Abstract
Aim
The cornerstone of diabetes management is the diagnosis of its risk factors and then applying appropriate therapy. The aim of the study was to find if dairy consumption increases the risk of T1DM in children.
Method
A systematic search of PubMed and Scopus was done up to 22 February 2020. The overall estimates and their 95% confidence intervals were calculated using a random effects model.
Result
Our meta-analysis was performed on 7 studies. We extracted 14 effect sizes for T1DM risk. The analysis indicated that dairy consumption increased the risk of T1DM (RR: 1.04, 95% CI: 1.01, 1.08), with high heterogeneity (I2 = 76.6%, P heterogeneity < 0.001).
Conclusion
This meta-analysis showed a significant association between the consumption of dairy products and increased risk of T1DM with considerable heterogeneity. Further, longitudinal studies are needed to determine the causal relationship between dairy products and T1DM occurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although type 1 diabetes mellitus (T1DM) can be diagnosed at any age, it is one of the most prevalent diseases in children [1]. It is reported that about 79,000 children aged 14 or under the age of 14 years are suffering from T1DM worldwide annually [2]. The peak of T1DM occurrence is between 5 and 7 years of age and at or near puberty [3]. T1DM is correlated with major chronic disease risk factors and increases morbidity and mortality [4, 5]. Death under 30 years usually results due to acute complications of diabetes, including diabetic ketoacidosis and hypoglycemia [6]; and cardiovascular disease is the major reason for death later in life [5, 6]. The basis of diabetes care is the identification of the risk factors and then using the appropriate treatment [7]. Genetics and exposure to environmental factors [8] may play a major role in the occurrence of type 1 diabetes. Among environmental risk factors, a significant one that may modify the risk of type 1 diabetes in early childhood is dietary quality. Limited breastfeeding duration and early exposure to complex dietary proteins are identified as risk factors for advanced beta-cell autoimmunity or type 1 clinical diabetes [6]. Supplementing breast milk with a highly hydrolyzed milk formula would reduce the cumulative incidence of diabetes-associated autoantibodies in such children [9]. All the above are factors that researchers will also investigate in order to evaluate the function of diet during early infancy and development of T1D, as they may intervene and serve as confounders as possible. However, the correlation of dairy products with health outcomes in children is not well-understood [10]. Also, there are inconsistencies about the impact of dairy on diabetes [11]. Therefore, the purpose of this research was to explore this main question, Can dairy intake increase the risk of T1DM? We aim to determine the association between dairy products intake and T1DM risk using observational and cohort evidences.
Method
Search strategy
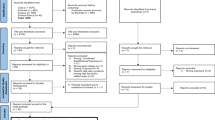
We systematically searched PubMed and Scopus up to 22 February 2020 with the following keywords: ((dairy product[tiab] OR milk[tiab] OR yogurt[tiab] OR cheese[tiab] OR kefir[tiab] OR butter[tiab] OR cream[tiab] OR “Dairy Products”[Mesh] OR Milk[Mesh] OR Yogurt[Mesh] OR Cheese[Mesh] OR Kefir[Mesh] OR Butter[Mesh] OR “Ice Cream”[Mesh]) AND (“type 1 diabetes”[tiab] OR “type 1 diabetic”[tiab] OR “type 1- diabetes mellitus”[tiab] OR “diabetes mellitus”[tiab] OR “diabetic patients”[tiab] OR DM[tiab] OR T1DM[tiab] OR TIDM[tiab] OR “insulin dependent”[tiab] OR IDDM[tiab] OR T1D[tiab] OR “Diabetes Mellitus, Type 1”[Mesh] OR “Diabetes Mellitus”[Mesh]) AND (“population-based” OR prospective OR “case control” OR longitudinal OR follow-up OR cohort OR retrospective OR nested OR “Longitudinal Studies”[Mesh] OR “Prospective Studies”[Mesh] OR “Case-Control Studies”[Mesh] OR “Cohort Studies”[Mesh] OR “Retrospective Studies”[Mesh])). Besides, a hand search of references of the published papers was done to detect other relevant articles. In Fig. 1, the details of the search strategy are illustrated. We also performed the systematic search for the second time in order to find any recent possible articles on 20 May 2020.
Eligibility criteria
Two trained reviewers (MSH and LSH) separately examined the eligibility of the studies twice. Studies were included if they met the following criteria: (a) observational study design; (b) involved subjects with <18 years old; (c) adequate information about any types of dairy products and the risk of type 1 diabetes mellitus; (d) publications that had provided estimates of relative risks (RRs) (odds ratios (ORs), hazard ratios (HRs), or rate ratios) with corresponding 95% confidence intervals (CIs); and (e) published in English.
