Abstract
The aim of this study was to describe the efficacy and safety of two insulin intensification strategies in patients recruited in India with type 2 diabetes mellitus inadequately controlled on basal insulin glargine with metformin and/or pioglitazone. This multinational, open-label, randomized, parallel-arm, noninferiority, phase IV clinical trial evaluated insulin lispro low mixture (LM25) and basal insulin glargine administered with prandial insulin lispro (IGL) for 24 weeks. Patients were male and female, aged ≥18 to ≤75 years, with screening glycosylated hemoglobin (HbA1c) concentration ≥7.5 to ≤10.5 % and fasting plasma glucose ≤121 mg/dL. The primary efficacy end point was change in HbA1c from baseline to 24 weeks of treatment. Secondary efficacy end points included change in HbA1c from baseline to 12 weeks and change in fasting blood glucose (FBG) from baseline to 12 and 24 weeks. Safety and tolerability were measured by treatment-emergent adverse events and the incidence, rate, and severity of hypoglycemic episodes. Of 81 patients randomized to LM25 (n = 40) or IGL (n = 41), 80 patients completed the trial and one patient discontinued due to subject decision. Mean (SD) change in HbA1c from baseline to week 24 was −1.2 % (1.11) for the LM25 group and −1.0 % (1.18) for the IGL group. Safety profile, mean (FBG), glycemic variability, hypoglycemic episodes per patient-year, and health outcome measures were numerically similar between the two groups. The results of this post hoc analysis in an Indian subpopulation were consistent with results reported for the trial-level population and provide information to the consideration of LM25 as treatment option for intensification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterized by progressive decline of insulin secretion by pancreatic β cells [1]. The International Diabetes Federation estimates that by 2035, the prevalence of diabetes will increase from 382 million to 592 million people, many of whom will reside in low and middle income countries and will be younger than 60 years [2]. The prevalence of diabetes is more than 65 million in India and is likely to reach 79.4 million by 2030 [2, 3]. T2DM is more predominant in India, where one of three patients with T2DM is overweight or obese [4]. The increase in prevalence in India is mainly due to urbanization, sedentary lifestyle, and rising prevalence of obesity [5].
Various guidelines have recommended different approaches for management of diabetes. The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) focused on patient-centered management that includes providing respectful care, understanding individual patient needs and preferences, and responding accordingly. Current guidelines from ADA/EASD recommend patient education and changes in lifestyle, including diet and exercise, as the foundation of any T2DM treatment regimen. Metformin is usually the first-line drug unless contraindications exist. If glycosylated hemoglobin (HbA1c) targets are not achieved or maintained after 3 months, two-drug combination therapy (i.e., metformin with a sulphonylurea, thiazolidinedione, dipeptidyl peptidase-4 inhibitor, glucagon-like peptide-1 receptor agonist, or basal insulin) is recommended. When two-drug combination therapy fails to achieve the desired HbA1c target, then three-drug combination therapy (with or without basal insulin) is started. Ultimately, complex insulin therapy alone or in combination with other agents is recommended when HbA1c remains uncontrolled [6–8].
Globally, basal insulin is often the starting insulin for patients with T2DM. When combination therapy with oral antihyperglycemic medication (OAM) and basal insulin fails to achieve adequate glycemic control, intensification of insulin therapy is recommended. Several recommended administration methods exist, including the gradual addition of prandial insulin to basal insulin therapy to minimize postprandial hyperglycemia caused by progressive β cell dysfunction [7]. Another strategy is to start premixed insulin (a fixed proportion of intermediate insulin with regular insulin or a rapid analog) administered traditionally twice daily [6].
Racial, cultural, and ethnic disparities are the key factors to consider when choosing antidiabetic therapy. Variation in glucose control in different ethnic populations is probably due to variations in dietary patterns, insulin resistance, glucose metabolism, etc. Guidelines from the Indian National Consensus Group on the use of insulin in patients with diabetes in India recommended premixed insulin as a safe, simple regimen that is easy to start and stay on [9].
Head-to-head data comparing insulin mixtures versus the addition of prandial insulin in patients inadequately controlled using basal-only insulin regimens are lacking. Therefore, this study described the efficacy and safety of two insulin intensification strategies in patients with inadequate glycemic control on once-daily basal insulin glargine plus metformin and/or pioglitazone. This manuscript describes the results of a post hoc analysis of the Indian subpopulation; the trial-level results have been described elsewhere [10].
