Abstract
Objective
To evaluate the effect of Helicobacter pylori (H. pylori) infection upon metabolic and inflammatory parameters in type 2 diabetes mellitus (T2DM).
Methods
A total of 72 patients with T2DM were included in the study. These patients were divided into two groups as H. pylori infection positive or negative. For each patient, the following data were collected: age, gender, duration of diabetics, anti-diabetic treatment, the body mass index (BMI), and laboratory parameters (lipid profile, GLU, HbA1c, HCY, HsCRP, ghrelin, leptin, leukocyte, and platelet counts).
Results
Totally 47 patients (65.28%) were H. pylori positive and 25 patients (34.72%) were H. pylori negative. Diabetic patients infected by H. pylori showed significantly increased Lpa (297.83 ± 299.51 vs 154.24 ± 83.63, p < 0.05), higher HbA1c (9.21 ± 2.15 vs 8.00 ± 1.77, p < 0.05), and decreased leptin (4.59 ± 7.55 vs 9.82 ± 10.76, p < 0.05) than non-infected patients. Additionally, 72.2% of the patients with HbA1c > 7% were found to be H. pylori positive and 44.4% of the patients with HbA1c ≤ 7% were H. pylori positive. The levels of other parameters were not significantly different between two groups (p > 0.05), although CRP levels determined by high-sensitivity assay showed mild and variable increases in H. pylori infection.
Conclusion
H. pylori affects glycemic control in T2DM and might promote the development of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Helicobacter pylori (H. pylori) is a Gram-negative, spiral-shaped pathogenic bacterium which can establish a persistent infection within human gastric mucosa, and affects approximately up to half of the world population [1]. This problem is particularly notable in developing countries, and the prevalence rate in China may be as high as 54.7% [2]. The effects of this organism may not only be confined to the gastrointestinal tract but also be associated with extra-intestinal ailments such as its role in diabetes and increased insulin resistance [3].
Diabetes mellitus is a group of metabolic diseases characterized by high levels of blood sugar (glucose). Type 2 diabetes mellitus (T2DM) is increasingly common and is responsible for the death of an estimated 3.8 million adults across the world [4]. Although major risk factors (e.g., lifestyle, genetic background, socioeconomic factors) for T2DM have been identified, they provide only partial explanations.
The relationship of H. pylori and DM was first explored in 1989 by Simon et al. [5]. But previous studies provided inconsistent conclusions concerning the association between various clinical manifestation of diabetes and H. pylori infection [6,7,8,9]. Recent study suggests the role of inflammation in the pathogenesis of T2DM, which is an important process induced by H. pylori infection [10]. It has been shown that H. pylori infection may increase insulin resistance through inducing chronic inflammation and affecting the levels of some insulin-regulating gastrointestinal hormones. This research sought to investigate the possible relationship between H. pylori infection and T2DM patients as well as the potential mediators concerning this correlation.
Patients and methods
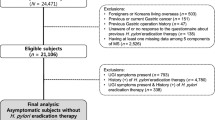
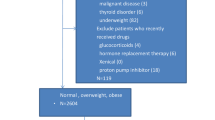
The study was a hospital-based, analytic observational study and performed through a cross-sectional method. T2DM patients aged 18–65 and recruited from the Second Affiliated Hospital of Bengbu Medical College were included into the study. Our research protocol was approved by ethical committee of Bengbu Medical College, and a written informed consent was signed by each participants according to national and institutional guidelines. Exclusion criteria were as follows: (1) patients over 65 years; (2) women patients who were currently pregnant or breastfeeding; (3) patients with a history of gastrointestinal tract surgery; (4) patients who were currently using antisecretory drugs (proton-pump inhibitor or H2 receptor blockers); (5) patients who had undergone or were currently undergoing H. pylori eradication therapy; (6) patients who were obliged to continuous use of antibiotics for various reasons.
We compiled a brief checklist covering demographic data and clinical parameters, including age, gender, duration of diabetics, and anti-diabetic treatment. The body mass index (BMI) is a statistical measure based on a person’s weight and height (weight/height2 = kg/m2). We also checked the laboratory parameters. GLU (glucose oxidase method); TC (CHOD-PAP substrate method); TG (GPO-PAP enzymatic method); HDL-C (selective inhibition method); LPa (latex-enhanced immunoturbidimetry); Apo A, Apo B, and HsCRP (turbidimetric immunoassay); and HCY (enzyme method) were measured on Beckman Coulter UniCel DxC 800 Synchron. LDL-C was calculated by the Friedewald formula. HbA1c was determined by ion-exchange HPLC method (HLC-723G8 automatic glycosylated hemoglobin analyzer). Commercially available human enzyme-linked immunosorbent assay (ELISA) for the determination of plasma ghrelin and leptin (eBioscience Inc., USA) was performed according to manufacturers’ instructions. The leukocyte and platelet counts were determined by flow cytometry, and Mindray BC-5800 automatic blood cell analyzer was used for the detection.