Exclusion criteria
Totally, 1457 articles were found in our initial search. We excluded 856 articles by reading the title and abstract. The other 194 papers were excluded because of the following reasons: age > 18 (n = 84), type 2 mellitus (n = 3), review studies (n = 4), and the article without sufficient data for outcomes (n = 103).
Data extraction
Two independent reviewers (MSH and FDj) extracted the data. Any disagreements and differences were resolved by the study supervisor (SS-b), if necessary. The following information of studies was extracted: the first author’s last name, date of publication, country, participants’ age range, gender, sample size, number of cases, duration of follow-up, method of measurement of exposure and outcome, comparisons, and ORs or RRs for type 1 diabetes mellitus.
Quality assessment
The quality of included studies was evaluated by means of the Newcastle-Ottawa Scale [12]. For cohort and case-control studies that were included in the analysis, we used their own specific methods. The NOS allocates a maximum of nine points to each study: four for selection, two for comparability, and three for assessment of outcomes (nine represented the highest quality). Any inconsistencies were set by discussion.
Statistical analysis
Random effects models were applied in the current meta-analysis. Therefore, the overall effect size was calculated. Between-study heterogeneity which refers to the variation in study outcomes among studies was assessed using Cochran’s Q test and I2 statistic. Sensitivity analysis was conducted to examine changes in pooled effect size when one study had been removed. Publication bias was assessed by visual inspection or using Egger’s regression asymmetry test. All statistical analyses were done through Stata software, version 12.0 (Stata Corp LP, College Station, TX, USA). A p value of 0.05 was considered as statistical significance.
Results
Study selection
We found 1457 publications in our initial search. Of those, 400 records were duplicates. We reviewed titles and abstracts of all remaining publications, and as a result, another 856 publications were excluded, yielding 201 studies for full text assessing (Fig. 1). Of those, 7 publications provided sufficient information for the present meta-analysis. Thus, 6 case-control studies [13,14,15,16,17,18] and 1 cohort [19] with 5067 participants were included in the final analyses.
Characteristics of the included studies
Most included studies reported the association of dairy products consumption and risk of type 1 diabetes in children, and only one study assessed the risk of beta-cell autoimmune as an outcome.
General characteristics of the studies are provided in Table 1. These studies were published between 1991 and 2012 and were conducted in Finland [16,17,18,19], Germany [15], Sweden [13], and the UK [14]. All of the studies included both genders. The exposure assessment tool in six studies was the dairy products consumption questionnaire, and only one study was relied on 3-day food recall. All studies were at high quality (≥7 stars) on the basis of the Newcastle-Ottawa Scale (Table 2).
Our meta-analysis was performed on 7 studies. We extracted 14 effect sizes for type 1 diabetes risk. The overall effect of an association between dairy products consumption and risk of T1DM is illustrated in Fig. 2. The analysis indicated that dairy products consumption increased the risk of T1DM (RR: 1.04, 95% CI: 1.01, 1.08), with high heterogeneity, (I2 = 76.6%, P heterogeneity < 0.001). Based on the visual inspected funnel plot (Fig. 3) and Egger test, the studies did not have publication bias (p = 0.161). Sensitivity analysis results showed that excluding any of the studies was not significantly changed.
Discussion
The current meta-analysis revealed a significant linkage on the consumption of dairy products and increased risk of T1DM in children with considerable heterogeneity. In 2006, Multinational Project for Childhood Diabetes (DIAMOND) was indicated that Finland, Sweden, and the UK are the first, third, and fifth, respectively, with the very high incidence of childhood type 1 diabetes, and Germany was classified as a high-risk group [20]. Culturally, racial variation in communities of European origin has shown a higher incidence compared to non-Europeans but may cause these differences, particularly among long-term immigrants to European countries [21]. The most important risk factors for type 1 diabetes as autoimmune diabetes are genetic, family history, and environment [22]. Although the evidences proved that the diet is a potential risk factor for the induction of diabetes autoantibodies in children [17, 23], whether dairy products protect against T1DM or not is a controversial issue. Surely, kind of milk consumption pattern and specially fresh cow milk in the north European population (Finland, Sweden, the UK) [24] is an important factor in diabetes incidence and prevalence. Previous epidemiological data have shown the relationship between dairy product consumption and risk of T1DM. In line with our study, Suvi et al. found that high intakes of dairy products during childhood may be diabetogenic in children with type 1 diabetes [16]. In 2018, Canada Clinical Practice published guidelines about reducing the risk of developing diabetes, and suggested nonlinear inverse associations were observed for total dairy products and yogurt, with most of the benefit being observed when increasing the intake of total dairy products from little to no dairy up to 300 to 400 g/day or yogurt up to 120 to 140 g/day, above which there was no further benefit [25].
