Abstract
The frequent clinical use of bioelectrical impedance analysis (BIA) devices has made clinical evaluations of BIA-based parameters mandatory. This study was performed to define new cutoff points for the fat mass index, fat-free mass index, and percent body fat for overweight and obese men living in Riyadh, KSA. Three hundred sixty-two male subjects aged 18 to 62 years were enrolled in this cross-sectional study. The participants were divided into two groups; one group was assessed with an InBody 720 (n = 179) device, and the other group was assessed with a Tanita BC-418 (n = 183) device. Fat mass, fat mass index (FMI), fat-free mass, fat-free mass index (FFMI), and percent body fat (PBF) were measured. In addition, anthropometric measures, including weight, height, body mass index (BMI), waist circumference, hip circumference, waist hip ratio, and mid-arm circumference were included. Pearson correlation coefficients, Kappa analyses, and ROC curves were used. FMI exhibited the strongest positive correlation with BMI among all of the measured BIA-based parameters in both the InBody and Tanita groups (r = 0.916 and 0.958, respectively, P < 0.00001). ROC curves indicated that FMI was the most accurate parameter for the diagnosis of obesity in both groups (AUCs = 0.970 and 0.980). The FMI, FFMI, and PBF cutoff values with the best sensitivities and specificities in both groups were 7.8 vs. 6.7 kg/m2, 19.2 vs. 20.7 kg/m2, and 27.8 vs. 24 %, respectively, based on the WHO criteria for the diagnosis of obesity in Asian population. FMI was the best predictor of obesity among all of the BIA-based parameters. Considerable differences were noted between the different machines. Knowledge of device-specific cutoff points would increase the clinical value of BIA in the diagnosis of obesity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obesity is a highly prevalent metabolic disorder that has reached pandemic proportions. Obesity affects approximately 300 million people around the world and is accompanied by increased mortality and reduced life expectancy [1]. Obesity is diagnosed based on excess body fat; therefore, measuring body fat is essential for the diagnosis of obesity and the assessment of its comorbidities [2].
The traditional methods for the measurement of body fat typically range from simple measures, such as waist circumference (WC), waist-to-hip ratio (WHR), body mass index (BMI), and subcutaneous skinfold thickness, to more complex methods, such as impedancemetry and dual-energy X-ray absorptiometry (DXA) [3]. The BMI is the most widely used tool for the diagnose and assessment of the degree of obesity within populations. This measure is a reliable and clinically valid method [4]. Furthermore, many studies have shown that higher BMIs are associated with increased risks of metabolic derangement-related diseases and that the BMI may be used as a predictor of these diseases [5]. However, many studies have observed that the BMI generally has low sensitivity for the diagnosis of obesity [6], while other studies have demonstrated that the BMI is most inaccurate in the diagnosis of obesity within intermediate BMI ranges because it cannot discriminate between body fat and lean mass [7, 8]. Additionally, there are many effects of ethnicity, gender, and age on the accuracy of the BMI for the detection of body adiposity [9].
Bioelectrical impedance analysis (BIA) is a relatively simple, safe, quick, and noninvasive method of body composition assessment. BIA is reliable, easy to perform and is widely used in clinical practice. According to the ESPEN guidelines, BIA is suggested to function well in healthy subjects and in patients with stable water and electrolyte balances and a validated BIA equation that accounts for age, sex, and race is available [10]. Fat-free mass (FFM), percent body fat (PBF), body cell mass (BCM), total body water (TBW), extracellular water (ECW), intracellular water (ICW), visceral fate area (VFA), and other parameters of body composition can be estimated with BIA devices via the use of many appropriate population-, age-, and pathology-specific BIA equations and established procedures [11]. Furthermore, it has been shown that PBF values calculated via BIA are significantly closer to the values obtained from DXA and hydrostatic weighing (HW); therefore, there is good agreement between BIA and DXA measures [12]. The clinical values of many BIA-based parameters, such as fat mass index (FMI), fat-free mass index (FFMI), and percent body fat (PBF), and their correlations with anthropometric measures such as BMI, WC, and WHR are under investigation. We performed the present study to define new cutoff points for FMI, FFMI, and PBF values for use as references for obesity diagnoses based on a male population that visited the weight reduction clinic in King Saud University, Riyadh, KSA.