In all patients, the diagnosis of H. pylori infection was determined by ELISA for anti-H. pylori IgG, with a reported sensitivity and specificity of 96% [11]. Measurement of specific anti-H. pylori IgG reveals an immune response that represents either a current infection or a previous exposure, since IgG disappears only several months after eradication of the microorganism. Then, we divided the subjects into two groups according to H. pylori infection as H. pylori–positive patients and H. pylori–negative patients. The association between H. pylori infection and demographic factors, and biochemical and anthropometric indicators was investigated in all patients.
Statistical analysis
Statistical Package for the Social Sciences (SPSS) 20.0 Package program was used for the analysis of data. The data were presented as mean ± SD. Adjustment to normality was checked through the Kolmogorov-Smirnov test. Quantitative variables with normal distribution were compared by H. pylori status using independent sample t test or the Mann-Whitney U test as appropriate. Qualitative parameters were analyzed with the chi-square test. Two-tailed p values of below 0.05 were considered significant.
Results
A total of 72 T2DM patients with a mean age of 54 (± 7) were enrolled in this study, including 44 males and 28 females. The presence of H. pylori infection was diagnosed in 47 (65.28%) of 72 diabetic patients. In male patients, the prevalence of H. pylori infection was 68.18% while it was 60.71% in females. The demographic and laboratory characteristics of the study population, divided into H. pylori–positive and H. pylori–negative groups, are displayed in Tables 1 and 2.
Patients infected with H. pylori and non-infected with H. pylori were not significantly different in terms of gender, age, BMI, duration of diabetics, and anti-diabetic treatment. The level of GLU, TC, TG, HDL-C, Apo A, Apo B, HsCRP, HCY, leukocyte, and platelet counts were not significantly different between H. pylori–positive and H. pylori–negative groups (p > 0.05), although CRP levels determined by high-sensitivity assay showed mild and variable increases in H. pylori infection. Diabetic patients infected by H. pylori showed significantly increased serum Lpa (297.83 ± 299.51 vs 154.24 ± 83.63, p < 0.05) and decreased leptin (4.59 ± 7.55 vs 9.82 ± 10.76, p < 0.05) than non-infected patients (Table 1). HbA1c, the major fraction of glycated hemoglobin, is most valuable glucose monitoring index for patients with diabetes [12]. A significant relationship between glycemic control and the presence of H. pylori was detected. Patients with H. pylori infection had higher HbA1c level (9.21 ± 2.15 vs 8.00 ± 1.77, p < 0.05). Additionally, 55.6% of the patients with HbA1c ≤ 7% were H. pylori negative and 72.2% of the patients with HbA1c > 7% were found to be H. pylori positive (Table 2), indicating that H. pylori–infected group had worse glycemic control.
Discussion
It is well known that diabetic patients have an increased risk of suffering chronic infections because of cellular and humoral immune deficiency. It has been reported that the prevalence of H. pylori infection varies between 30 and 80% in diabetic patients [13,14,15,16]. In this recent study of our institute, the prevalence of H. pylori infection in diabetics was found to be 65.28% and this rate was concordant with results observed in different related studies.
H. pylori plays a major pathogenic role in a wide array of gastric disorders, including simple gastritis, peptic ulcers, and gastric malignancies. During the last two decades, the associations among H. pylori and several extragastric manifestations, such as iron deficiency anemia, cardiovascular disease, as well as diabetes mellitus (DM), and other metabolic syndromes [17,18,19,20], strongly emerged in literature. The relationship between H. pylori and DM was first explored in 1989. From then on, it has been suggested that infection with H. pylori is potentially linked to DM in many aspects [21,22,23,24]. However, the question of whether H. pylori infection is associated with poorer glycemic control in patients with DM remains controversial.
This study showed a positive association between H. pylori status and HbA1c levels, a valid and sensitive biomarker for long-term glycemic control among a group of middle-aged to elderly Chinese subjects with T2DM. Patients with H. pylori infection had significantly elevated HbA1c level. Additionally, compared with low HbA1c (≤ 7%), the patients with HbA1c > 7% showed a significantly higher H. pylori–infection rate (72.2% vs 44.4%), indicating that H. pylori–infected group had worse glycemic control.
