Abstract
Background
During the past few decades, overweight and obesity have become a global health hazard. The estimation and documentation of obesity are important in countries like India that have a broad diversity of populations. However, there is discrepancy in the various adiposity indicators used to estimate obesity. The present study examines population-specific associations between percent body fat (%BF) and adiposity variables among females in three population groups.
Materials and methods
A cross-sectional study was conducted among Jat, Mizo, and Liangmai communities of India. Data were collected using interview schedules and somatometric measurements based on ISAK protocols. Body density was calculated from skinfold measurements and the Siri equation was used to determine %BF. WHO cut-offs were used for waist circumference (WC), waist-to-hip ratio (WHR), and body mass index (BMI), whereas Ashwell and Gibson and American Council for Exercise cut-offs were used for waist-to-height ratio (WHtR) and %BF, respectively.
Results
Obesity variables are differentially distributed across the three populations. The mean values of %BF and WHtR were the highest among the Liangmai, whereas BMI, WC, and WHR were the highest among the Mizo.
Conclusion
All of the selected adiposity indicators (WC, WHR, WHtR, and BMI) in all three populations were significantly positively correlated with %BF. Thus, %BF should be incorporated with other adiposity indicators as well, for a better understanding and categorisation of obesity among different populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overweight and obesity have considerably become a global health hazard as the prevalence of obesity has increased during the last few decades [1]. It has also become the most frequently diagnosed chronic disease in many countries affecting all age groups. Whenever excessive fat gets accumulated in the body leading to health impairment, the person is said to be suffering from obesity [2]. Obesity is diagnosed on the basis of excess body fat; thus, measuring body fat is important for the diagnosis of obesity and its associated comorbidities [3]. Years ago, it was considered as a problem of high-income countries but now, low- and middle-income countries like India have shown an increase in the prevalence of obesity [4].
Obesity can be characterised by various adiposity indicators, with body mass index (BMI) being most frequently used followed by waist circumference (WC), waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR). But, all of these adiposity indicators have been shown to be of limited use for distinguishing lean mass from fat mass [5]. If BMI is the sole measure for estimation of obesity, individuals with high percent body fat (%BF) and low BMI will escape the detection of obesity-related diseases [6, 7]. Moreover, the prediction of central obesity through WC, WHR, and WHtR using a standard cut-off seems to be less accurate, as differences in body composition exist between different age groups and ethnic groups [8]. Thus, the estimation of %BF has now become an important measure for assessing obesity [9]. Among women, a higher level of essential body fat is observed as compared to men, because sex-characteristic fat is essential for child-bearing. Moreover, the fat deposition and weight gain before, during, and after pregnancy not only affect current pregnancy but also act as primary contributors to the development of obesity among women in later stages of life [10].
Although a number of obesity variables have been studied and reported, identification of feasible, cost-effective, and relatively common variable is needed. The number of deaths due to non-communicable diseases is rapidly increasing; therefore, it is essential to spread awareness among the general population about maintenance of desirable amount of fat in body. Thus, estimation of body fat will not only provide an overall impression of health but will also set a good baseline, and later provide a quantitative number on which a fitness programme can be developed for any population. Specifically in Indian context, where there is a huge diversity at every level, population-based studies associating percent body fat with other adiposity variables using anthropometry are almost negligible. Thus, the present study aims to estimate obesity using %BF and by comparing it with other adiposity indicators (WC, WHR, BMI, WHtR) among tribal (Mizo and Liangmai) and non-tribal (Jat) females of India.
Materials and methods
Study population
A cross-sectional study was conducted across three different communities, namely, the Jat, Mizo, and Liangmai. Data were collected from 1483 females among whom 1070 were from the Jat community residing in the Palwal district of Haryana, 227 were from the Mizo community from the Churachandpur district of Manipur, and 186 were from the Liangmai community from the Tamenglong and Senapati districts of Manipur, India. The age group of the Jat females was 30 to 65 years and the age groups of the Mizo and Liangmai females were 18 to 65 years. The presently studied non-tribal population Jat inhabits the plain area of Haryana, whereas the studied tribal populations Mizo and Liangmai inhabit the hilly terrain of Manipur. Jat has a Eurasian ancestry and speaks language belonging to Indo-European linguistic group. Their major staple food is wheat and they follow vegetarianism. In contrast, Mizo and Liangmai females belonged to East-Asian ancestry and speak languages categorised under Tibeto-Burman linguistic group. Their major staple food is rice and they are non-vegetarians.