Methods
Study population
All subjects were visitors or cases of the weight reduction clinic of the College of Applied Medical Sciences (CAMS), male sector, King Saud University. Three hundred sixty-two male subjects aged 18 to 62 years were enrolled in a cross-sectional study. Participants were divided into two groups: InBody group (n = 179) that were assessed with an InBody 720 device or a Tanita group (n = 183) that were assessed via the Tanita BC-418. Subjects with edema, dehydration, metal implants, having pacemakers, cancer, severe disabilities, and severe psychiatric disturbances were excluded. Informed consent was obtained from each participant before enrollment. The CAMS research ethics committee approved the study protocol.
Demographic variables
Socioeconomic status questionnaires that accounted for variables such as ethnicity, Saudi and non-Saudi status, marital status, and annual income were completed by the participants.
Anthropometric measures
A clinician or a trained assistant measured the weight, height, waist circumference (WC), hip circumference (HC), and mid-arm circumference (MAC) of the participants. The body weights and heights were measured using a Seca digital scale with a non-stretchable stadiometer (Seca Co., Germany). BMI was calculated as the body weight in kilograms divided by the square of the height in meters. The cutoff points of BMI in this study were based on the WHO international criteria for all populations (>30 kg/m2) [13] and WHO criteria for Asian populations with suggested public health actions (>27.5 kg/m2) [14]. WC was determined by measuring the waist diameter at the midpoint between the iliac crest and the lower border of the tenth rib. The average of two measurements was taken as the WC. The HC was assessed in the lateral position by measuring the circumference at the most prominent point, and the average of two measurements was also used as the HC. Waist hip ratio (WHR) was calculated by dividing WC on HC. Because of the local culture, WC, HC, and MAC were measured on wearing one layer of clothes, but weight and height were measured with no shoes.
Bioelectrical impedance analysis
The BIA analyses were done using InBody 720 (InBody, Biospace, Korea) and Tanita BC-418 (Tanita Corporation, Japan) devices. The subject was asked to first wipe the soles of his feet and the palms of his hands with an electrolyte tissue, and then to stand over the foot electrodes of the machine. The participant’s data were entered, and then he gently asked to handle the hand electrodes. The parameters recorded with the InBody 720 included body weight, BMI (height was manually entered), degree of obesity, protein mass, mineral mass, total body water, intracellular and extracellular water, skeletal muscle mass, fat-free mass (FFM), fat mass (FM), percent body fat (PBF), visceral fat area (VFA), and fitness score based on the target values for the ideal body fitness. In contrast, the Tanita BC-418 device was used to measurethe body weight, BMI, total body water, fat-free mass (FFM), fat mass (FM), percent body fat (PBF), and visceral fat rating. Furthermore, both devices calculated the regional fat and muscle distributions in the trunk and extremities. The BIA devices emit many frequencies of electric current (InBody 720 emits 1, 5, 50, 250, 500 kHz, and 1 MHz, while Tanita emits 50 and 500 kHz). This multifrequency technology, particularly at the 5, 50, and 250 kHz frequencies, was used to more accurately to measure the resistance, reactance, and components of body impedance that were then used to accurately calculate body water, FM, FFM, etc. The fat mass index (FMI) was calculated by dividing the FM in kilogram by the square of the height in meters, and the results are expressed in kg/m2. The fat-free mass index was calculated in the same manner (FFM (kg)/wt2 (m2)) [15].
Statistical analysis
The statistical analyses were performed using SPSS for Windows (version 22; SPSS Inc., Chicago, IL, USA). The data were summarized with the means, standard deviations (SD), and the ranges. Mann-Whitney test was used to differentiate between the ethnic groups. Pearson’s correlation coefficient was used to examine the correlations between the study parameters. P ≤ 0.05 was considered statistically significant. Kappa analyses were used to study the agreements of the BMI with the FMI and PBF values with the 95 % confidence intervals. ROC curves were used to detect obesity and to identify new cutoff points with higher sensitivities (true positive rates) and specificities (true negative rates) for the FMI, FFMI and PBF.
Results
All descriptive characteristics and ethnic comparisons of all study populations are shown in Table 1 in form of the means ± SDs and the ranges of the measures. There were no significance ethnic differences between the Saudi and non-Saudi males based on Mann-Whitney test. Table 2 shows the correlation matrix with the Pearson correlation coefficients of the measured variables.