The mechanisms linking H. pylori to glycemic control in T2DM are complicated. It is well known that insulin resistance and abnormal insulin secretion are the main pathogenic factors in T2DM. H. pylori might affect pathophysiological process of insulin resistance through subclinical chronic inflammation, by which the bacterium influences glycemic control in diabetics [25]. An alternative hypothesis is that gastrointestinal conditions resulting from H. pylori infection could delay gastric emptying and consequently causes poor glucose control. Furthermore, H. pylori–induced gastritis can potentially affect the secretion of gastric-related hormones, which are secreted from gastric mucosa and are involved in energy homeostasis, modulating insulin sensitivity and glucose homeostasis. Leptin, a multifunctional polypeptide primarily produced by adipocytes, favors energy expenditure increase and food intake reduction [26]. In recent years, increased evidence indicates that high levels of leptin may impair glucose-stimulated insulin secretion and induce apoptosis of β cells. Our study showed that H. pylori infection elevated the production of leptin among patients with T2DM, and thus might promote the development of diabetes.
DM is always a multifactorial metabolic disorder characterized by changes in the metabolism of carbohydrates, fats, and protein. A growing body of evidence has demonstrated a significant relationship between lipid profiles and thrombotic activation–related anti-thrombin (AT)-III and H. pylori infection [27]. In the present study, lipid profile was not significantly different between H. pylori–positive and H. pylori–negative groups, but LPa levels were found to be significantly increased in infected diabetic patients. LPa is a new novel marker of cardiac events, and serum elevated level of LPa is an independent risk factor for coronary heart disease (CHD) [28], so H. pylori infection has been hypothesized to predispose diabetics to cardiovascular and cerebral diseases.
In conclusion, our results demonstrated a significant association between H. pylori infection and impaired glycemic control in type 2 diabetic patients. Leptin appeared to mediate this effect. The relationship between H. pylori and LPa level remains speculative, but a greater understanding may give important insights into the cardiac events in diabetic patients. A few limitations warrant consideration: first, this was a single-center study, and the small sample size used in this study remains as a limitation. Further investigations are recommended, with a larger subject population, to validate the findings reported here. Second, we did not investigate the patients with H. pylori infection after treatment. More investigations will be required to evaluate the effects of H. pylori eradication on the metabolic status in diabetic patients.
References
Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, van der Merwe S, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis. 2011;20(3):299–304.
Cheng H, Hu F, Zhang L, Yang G, Ma J, Hu J, et al. Prevalence of Helicobacter pylori infection and identification of risk factors in rural and urban Beijing. China. Helicobacter. 2009;14(2):128–33.
Rizzatti G, Matteo MV, Ianiro G, Cammarota G, Franceschi F, Gasbarrini A. Helicobacter pylori in metabolic related diseases. Minerva Gastroenterol Dietol. 2018;64(3):279–309.
Van DS, Beulens JW, Van YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(Suppl 1):3–8.
Simon L, Tornóczky J, Tóth M, Jambor M, Sudar Z. The significance of Campylobacter pylori infection in gastroenterologic and diabetic practice. Orv Hetil. 1989;130(25):1325–9.
Kayar Y, Pamukçu Ö, Eroğlu H, Erol KK, Ilhan A, Kocaman O. Relationship between Helicobacter pylori infections in diabetic patients and inflammations, metabolic syndrome, and complications. International Journal of Chronic Diseases. 2015;2015(290128):6.
Yang GH, Wu JS, Yang YC, Huang YH, Lu FH, Chan CJ. Gastric Helicobacter pylori infection associated with risk of diabetes mellitus, but not prediabetes. Journal of Gastroenterology and Hepatology. 2014;29(10):1794–9.
Zhou F, Zhong X, Chen J, Li C, Shang M, Jiang C, et al. Helicobacter pylori infection associated with type 2 diabetic nephropathy in patients with dyspeptic symptoms. Diabetes Research and Clinical Practice. 2015;110(3):328–34.
Sotuneh N, Hosseini SR, Shokri-Shirvani J. Bijani Ali and Ghadimi R. Helicobacter pylori infection and metabolic parameters: is there an association in elderly population? Int J Prev Med. 2014;5(12):1537–42.
Guo X, Zhao BH, Zhang MX. Risk factors of Helicobacter pylori infection among adults in northern China. Hepato-Gastroenterology. 2011;58(106):306–10.
Figura N, Palazzuoli A, Vaira D, Campagna M, Moretti E, Iacoponi F, et al. Cross-sectional study: CagA-positive Helicobacter pylori infection, acute coronary artery disease and systemic levels of B-type natriuretic peptide. J Clin Pathol. 2014;67(3):251–7.
Krabbe CEM, Schipf S, Ittermann T, Dörr M, Nauck M, Chenot JF, et al. Comparison of traditional diabetes risk scores and HbA1c to predict type 2 diabetes mellitus in a population based cohort study. J Diabetes Complications. 2017;31(11):1602–7.