The data used in the present study were collected through household survey carried out as part of different projects where genetic analyses were done. Thus, the major criteria for recruitment of participants in this study were that the participants should not be blood related up to at least the first cousin. The study was approved by the Ethical Committee of the Department of Anthropology, University of Delhi, Delhi, India.
Data collection
Data related to general information like age, sex, educational status, lifestyle, occupation, and dietary pattern were collected with the help of pre-tested and modified interview schedules. Eight somatometric measurements were carried out, namely height, body weight, waist circumference, hip circumference, and skinfold at biceps, triceps, and subscapular and supra-spinale locations using standardised protocols established by the International Society for the Advancement of Kinanthropometry (ISAK) [11]. Height was measured in centimetres (cm) using an anthropometric rod and was recorded to the nearest 0.1 cm. Body weight was measured to the nearest 0.1 kilogrammes (kg) using a portable spring balance. Waist circumference (WC) and hip circumference (HC) were taken using a flexible steel tape in centimetres. WC was measured at the level of the narrowest point between the lower costal (10th rib) border and the iliac crest. HC was measured at the level of the greatest posterior protuberance of the buttocks which usually corresponds anteriorly to about the level of the symphysis pubis.
Skinfold thickness was measured in millimetres (mm) using Harpenden skinfold calliper to the nearest of 0.1 mm. Skinfold at biceps and triceps was taken at the place where the mid upper arm circumference has been measured and above the biceps and triceps muscles, respectively, when the arm is hanging freely. Subscapular skinfold was taken at the point immediately below the inferior angle of the scapula and skinfold at supra-spinale was taken above the anterior superior iliac spine on a line to the anterior axillary border and on a diagonal line going downwards and medially at 45°.
Percent body fat and other adiposity parameters
Body density was calculated using the age- and sex-specific Durnin and Womersley equation (1974) [12] and, after that, the Siri equation (1961) [13] was used to determine %BF. The age- and sex-specific equations of the Durnin and Womersley (where D= predicted density of the body (g/ml), and L= log of the total of the 4 skinfolds) and Siri equation are as follows:
-
1.
Age—Less than 17 years
-
2.
Age—17–19 years
-
3.
Age—20–29 years
-
4.
Age—30–39 years
-
5.
Age—40–49 years
-
6.
Age—more than 50 years
Various adiposity indicators like WC, BMI, WHR, and WHtR were calculated. BMI was calculated as weight in kilogrammes divided by height in metres square. Waist-to-hip ratio was calculated as waist circumference divided by hip circumference. Waist-to-height ratio was calculated as waist circumference divided by height. Females with WHR > 0.85, WC ≥ 80 cm, BMI ≥ 27.5 [2, 14], WHtR ≥ 0.5 [15], and %BF ≥ 32.00 were considered to be at risk [16].
Statistical analysis
Statistical analyses were carried out using MS Excel 2010 and SPSS 20. Basic descriptive statistics (mean and standard deviation) were calculated to understand the diversity of various adiposity indicators and somatometric measurements among females in all three population groups. Between-group differences in somatometric variables were tested using one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. Pearson’s correlation coefficients (r) were calculated to determine the relation of %BF with BMI, WC, WHR, and WHtR. Results with p values < 0.05 were considered significant.
Results
A differential distribution of various adiposity indicators was found, where high WHR was observed in 62.3% (667/1070) Jat females, whereas 52.8% (565/1070) females had central obesity, 48.9% (524/1070) females had high %BF, 37.6% (403/1070) females had high WHtR, and 21.21% (227/1070) females had high BMI. On the other hand, 19.5% (209/1070) Jat females were found to be underweight. In the case of Mizo females, 80.1% (182/227) had high WHR, 73.1% (166/227) had central obesity, 52.8% (120/227) had high WHtR, 45.81% (104/227) had high BMI, and 29.9% (68/227) had high %BF. On the other hand, 3.5% (8/227) females were found to be underweight. Also, among Liangmai, high WHR was observed in 84.9% (158/186) females, whereas 73.1% (136/186) females had central obesity (WC), 63% (119/186) had high WHtR, 53.7% (100/186) had high %BF, and 43.01% (80/186) had high BMI. On the other hand, 1% (2/186) females were found to be underweight (Table 1).