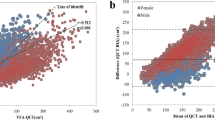
The Pearson correlations showed that the FMI as measured with the InBody device exhibited a strong positive correlation with BMI (r = 0.916, P < 0.00001, Fig. 1a). The correlation was also strong between PBF and BMI, but it was significantly less than that with FMI (r = 0.752, P < 0.00001, Fig. 1b). Regarding the Tanita measurements the Pearson correlation coefficient was higher between the FMI and BMI than between the PBF and BMI (r = 0.958 vs. 0.896, respectively, P < 0.00001, Fig. 2). In contrast, the FFMI was moderately correlated with the BMI in both the InBody and Tanita groups (r = 0.672 and 0.876, respectively, P < 0.00001, Fig. 3).
Kappa analyses revealed that the BMI was more strongly related to the FMI than the other InBody-related parameters (k = 0.560, P < 0.00001), and the kappa value that resulted from the BMI according to the Asian cutoff value (27.5 kg/m2) was greater than that resulting from the international cutoff value (k = 0.671 vs. k = 0.560, P < 0.00001). Furthermore, the kappa analyses demonstrated that the agreement of the BMI with the and PBF was lower than that with the FMI (k = 0.474 vs. k = 0.560 when using the BMI cutoff value of 30 kg/m2 and k = 0.563 vs. k = 0.671 when using BMI cutoff value of 27.5 kg/m2, P < 0.00001).
The application of the WHO criteria for Asian populations (BMI ≥ 27.5) to the participants in InBody group resulted in an area under the ROC curve of 0.970 (Fig. 4a), indicating that the FMI was an accurate classifier of obesity. The FMI cutoff value with the best sensitivity and specificity (96.4 and 81.8 %, respectively) was 7.83 kg/m2. The other main parameters, including FFMI and PBF, could also be used as classifiers of obesity. The AUCs of the FFMI and PBF ROC curves were much lower than that of the FMI (0.916 and 0.929, respectively, Fig. 4a). The cutoff value for the FFMI with 87.6 % sensitivity and 81.8 % specificity was 19.19 kg/m2, and the cutoff value for the PBF with 94.2 % sensitivity and 77.3 % specificity was 27.80 %. The conditions were slightly different when the WHO international criteria (BMI ≥30) were applied. The areas under the FMI, FFMI, and PBF curves were 0.972, 0.844, and 0.931, respectively (Fig. 4b). The FMI cutoff value with the best sensitivity and specificity (95.1 and 94.4 %, respectively) was 9.77 kg/m2. The FFMI cutoff value with 80.5 % sensitivity and 77.8 % specificity was 19.66 kg/m2, and the cutoff value of the PBF with 87 % sensitivity and 83.3 % specificity was 32.85 %.
ROC curve showing sensitivity and specificity of FMI, FFMI, and PBF values that were measured by InBody device, at different cutoff points, in addition to area under the curves (AUC); arrows are pointing to suggested cutoff values of the best sensitivity and specificity; a using BMI criteria for Asia i.e., ≥27.5, b using BMI criteria for international population i.e., ≥30
Figure 5a shows the ROC curves for the FMI, FFMI, and PBF of the Tanita group based on the WHO criteria for Asian men. The AUCs were 0.980, 0.945, and 0.959, respectively. FMI cutoff value with the best sensitivity and specificity (93.2 and 92.3 %, respectively) was approximately 6.70 kg/m2. The FFMI cutoff value with 89.8 % sensitivity and 86.2 % specificity was 20.75 kg/m2, and the cutoff value of the PBF with 90.7 % sensitivity and 89.2 % specificity was 24.05 %. The application of the international criteria for the diagnosis of obesity resulted in cutoff values for the FMI, FFMI, and PBF of 8.09, 21.53, and 26.95, respectively (Fig. 5b). These suggested values resulted in the best sensitivities and specificities (94.4 & 89.2, 90 & 86, and 90 & 86 %, respectively). The AUCs of the three parameters were 0.974, 0.926, and 0.945, respectively.