As our knowledge, accumulating evidence-supported dairy products intake can increase the risk of type 1 diabetes [14, 15, 17, 18, 26]. Numerous mechanistic pathways suggested about the correlation between dairy products consumption and T1DM risk [27]. Most dairy products are milk based [28]; therefore, we have tried to point the mechanisms for the impact of milk on T1DM risk. Moreover, a series of studies have shown that children with newly diagnosed type 1 diabetes had elevated levels of antibodies, in particular to food antigens, lactose intolerance, and cow’s milk proteins [17, 29,30,31,32]. The role of genetics, as one of other risk factors in T1DM occurrence, has been of recent interest. T1DM as a chronic immune-mediated disease with a subclinical prodromal period is characterized by selective loss of insulin-producing β-cells in the pancreatic [16] islets in genetically susceptible subjects. Auto-reactive T cells, CD4 and CD8 cells, and a series of auto-antigens like glutamic acid decarboxylase (GAD) have been implicated as active players in β-cell destruction [19, 33]. The issue of whether there is any primary autoantigen in T1D has remained controversial. Given that there are two major HLA haplotypes conferring disease susceptibility, i.e., the DR3–DQ2 haplotype and the DR4–DQ8 haplotype, one may assume that there will be at least two primary antigens in T1D [16, 22, 34]. The first signs of β-cell autoimmunity might appear already during the first year of life [33]. The enormous studies reported that a strong connection between the longitudinal consumption of cow milk in the children and the development of advanced β-cell autoimmunity is related to several previous case-control and cohort results with endpoints varied from early pre-type 1 diabetes to clinical disease [16, 19, 35]. Lamb et al., clearly explained that higher intakes of cow’s milk may promote progression to type 1 diabetes in children with autoimmune islets [36], an effect that could be induced by certain fatty acids in cow’s milk and meat, such as myristic [34]. Also, lipid-mediated signals can play an important role in lipotoxicity induced by fatty acids [37]. A nested case control determined that there was no relationship between the consumption of sour milk products and cheese with advanced β-cell autoimmunity [17]. In this article, the mechanism of the effect of sour milk and cheese on T1DM was mentioned kind of protein that is partly hydrolyzed to smaller peptides and amino acids. Familial history is another risk factor for type 1 diabetes [38]. Parkkola’s article indicated the children with familial type 1 diabetes have an autoantibody profile, implying similar pathogenic disease mechanisms [39] Further, the duration of breastfeeding has the potential for being T1DM [40].
The recent trial published that cow’s milk does not play a critical role in the development of type 1 diabetes [41]. It was reported that camel milk is safe and efficacious in improving long-term glycemic control, with a significant reduction in the doses of insulin in type 1 diabetic patients [42]. To dispose the controversy in evidence with about effect of cow milk on diabetes mellitus [16, 19, 35, 41], a systematic review published in 2017 supported that consumption of camel milk can decease blood sugar and insulin resistance [43]. Another hypothesis was discussed by Sørensen et al. that found an association between higher serum 25-hydroxyvitamin D in late pregnancy and lower risk of type 1 diabetes in offspring [44]. Dairy products are known to be rich in vitamin D. This has been demonstrated that vitamin D changes the balance of the T cell response in the body towards downregulation of the T-helper-1 immune response [45] and beneficial factor as it plays an important function in regulating the immune system, as well as diabetes-relevant metabolic pathways.
Our meta-analysis has several strengths; for example, our search strategy was very accurate and covered multiple databases. Further, our statistical examinations indicated no evidence of publication bias in our analyses, and, finally, to the best of knowledge, this is the first meta-analysis to be performed in this regard, although some limitations exist. At first, these studies that are selected to be included have heterogeneous risk measurement methods. The high heterogeneity is another limitation that could impact our findings powerfully. Second, owing to the small number of studies, there was no ability to better examination of the association between childhood dairy products consumption with risk of T1DM. Third, most of the studies presented some form of bias, and thus, it is hard to reach a certain conclusion. Another limitation that is necessary to mention is the quantity of milk that is different in various studies.
Conclusion
We found a positive association between dairy products consumption and odds of type 1 diabetes mellitus in children. Our team suggested that these findings need to be confirmed by larger trials in order to conclusively determine any relationship between dairy products intake and T1DM.
References
Gale E. Type 1 diabetes in the young: the harvest of sorrow goes on. Springer; 2005.