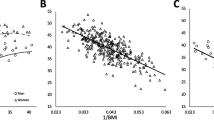
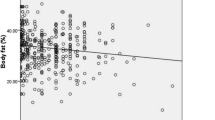
Correlation analyses indicated that all the selected adiposity indicators (BMI, WC, WHR, WHtR) in all three populations demonstrated statistically significant positive correlations with %BF (Table 2).
All the adiposity indicators in all three populations are found to be higher than the normal ranges except for BMI in the Jat population. The mean values of BMI (21.99 kg/m2), WC (81.16 cm), WHR (0.87), and WHtR (0.52) were lowest among Jat females when compared with Mizo and Liangmai females. However, significantly higher mean values of BMI (24.84 kg/m2) and WC (86.61 cm) were found among Mizo females. On the other hand, Liangmai females have comparatively higher statistically significant mean values of %BF (31.73%), WHR (0.92), and all skinfold thicknesses (S-Table 1).
The percentages of Jat females classified by BMI as being normal body weight and as overweight but classified as obese by %BF were 41.3% and 75%, respectively. In Mizo females, 11.1% and 35.7% of those classified by BMI as being normal body weight and as overweight, respectively, were found to be obese by %BF. In Liangmai females, 25% and 66.2% of those classified by BMI as being normal body weight and as overweight, respectively, were found to be obese by %BF. In all the three populations, 89.7%, 50%, and 80.9% of those classified as obese by BMI were also found to be obese by %BF among Jat, Mizo, and Liangmai females, respectively (S-Table 2a).
The percentages of Jat females classified by WC as being normal body weight and at risk for obesity but classified as obese by %BF were 21.3% and 73.6%, respectively. In the case of Mizo females, 1.63% and 40.4% of those classified by WC as being normal body weight and at risk for obesity, respectively, were found to be obese by %BF. In Liangmai females, 20% and 66.2% of those classified by WC as being normal body weight and at risk for obesity, respectively, were found to be obese by %BF (S-Table 2b).
The percentages of Jat females classified by WHR as being normal body weight and at risk for obesity but classified as obese by %BF were 15% and 55.8%, respectively. In the case of Mizo females, 5.5% and 32.1% of those classified by WHR as being normal body weight and at risk for obesity, respectively, were found to be obese by %BF. In Liangmai females, 25.1% and 58.6% of those classified by WHR as being normal body weight and at risk for obesity, respectively, were found to be obese by %BF (S-Table 2c).
The percentages of Jat females classified by WHtR as being normal body weight and at morbidly high risk for obesity but classified as obese by %BF were 19.1% and 86.4%, respectively. In the case of Mizo females, 53.5% of those classified by WHR as being at morbidly high risk for obesity were found to be obese by %BF. In Liangmai females, 14.8% and 83.3% of those classified by WHtR as being normal body weight and at morbidly high risk for obesity, respectively, were found to be obese by %BF (S-Table 2d).
Discussion
The identification of true obese individuals in a population is highly essential for the detection of obesity-related diseases, specifically in countries like India where the levels of obesity have increased extensively in the past few years [17]. The results of the present study showed a significant difference in estimates of body composition among all three populations. Even though a modified criterion for defining overweight and obesity in the Indian population was used, it does not always indicate the degree of obesity. According to this criterion, all the adiposity indicators in all three populations were found to be higher than the prescribed normal ranges [2, 15] except for BMI in the Jat population (S-Table 1 and Table 1). This seems to indicate a burden of obesity in the three selected populations in terms of various adiposity indicators. However, the mean %BF was in normal range in all the populations (S-Table 1).