Oldenburger B, Diepersloot RJ, Hoekstra JB. High seroprevalence of Helicobacter pylori in diabetes mellitus patients. Dig Dis Sci. 1996;41(3):458–61.
Gentile S, Turco S, Oliviero B, Torella R. The role of autonomic neuropathy as a risk factor of Helicobacter pylori infection in dyspeptic patients with type 2 diabetes mellitus. Diab Res Clin Pract. 1998;42(1):41–8.
Marrollo M, Latella G, Melide D, Storelli E, Iannarelli R, Stornelli P, et al. Increased prevalence of Helicobacter pylori in patients with diabetes mellitus. Dig Liver Dis. 2001;33(1):21–9.
Oluyemi A, Anomneze E, Smith S, Fasanmade O. Prevalence of a marker of active Helicobacter pylori infection among patients with type 2 diabetes mellitus in Lagos. Nigeria. Bmc Res Notes. 2012;5(1):284.
Kato S, Osaki T, Kamiya S, Zhang XS, Blaser MJ. Helicobacter pylori sabA gene is associated with iron deficiency anemia in childhood and adolescence. PLoS One. 2017;12(8):e0184046.
Wang JW, Tseng KL, Hsu CN, Liang CM, Tai WC, Ku MK, et al. Association between Helicobacter pylori eradication and the risk of coronary heart diseases. PLoS One. 2018;13(1):e0190219.
Kountouras J, Boziki M, Polyzos SA, Katsinelos P, Gavalas E, Zeglinas C, et al. The emerging role of Helicobacter Pylori-induced metabolic gastrointestinal dysmotility and neurodegeneration. Curr Mol Med. 2017;17(6):389–404.
German SV, Bobrovnitsky IP. New aspects of Helicobacter pylori infection: association with metabolic disturbances. Ter Arkh. 2017;89(10):102–7.
Sakitani K, Hirata Y, Suzuki N, Shichijo S, Yanai A, Serizawa T, et al. Gastric cancer diagnosed after Helicobacter pylori eradication in diabetes mellitus patients. BMC Gastroenterol. 2015;15:143.
Vafaeimanesh J, Rajabzadeh R, Ahmadi A, Moshtaghi M, Banikarim S, Hajiebrahimi S, et al. Effect of Helicobacter pylori eradication on glycaemia control in patients with type 2 diabetes mellitus and comparison of two therapeutic regimens. Arab J Gastroenterol. 2013;14(2):55–8.
Baradaran A, Nasri H. Helicobacter pylori specific IgG antibody and serum magnesium in type-2 diabetes mellitus chronic kidney disease patients. Saudi J Kidney Dis Transpl. 2011;22(2):282–5.
Lazaraki G, Kountouras J, Metallidis S, Vrettou E, Alevizos M, Tzioufa V, et al. Endothelial nitric oxide synthase (eNOS) is not upregulated in gastric mucosa of Helicobacter pylori (H. pylori)-positive patients with type 2 diabetes mellitus. Dig Liver Dis. 2009;41(4):253–62.
Aydemir S, Bayraktaroglu T, Sert M, Sokmen C, Atmaca H, Mungan G, et al. The effect of Helicobacter pylori on insulin resistance. Dig Dis Sci. 2005;50(11):2090–3.
Franceschi F, Annalisa T, Teresa DR. Giovanna D’A, Ianiro G, Franco S, et al. Role of Helicobacter pylori infection on nutrition and metabolism. World J Gastroenterol. 2014;20(36):12809–17.
Sun Y, Fu D, Wang YK, Liu M, Liu XD. Prevalence of Helicobacter pylori infection and its association with lipid profiles. Bratisl Lek Listy. 2016;117(9):521–4.
Caplice NM, Panetta C, Peterson TE, Kleppe LS, Mueske CS, Kostner GM, et al. Lipoprotein (a) binds and inactivates tissue factor pathway inhibitor: a novel link between lipoproteins and thrombosis. Blood. 2001;98(10):2980–7.
Acknowledgements
This study was supported by the Scientific Research Innovation Team Project of Anhui Colleges and Universities (2016-40), the University Natural Science Project of Anhui Province (KJ2017A229), the Project of Anhui province university outstanding youth talent support program (gxyq2018037), and the Science and technology development fund project of Bengbu medical college (BYKF1709).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
Our research protocol was approved by ethical committee of Bengbu Medical College, and a written informed consent was signed by each participant according to national and institutional guidelines.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, W., Kashif, M.R., Yuping, Y. et al. Association of Helicobacter pylori infection with metabolic and inflammatory profile in type 2 diabetes mellitus. Int J Diabetes Dev Ctries 40, 47–51 (2020). https://doi.org/10.1007/s13410-019-00757-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-019-00757-8