Patterson C, Guariguata L, Dahlquist G, Soltész G, Ogle G, Silink M. Diabetes in the young–a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res Clin Pract. 2014;103(2):161–75.
Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371(9626):1777–82.
Wong J, Constantino M, Yue DK. Morbidity and mortality in young-onset type 2 diabetes in comparison to type 1 diabetes: where are we now? Curr Diab Rep. 2015;15(1):566.
Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny County type 1 diabetes registry. Diabetes Care. 2010;33(12):2573–9.
Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes. 2010;59(12):3216–22.
Morris SF, Wylie-Rosett J. Medical nutrition therapy: a key to diabetes management and prevention. Clin Diabetes. 2010;28(1):12–8.
Scott A, Chambers D, Goyder E, O’Cathain A. Socioeconomic inequalities in mortality, morbidity and diabetes management for adults with type 1 diabetes: a systematic review. PLoS One. 2017;12(5).
Knip M, Virtanen SM, Seppä K, Ilonen J, Savilahti E, Vaarala O, et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010;363(20):1900–8.
Dror DK, Allen LH. Dairy product intake in children and adolescents in developed countries: trends, nutritional contribution, and a review of association with health outcomes. Nutr Rev. 2014;72(2):68–81.
Turner KM, Keogh JB, Clifton PM. Dairy consumption and insulin sensitivity: a systematic review of short- and long-term intervention studies. Nutr Metab Cardiovasc Dis. 2015;25(1):3–8.
Wells GA, Tugwell P, O’Connell D, Welch V, Peterson J, Shea B, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2015.
Dahlquist G, Blom L, Lonnberg G. The Swedish Childhood Diabetes Study--a multivariate analysis of risk determinants for diabetes in different age groups. Diabetologia. 1991;34(10):757–62.
Marshall A, Chetwynd A, Morris J, Placzek M, Smith C, Olabi A, et al. Type 1 diabetes mellitus in childhood: a matched case control study in Lancashire and Cumbria, UK. Diabet Med. 2004;21(9):1035–40.
Rosenbauer J, Herzig P, Kaiser P, Giani G. Early nutrition and risk of type 1 diabetes mellitus--a nationwide case-control study in preschool children. Exp Clin Endocrinol Diabetes. 2007;115(8):502–8.
Virtanen SM, Laara E, Hypponen E, Reijonen H, Rasanen L, Aro A, et al. Cow's milk consumption, HLA-DQB1 genotype, and type 1 diabetes: a nested case-control study of siblings of children with diabetes. Childhood diabetes in Finland study group. Diabetes. 2000;49(6):912–7.
Virtanen SM, Nevalainen J, Kronberg-Kippilä C, Ahonen S, Tapanainen H, Uusitalo L, et al. Food consumption and advanced β cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes: a nested case-control design. Am J Clin Nutr. 2012;95(2):471–8.
Virtanen SM, Saukkonen T, Savilahti E, Ylonen K, Rasanen L, Aro A, et al. Diet, cow’s milk protein antibodies and the risk of IDDM in Finnish children. Childhood diabetes in Finland Study Group. Diabetologia. 1994;37(4):381–7.
Virtanen S, Hyppönen E, Läärä E, Vähäsalo P, Kulmala P, Savola K, et al. Cow’s milk consumption, disease-associated autoantibodies and type 1 diabetes mellitus: a follow-up study in siblings of diabetic children. Diabet Med. 1998;15(9):730–8.
Group DP. Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23(8):857–66.
Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine. 2010;38(11):602–6.
DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449–62.
Wahlberg J, Vaarala O, Ludvigsson J, Group A-S. Dietary risk factors for the emergence of type 1 diabetes-related autoantibodies in 2½-year-old Swedish children. Br J Nutr. 2006;95(3):603–8.
Vuorisalo T, Arjamaa O, Vasemägi A, Taavitsainen J-P, Tourunen A, Saloniemi I. High lactose tolerance in north Europeans: a result of migration, not in situ milk consumption. Perspect Biol Med. 2012;55(2):163–74.
Prebtani AP, Bajaj HS, Goldenberg R, Mullan Y. Reducing the risk of developing diabetes. Can J Diabetes. 2018;42:S20–S6.
Fava D, Leslie RDG, Pozzilli P. Relationship between dairy product consumption and incidence of IDDM in childhood in Italy. Diabetes Care. 1994;17(12):1488–90.
Knip M, Virtanen SM, Åkerblom HK. Infant feeding and the risk of type 1 diabetes. Am J Clin Nutr. 2010;91(5):1506S–13S.
Early R. Dairy products and milk-based food ingredients. Natural Food Additives, Ingredients and Flavourings. Elsevier; 2012. p. 417–45.