Generally, obesity has been measured using various adiposity indicators like BMI, WC, WHR, and WHtR, but until now, no common consensus has been derived on the best predictor of obesity. Earlier, BMI was considered to be a better predictor of obesity and its associated risk factors; then later on, evidence suggested a greater association of WC and WHR with obesity [18] and metabolic syndromes in comparison with BMI [19,20,21,22]. Few recent studies have also revealed WHtR to be a better predictor of obesity and metabolic risk compared with other adiposity indicators [15, 23,24,25]. The reason being, height of an individual influences the distribution of body fat, and so, by keeping waist circumference to less than half of height, the body fat will get distributed evenly. However, there is an inconsistency in the use of these adiposity indicators for predicting the levels of obesity and its related disorders, in terms of different ethnicities [26].
Further, the estimation of general and central obesity through BMI, WC, and WHtR does not take body fat distribution into account. All these adiposity indicators poorly distinguish between total body fat, total body lean mass, and bone mass [27]. The existing literature also suggests %BF to be a relatively better measure of excess adiposity and obesity [28,29,30]. Also, individuals with high %BF will tend to escape from being screened for the risk of obesity and its related diseases even if other adiposity variables are found to be normal. The similar type of discrepancy has been found in our results, where 41.3%, 11.1%, and 25% females classified earlier as normal by BMI were found to be obese by %BF among Jat, Mizo, and Liangmai, respectively (S-Table 2a). Thus, these BMI-classified normal females would be at higher risk for obesity-related diseases, if %BF was not measured simultaneously. Moreover, the prediction of obesity using %BF among the obese individuals by BMI is different in different populations, as 89.7% and 80.9% females were identified as obese by both BMI and %BF among Jat and Liangmai, respectively. On the other hand, only 50% females were identified as obese by both BMI and %BF among Mizo.
Another interesting finding of the present study is that, in the case of Jat females who were classified as underweight by BMI, 8.6% turned out to be obese when their %BF was measured. Similar results have been found for other adiposity indicators (WC, WHR, and WHtR) where females have been misclassified as normal according to these adiposity indicators (S-Table 2b, c, and d). These results are in accordance with the existing literature that suggests individuals with high %BF and normal BMI are more likely to develop metabolic syndrome [31] and cardiovascular diseases [32,33,34]. Also, various previous studies reported that increased cardiovascular risk is associated with high %BF regardless of BMI which may lead to underestimation of participants with CVD risk factors [35, 36].
Thus, for assessing obesity among populations, BMI and other adiposity indicators like WC, WHR, and WHtR alone could not be considered as sole indicators [37, 38], as individuals get masked from the screening of underlying excess adiposity characterised by an increased percentage of fat mass and reduced muscle mass. Rather, percent body fat using appropriate formula along with other adiposity indicators should be considered to capture and categorise obesity in populations [39, 40].
Conclusion
The study concludes that %BF measurement should be incorporated with other adiposity variables in order to reduce the discrepancy in the classification of true obese individuals and for a better understanding of obesity-related diseases. However, the results of the present study need to be validated with larger sample size including males.
References
Hurt RT, Kulisek C, Buchanan LA, McClave SA. The obesity epidemic: challenges, health initiatives, and implications for gastroenterologists. Gastroenterol Hepatol. 2010;6(12):780–92.
World Health Organization, Western Pacific Region, International Association for the Study of Obesity, International Obesity Task Force. Asia Pacific Perspective: Redefining obesity and its treatment. Australia: Health Communications Australia; 2000.
Luciano A, Livieri C, Di Pietro ME, Bergamaschi G, Maffeis C. Definition of obesity in childhood: criteria and limits. Minerva Pediatr. 2003;55(5):453–9.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81.
Meeuwsen S, Horgan GW, Elia M. The relationship between BMI and percent body fat, measured by bioelectrical impedance, in a large adult sample is curvilinear and influenced by age and sex. Clin Nutr. 2010;29(5):560–6.
Wilson HJ, Dickinson F, Griffiths PL, Azcorra H, Bogin B, InÊS Varela-Silva M. How useful is BMI in predicting adiposity indicators in a sample of Maya children and women with high levels of stunting? Am J Hum Biol. 2011;23(6):780–9.
Padwal R, Leslie WD, Lix LM, Majumdar SR. Relationship among body fat percentage, body mass index, and all-cause mortality: a cohort study. Ann Intern Med. 2016;164(8):532–41.
Favano A, WHO. Obesity: preventing and controlling the global epidemic. In: WHO consultancy report. São Paulo: Roca; 2004.
Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, et al. Health consequences of obesity. Arch Dis Child. 2003;88(9):748–52.
Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin. 2009;36(2):317–32.
ISAK. International Standards for Anthropometric Assessment. Underdale, S.A: International Society for the Advancement of Kinanthropometry; 2001.
Durnin JV, Womersley JV. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32(1):77–97.
Siri WE. Body composition from fluid spaces and density: analysis of methods. In: Brozek J, Henschel A, editors. Techniques for measuring body composition. Washington, DC: National Academy of Sciences; 1961. p. 223–44.
Kesavachandran CN, Bihari V, Mathur N. The normal range of body mass index with high body fat percentage among male residents of Lucknow city in north India. Indian J Med Res. 2012;135(1):72.
Ashwell M, Gibson S. Waist to height ratio is a simple and effective obesity screening tool for cardiovascular risk factors: analysis of data from the British National Diet and Nutrition Survey of adults aged 19-64 years. Obes Facts. 2009;2:97–103.
Muth ND. What are the guidelines for percentage of body fat loss? ACE Blog 2009 December 2. In ACE blog California. Available: https://www.acefitness.org/acefit/healthy-living-article/60/112/what-are-theguidelines-for-percentage-of/. Accessed 9 Oct 2017.
Pradeepa R, Anjana RM, Joshi SR, Bhansali A, Deepa M, Joshi PP, Dhandania VK, Madhu SV, Rao PV, Geetha L, Subashini R. Prevalence of generalized & abdominal obesity in urban & rural India-the ICMR-INDIAB Study (Phase-I)[ICMR-INDIAB-3]. Indian J Med Res 2015;142(2):139, 150.
Song X, Jousilahti P, Stehouwer CD, Söderberg S, Onat A, Laatikainen T, et al. Comparison of various surrogate obesity indicators as predictors of cardiovascular mortality in four European populations. Eur J Clin Nutr. 2013;67(12):1298–302.
Brundavani V, Murthy S, Kurpad A. Estimation of deep-abdominal-adipose-tissue (DAAT) accumulation from simple anthropometric measurements in Indian men and women. Eur J Clin Nutr. 2006;60:658–66.
Wang F, Wu S, Song Y, Tang X, Marshall R, Liang M, et al. Waist circumference, body mass index and waist to hip ratio for prediction of the metabolic syndrome in Chinese. Nutr Metab Cardiovasc Dis. 2009;19(8):542–7.
Park SH, Choi SJ, Lee KS, Park HY. Waist circumference and waist-to-height ratio as predictors of cardiovascular disease risk in Korean adults. Circ J. 2009;73(9):1643–50.
Gharipour M, Sarrafzadegan N, Sadeghi M, Andalib E, Talaie M, Shafie D, et al. Predictors of metabolic syndrome in the Iranian population: waist circumference, body mass index, or waist to hip ratio? Cholesterol. 2013;2013:1–6. https://doi.org/10.1155/2013/198384.
Hsieh SD, Muto T, Yoshinaga H, Tsuji H, Arimoto S, Miyagawa M, et al. Waist-to-height ratio, a simple and effective predictor for metabolic risk in Japanese men and women. InInt Congr Ser. 2006;1294:186–9.
Rajput R, Rajput M, Bairwa M, Singh J, Saini O, Shankar V. Waist height ratio: a universal screening tool for prediction of metabolic syndrome in urban and rural population of Haryana. Indian J Endocrinol Metab. 2014;18(3):394–9.
Verma M, Rajput M, Sahoo SS, Kaur N, Rohilla R. Correlation between the percentage of body fat and surrogate indices of obesity among adult population in rural block of Haryana. J Fam Med Prim Care. 2016;5(1):154–9.
Nice P. Assessing body mass index and waist circumference thresholds for intervening to prevent ill health and premature death among adults from black, Asian and other minority ethnic groups in the UK. NICE July. 2013;46.
Goonasegaran AR, Nabila FN, Shuhada NS. Comparison of the effectiveness of body mass index and body fat percentage in defining body composition. Singap Med J. 2012;53(6):403–8.