ROC curve showing sensitivity and specificity of FMI, FFMI, and PBF values that were measured by Tanita device at different cutoff points, in addition to area under the curves (AUC); arrows are pointing to suggested cutoff values of the best sensitivity and specificity; a using BMI criteria for Asia i.e., ≥27.5, b using BMI criteria for international population i.e., ≥30
Discussion
Bioelectric impedance analysis (BIA) devices are commonly used for obesity assessment and in many specialities of clinical practice. BIA-based parameters are frequently used and some of them are valuable for assessment of body composition particularly in the fields of obesity and metabolism. In present clinical practice, BMI is typically considered a surrogate marker of excess adiposity in terms of overweight and obesity. People with the same BMIs may have very different body compositions, which may result in considerable variability in the susceptibilities for different metabolic comorbidities of obesity. Therefore, it is better to measure and express body composition parameters such as the FMI and FFMI than the crude BMI [16]. Indeed, the ideal alternative should actually represent the fatness of the body.
This study suggested the FMI as a good alternative measure for the diagnosis of obesity due to its higher sensitivity and specificity for obesity diagnose. The FMI exhibited a strong positive correlation with the BMI. Mathematically, the BMI equal the FMI plus FFMI, and pathologically excess body adiposity is the main finding in all obesity phenotypes including sarcopenic obesity [17]. Therefore, the FMI should be considered a strong alternative to the BMI. Furthermore, the kappa analyses revealed that the agreement between the BMI and FMI was higher than that of the BMI with the PBF; thus, it is logical to use the FMI rather than the BMI as a diagnostic parameter for obesity.
These findings are in line with those of Schutz et al. [15] who created reference percentiles for FFMI and FMI values and stated that these percentiles could be of practical value, especially for the clinical evaluation of sarcopenic obesity, by complementing the classical concept of BMI in a more qualitative manner. Furthermore, Kang et al. [18] found that FMI and PBF are also useful parameters for studies of the relationship between osteoporosis and obesity. In contrast, Ribeiro et al. [19] preferred the use of the BMI, WC, and waist-to-height ratio as diagnostic tests for the identification of excess body fat in children from the ages of seven to ten years. Another recent study of schoolchildren concluded that BMI is a more reliable indicator of body fat percentage, which predicts adiposity in children better than the body adiposity index [20]. This discrepancy may be due to the different ages of the study populations. Moreover, Habib [21] used PBF and BMI as references for the assessment of obesity and its prevalence in an adult sample.
This study suggested new cutoff values of the FMI as well as the FFMI and PBF based on data from two different BIA machines. For example, the ROC curve analysis revealed that the FMI cutoff value with the best sensitivity and specificity was 7.83 kg/m2 based on definition of obesity at the BMI = 27.5 kg/m2. This finding was based on the measurements from the InBody device, and the results from the Tanita device produced a different cutoff value of 6.7 kg/m2. The FMI cutoff value has been defined previously in an Indian sample at 6.6 kg/m2 for men in the prediction of 25 % body fat. In that study, Rao et al. calculated the fat mass using skinfold measurements [22]. Another FMI cutoff point was defined by Schutz et al. [15]; these authors calculated the FM with BIA method and found that the 95th percentile for the FMI was 7.0 kg/m2 in Caucasian young adult males and progressively increased with age (by 2 units). Additionally, Liu et al. [23] also reported the same 7 kg/m2 value, and considered this value to be an independent screening factor for metabolic syndrome in Korean men.
The FFMI cutoff values suggested in the present study are slightly different from the reference values determined by Franssen et al. [24] who derived the FFMI values in a prospective analysis of 186,975 individuals from the national UK Biobank study (45.9 % men; age 56.9 ± 6.8 years; BMI 26.5 ± 3.6 kg/m2) to be 18.3 ± 2.4 kg/m2 (mean ± SD).In our study, the mean ± SD for the FFMI in the Tanita group was 21.60 ± 2.71 kg/m2. Despite the use of a similar device (Tanita BC-418MA), these authors did not use ROC curves to test the suggested reference value of the FFMI. Different statistical methodology and sample sizes caused this discrepancy. Regarding the PBF, our results are similar to those of a Spanish study that determined that a PBF of 25 % in males corresponded to a BMI of 27.5 kg/m2 [25].