Savilahti E, Akerblom H, Tainio V, Koskimies S. Children with newly diagnosed insulin dependent diabetes mellitus have increased levels of cow’s milk antibodies. Diabetes Res. 1988;7(3):137–40.
Dahlquist G, Savilahti E, Landin-Olsson M. An increased level of antibodies to β-ltoglobulin is a risk determinant for early-onset type 1 (insulin-dependent) diabetes mellitus independent of islet cell antibodies and early introduction of cow’s milk. Diabetologia. 1992;35(10):980–4.
Saukkonen T, Savilahti E, Vaarala O, Virtala ET, Tuomilehto J, Åkerblom HK, et al. Children with newly diagnosed IDDM have increased levels of antibodies to bovine serum albumin but not to ovalbumin. Diabetes Care. 1994;17(9):970–6.
Nicklas TA, Qu H, Hughes SO, He M, Wagner SE, Foushee HR, et al. Self-perceived lactose intolerance results in lower intakes of calcium and dairy foods and is associated with hypertension and diabetes in adults. Am J Clin Nutr. 2011;94(1):191–8.
Knip M, Siljander H. Autoimmune mechanisms in type 1 diabetes. Autoimmun Rev. 2008;7(7):550–7.
Virtanen SM, Niinistö S, Nevalainen J, Salminen I, Takkinen H-M, Kääriä S, et al. Serum fatty acids and risk of advanced β-cell autoimmunity: a nested case–control study among children with HLA-conferred susceptibility to type I diabetes. Eur J Clin Nutr. 2010;64(8):792–9.
Verge CF, Howard NJ, Irwig L, Simpson JM, Mackerras D, Silink M. Environmental factors in childhood IDDM: a population-based, case-control study. Diabetes Care. 1994;17(12):1381–9.
Lamb MM, Miller M, Seifert JA, Frederiksen B, Kroehl M, Rewers M, et al. The effect of childhood cow’s milk intake and HLA-DR genotype on risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young. Pediatr Diabetes. 2015;16(1):31–8.
Choi S-E, Jung I-R, Lee Y-J, Lee S-J, Lee J-H, Kim Y, et al. Stimulation of lipogenesis as well as fatty acid oxidation protects against palmitate-induced INS-1 β-cell death. Endocrinology. 2011;152(3):816–27.
Sparud-Lundin C, Öhrn I, Danielson E. Redefining relationships and identity in young adults with type 1 diabetes. J Adv Nurs. 2010;66(1):128–38.
Parkkola A, Härkönen T, Ryhänen SJ, Ilonen J, Knip M. Extended family history of type 1 diabetes and phenotype and genotype of newly diagnosed children. Diabetes Care. 2013;36(2):348–54.
Pérez-Bravo F, Oyarzún A, Carrasco E, Albala C, Dorman JS, Santos JL. Duration of breast feeding and bovine serum albumin antibody levels in type 1 diabetes: a case-control study. Pediatr Diabetes. 2003;4(4):157–61.
Knip M, Åkerblom HK, Al Taji E, Becker D, Bruining J, Castano L, et al. Effect of hydrolyzed infant formula vs conventional formula on risk of type 1 diabetes: the TRIGR randomized clinical trial. Jama. 2018;319(1):38–48.
Agrawal R, Jain S, Shah S, Chopra A, Agarwal V. Effect of camel milk on glycemic control and insulin requirement in patients with type 1 diabetes: 2-years randomized controlled trial. Eur J Clin Nutr. 2011;65(9):1048–52.
Mirmiran P, Ejtahed H-S, Angoorani P, Eslami F, Azizi F. Camel milk has beneficial effects on diabetes mellitus: a systematic review. Int J Endocrinol Metab. 2017;15(2):e42150-e.
Sørensen IM, Joner G, Jenum PA, Eskild A, Torjesen PA, Stene LC. Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes. 2012;61(1):175–8.
Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387(10035):2340–8.
Author information
Authors and Affiliations
Contributions
MSH and SS-b designed the study. MRA and MSH did the literature search and screening data. FDj, MSH, and LSH performed data extraction and quality assessment, independently. FDj, LSH, FSH, and HSH analyzed and interpreted data and wrote the manuscript. SS-b finalized the manuscript and supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahavandi, M., Djafari, F., Sheikhi, L. et al. The association between dairy products consumption with risk of type 1 diabetes mellitus in children: a meta-analysis of observational studies. Int J Diabetes Dev Ctries 41, 369–376 (2021). https://doi.org/10.1007/s13410-021-00923-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-021-00923-x