Ablove T, Binkley N, Leadley S, Shelton J, Ablove R. Body mass index continues to accurately predict percent body fat as women age despite changes in muscle mass and height. Menopause. 2015;22(7):727–30.
Ho-Pham LT, Lai TQ, Nguyen MT, Nguyen TV. Relationship between body mass index and percent body fat in Vietnamese: implications for the diagnosis of obesity. PLoS One 2015;27;10(5):e0127198.
González-Agüero A, Matute-Llorente Á, Gómez-Cabello A, Vicente-Rodríguez G, Casajús JA. Percentage of body fat in adolescents with Down syndrome: estimation from skinfolds. Disabil Health J. 2017;10(1):100–4.
Ramírez-Vélez R, Correa-Bautista JE, Sanders-Tordecilla A, Ojeda-Pardo ML, Cobo-Mejía EA, Castellanos-Vega RDP, et al. Percentage of body fat and fat mass index as a screening tool for metabolic syndrome prediction in Colombian university students. Nutrients. 2017;9(9):1009.
Oreopoulos A, Ezekowitz JA, McAlister FA, Kalantar-Zadeh K, Fonarow GC, Norris CM, et al. 2010. Association between direct measures of body composition and prognostic factors in chronic heart failure. In Mayo Clin Proc. 2010;85(7):609–17.
Gómez-Ambrosi J, Silva C, Galofré JC, Escalada J, Santos S, Gil MJ, et al. Body adiposity and type 2 diabetes: increased risk with a high body fat percentage even having a normal BMI. Obesity (Silver Spring). 2011;19(7):1439–44.
Shea JL, King MT, Yi Y, Gulliver W, Sun G. Body fat percentage is associated with cardiometabolic dysregulation in BMI-defined normal weight subjects. NutrMetab Cardiovasc Dis. 2012;22(9):741–7.
Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: the Heritage Family Study. Int J Obes. 2002;26(6):789–96.
Gómez-Ambrosi J, Silva C, Galofré JC, Escalada J, Santos S, Millán D, et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes. 2012;36(2):286–94.
Etchison WC, Bloodgood EA, Minton CP, Thompson NJ, Collins MA, Hunter SC, et al. Body mass index and percentage of body fat as indicators for obesity in an adolescent athletic population. Sports Health. 2011;3(3):249–52.
Zeng Q, Dong SY, Sun XN, Xie J, Cui Y. Percent body fat is a better predictor of cardiovascular risk factors than body mass index. Braz J Med Biol Res. 2012;45(7):591–600.
Peixoto MD, Benício MH, Latorre MD, Jardim PC. Waist circumference and body mass index as predictors of hypertension. Arq Bras Cardiol. 2006;87(4):462–70.
Tanamas SK, Lean ME, Combet E, Vlassopoulos A, Zimmet PZ, Peeters A. Changing guards: time to move beyond body mass index for population monitoring of excess adiposity. QJM. 2015;109(7):443–6.
Acknowledgements
The authors are grateful to the Department of Biotechnology, Ministry of Science and Technology and Research, and Development Grant, 2014–2015, University of Delhi, for providing grants to carry out this study and all the participants of Jat, Mizo, and Liangmai populations for their generous participation in this study.
Grant sponsorship
This study was sponsored by Research and Development Grant, 2014–2015, University of Delhi, and Department of Biotechnology, Ministry of Science and Technology.
Author information
Authors and Affiliations
Contributions
Shivani Chandel, Monika Kulshreshtha, and Sukhmani Kaur analysed the data and drafted the manuscript. Kallur Nava Saraswathy and Naorem Kiranmala Devi designed the study and directed implementation and data collection. Somorjit Singh Ningombam, Masan Kambo Newmei, and Varhlun Chhungi collected the data and Suniti Yadav provided necessary logistic support. Shivani Chandel, Monika Kulshreshtha, Sukhmani Kaur, and Kallur Nava Saraswathy edited the manuscript for intellectual content and provided critical comments on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
An informed written consent was obtained from all the recruited participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chandel, S., Kulshreshtha, M., Kaur, S. et al. Percent body fat and adiposity indicators: a study among tribal and non-tribal females of India. Int J Diabetes Dev Ctries 39, 715–720 (2019). https://doi.org/10.1007/s13410-019-00733-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-019-00733-2