The most important finding of the present study was that the different BIA machines produced different quantifications of the fat mass that resulted in different cutoff values for the FMI, FFMI, and PBF. Therefore, it is essential to use the same device for frequent monitoring of obese patients and to use device-specific cutoff values for diagnoses and the setting of goals for the management of obesity. All BIA devices use the same principle but variations in current frequencies and the number of measuring electrodes lead to variable impedance and reactance measurements. This supposition is in line with the results of Ramsey et al. [26] who compared three BIA devices (i.e., a foot-to-foot Tanita 300-GS, a hand-to-foot Impedimed DF50, and a bioelectrical impedance spectroscopy Impedimed SFB7) in the measurement of fat-free mass. The results differed significantly from those measured with the foot-to-foot and hand-to-foot BIAs. Moreover, Pateyjohns et al. [27] compared some BIA-related parameters (i.e., PBF, fat mass, and fat-free mass) using three different devices against DXA in overweight and obese men and concluded that the Tanita device, in contrast to the other two, demonstrated little bias and may be useful for group comparisons but that the utility of this device for the assessment of the body composition of individuals is limited.
Finally, this study has several limitations. First, no female subjects were included due to the limited access to female cases in Saudi Arabia. Second, there were no comparisons of the BIA-based results with those from CT, MRI, DXA or air-displacement plethysmography; however, the BIA accuracy and validity of BIA measures have been well demonstrated in many studies that have investigated various ages, genders, and pathologies [28–32]. Third, the samples were not taken from different regions of SA. Despite these limitations, we can conclude based on the results of this study that the FMI seems to be a better indicator than the BMI for use in the screening for the presence of obesity in men living in Riyadh, KSA. Moreover, we have suggested new local cutoff FMI, FFMI, and PBF values for body composition assessment and obesity diagnosis that could be used as a guide for practitioners in obesity medicine. Future studies with larger sample sizes that include many regions of the kingdom and both males and females are needed to further clinically validate these results.
References
Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA. 2003;289:187–93.
Luciano A, Livieri C, Di Pietro ME, Bergamaschi G, Maffeis C. Definition of obesity in childhood: criteria and limits. Minerva Pediatr. 2003;55(5):453–9.
Zeng Q, Dong SY, Sun XN, Xie J, Cui Y. Percent body fat is a better predictor of cardiovascular risk factors thanbody mass index. Braz J Med Biol Res. 2012;45:591–600.
Carpenter CL, Yan E, Chen S, Hong K, Arechiga A, Kim WS, et al. Body fat and body-mass index among a multi-ethnic sample of college-age men and women. J Obes Hindawi Publ Corp. 2013;790654:1–7.
Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57prospective studies. Lancet. 2009;373:1083–96.
Evans EM, Rowe DA, Racette SB, Ross KM, McAuley E. Is the current BMI obesity classification appropriate for black and white postmenopausal women? IntJ Obes. 2006;30(5):837–43.
Frankenfield DC, Rowe WA, Cooney RN, Smith JS, Becker D. Limits of body mass index to detect obesity and predictbody composition. Nutrition. 2001;17(1):26–30.
Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond). 2008;32(6):959–66.
Chang CJ, Wu CH, Chang CS, et al. Low body mass index but high percent body fat in Taiwanese subjects: implications of obesity cutoffs. Int J Obes (Lond). 2003;27(2):253–9.
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, et al. ESPEN. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23(6):1430–53.
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, et al. ESPEN. Bioelectrical impedance analysis-part I: review of principles and methods. Clin Nutr. 2004;23(5):1226–43.
Gupta N, Balasekaran G, Victor Govindaswamy V, et al. Comparison of body composition with bioelectric impedance (BIA) and dual energy X-ray absorptiometry (DEXA) among Singapore Chinese. J Sci Med Sport. 2011;14(1):33–5.
WHO Expert Committee on Physical Status. Physical status: the use and interpretation of anthropometry. WHO Tech Rep Ser. 1995;854:1–416.
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and interventionstrategies. Lancet. 2004;363:157–63.
Schutz Y, Kyle UUG, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 years. Int J Obes. 2002;26:953–60.
Wang J, Rennie KL, Gu W, Li H, Yu Z, Lin X. Independent associations of body-size adjusted fat mass and fat-free mass with the metabolic syndrome in Chinese. Ann Hum Biol. 2009;36:110–21.
