Consent to participate
No additional written consent was needed from the participants because all the data were extracted from the computerized data system of hospital authority and no personal data were collected for this study.
Consent for publication
The article is the authors’ original work. The article has not received prior publication and is not under consideration for publication elsewhere. All authors have seen and approved the manuscript being submitted.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Xie, Z., Wang, C. et al. Association between uric acid and renal impairment in non-albuminuric diabetes kidney disease of type 2 diabetes. Int J Diabetes Dev Ctries 43, 677–686 (2023). https://doi.org/10.1007/s13410-022-01132-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-022-01132-w