Prado CM, Siervo M, Mire E, Heymsfield SB, Stephan BC, Broyles S, et al. A population-based approach to define body-composition phenotypes. Am J Clin Nutr. 2014;99(6):1369–77.
Kang DH, Guo LF, Guo T, Wang Y, Liu T, Feng XY, Che XQ. Association of body composition with bone mineral density in northern Chinese men by different criteria for obesity. J Endocrinol Investig. 2014. [Epub ahead of print].
Ribeiro EA, Leall DP, Assis MA. Diagnostic accuracy of anthropometricindices in predicting excess body fat among 7 to 10-year-old children. Rev Bras Epidemiol. 2014;243–254.
Zhao D, Zhang Y. Body mass index (BMI) predicts percent body fat better than body adiposity index (BAI) in school children. Anthropol Anz. 2015. doi:10.1127/anthranz/2015/0499 [Epub ahead of print].
Habib SS. Body mass index and body fat percentage in assessment of obesity prevalence in Saudi adults. Biomed Environ Sci. 2013;26(2):94–9.
Rao KM, Arlappa N, Radhika MS, Balakrishna N, Laxmaiah A, Brahmam GN. Correlation of fat mass index and fat-free mass index with percentage body fat and their association with hypertension among urban South Indian adult men and women. Ann Hum Biol. 2012;39(1):54–8.
Liu P, Ma F, Lo U, Liu Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Public Health. 2013;13:629.
Franssen FME, Rutten EPA, Groenen MTJ, Vanfleteren LA, Wouters EFM, Spruit MA. New reference values for body composition by bioelectrical impedance analysis in the general population: results from the UK Biobank. JAMDA. 2014;30:e1–6. doi:10.1016/j.jamda.2014.03.012.
Fernández-Real JM, Vayreda M, Casamitjana R, Saez M, Ricart W. Body mass index (BMI) and percent fat mass. A BMI >27.5 kg/m2 could be indicative of obesity in the Spanish population. Med Clin (Barc). 2001;117(18):681–4.
Ramsey R, Isenring E, Daniels L. Comparing measures of fat-free mass in overweight older adults using three different bioelectrical impedance devices and three prediction equations. J Nutr Health Aging. 2012;16(1):26–30.
Pateyjohns IR, Brinkworth GD, Buckley JD, Noakes M, Clifton PM. Comparison of three bioelectrical impedance methods with DXA in overweight and obese men. Obesity (Silver Spring). 2006;14(11):2064–70.
Wibæk R, Kæstel P, Skov SR, Christensen DL, Girma T, Wells JC, et al. Calibration of bioelectrical impedance analysis for body composition assessment in Ethiopian infants using air-displacement plethysmography. Eur J Clin Nutr. 2015. doi:10.1038/ejcn.2015.51 [Epub ahead of print].
Fakhrawi DH, Beeson L, Libanati C, Feleke D, Kim H, Quansah A, et al. Comparison of body composition by bioelectrical impedance and dual-energy X-ray absorptiometry in overweight/obese postmenopausal women. J Clin Densitom. 2009;12(2):238–44.
Boneva-Asiova Z, Boyanov MA. Body composition analysis by leg-to-leg bioelectrical impedance and dual-energy X-ray absorptiometry in non-obese and obese individuals. Diabetes Obes Metab. 2008;10(11):1012–8.
Thomson R, Brinkworth GD, Buckley JD, Noakes M, Clifton PM. Good agreement between bioelectrical impedance and dual-energy X-ray absorptiometry for estimating changes in body composition during weight loss in overweight young women. Clin Nutr. 2007;26(6):771–7.
Lazzer S, Boirie Y, Meyer M, Vermorel M. Which alternative method to dual-energy X-ray absorptiometry for assessing body composition in overweight and obese adolescents? Arch Pediatr. 2005;12(7):1094–101.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saudi University, KSA, for funding the work through this research group project no RGP-VPP-193).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abulmeaty, M.M.A., Almajwal, A.M. & Hassan, H.M.Z. New cutoff values for fat mass index, fat-free mass index and percent body fat in overweight and obese men living in Riyadh, SA. Int J Diabetes Dev Ctries 36, 204–212 (2016). https://doi.org/10.1007/s13410-015-0402-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-015-0402-4