Materials and methods
Design
This was a multinational, multicenter, open-label, randomized, parallel-arm, noninferiority (margin of 0.4 %), phase IV clinical trial. Patients were enrolled in 55 study centers in 11 countries (Argentina, Brazil, China, Egypt, India, Republic of Korea, Mexico, Romania, Russian Federation, Spain, and Turkey). In India, patients were enrolled in eight study centers.
Eligible patients were randomized in a 1:1 ratio to subcutaneous insulin lispro low mixture (LM25) twice daily or basal insulin glargine once daily, administered with prandial insulin lispro once daily (IGL) for 24 weeks and a stable dose of metformin and/or pioglitazone. LM25 was administered before breakfast and dinner (100 U/mL prefilled pens), basal insulin glargine at bedtime, and prandial insulin lispro before the largest meal of the day (100 U/mL prefilled pens). The largest meal of the day was defined as the meal with the highest 2-h postprandial blood glucose concentration recorded during the 2-week screening period using three separate 7-point self-monitoring of blood glucose (SMBG) profiles. The patient’s last dose of insulin glargine during the screening period was considered the initial total dose of LM25 and split into two equal doses per day. Patients in the IGL group were initiated with insulin lispro 4 IU daily and continued on the same dose of insulin glargine they received during the screening period. Randomization was stratified by country and HbA1c concentration at baseline (<8.5 or ≥8.5 %) [10]. All patients provided written informed consent, and the study was conducted in accordance with the ethical principles of the Declaration of Helsinki and is consistent with good clinical practices and applicable local laws and regulations.
Patients
Male and female patients aged ≥18 to ≤75 years with a diagnosis of T2DM based on history and clinical impression consistent with the World Health Organization’s Classification of Diabetes [11] were recruited. At screening, patients were to have had HbA1c concentrations ≥7.5 to ≤10.5 %, be taking stable doses of metformin and/or pioglitazone, and have received treatment with basal insulin glargine injected once daily for ≥90 days before the screening visit. Patients were also required to have a fasting plasma glucose (FPG) concentration ≤121 mg/dL, determined by the central laboratory, or >121 mg/dL if the investigator determined further titration of basal insulin glargine was not possible for safety reasons. Patients were excluded from the study if they had a screening body mass index >45 kg/m2 or more than one episode of severe hypoglycemia within 24 weeks before screening [10].
Outcome measures and assessments
The primary efficacy end point was change in HbA1c from baseline to 24 weeks of treatment. Secondary efficacy end points were change in HbA1c from baseline to 12 weeks of treatment; change in fasting blood glucose (FBG) concentration from baseline to 12 and 24 weeks; 7-point SMBG profiles at 12 and 24 weeks; glycemic variability (defined as the SD in 7-point SMBG profiles) at 12 and 24 weeks; daily total, basal, and prandial insulin doses at 12 and 24 weeks; and change in weight from baseline at 12 and 24 weeks.
Safety end points were measured by treatment-emergent adverse events (TEAEs); incidence, rate, and severity of hypoglycemic episodes (categorized as documented symptomatic [≤70 mg/dL], nocturnal, and severe); and vital signs. Patients insulin treatment satisfaction was assessed using the 22 grouped items on the Insulin Treatment Satisfaction Questionnaire (ITSQ). In addition, patients perceptions about the acceptability and effectiveness of their diabetes medications and perceived emotional and physical adverse events were assessed using the 21-item Perceptions About Medications-Diabetes 21 (PAM-D21) questionnaire, comprising four subscales (convenience/flexibility, perceived effectiveness, emotional effects, and physical effects) [10].
Statistical analysis
The intention-to-treat (ITT) population comprised all randomized patients who received at least one dose of study medication. The per-protocol (PP) population comprised all randomized patients except those who did not complete the study, received study drug different from their randomized study treatment, had any significant protocol violations, or were significantly noncompliant [10].
All measures were summarized using descriptive statistics including counts and percentages for categorical variables, and mean, SD, median, and interquartile range (IQR) for continuous variables. No treatment comparisons were performed because this was a subgroup analysis with a relatively small sample size. Noninferiority was not assessed in this Indian subpopulation.
Results
Patient disposition
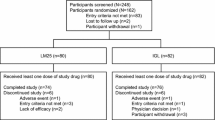
A total of 143 patients were screened for study entry in India (Fig. 1), of which 81 patients were randomized to 24 weeks of treatment either with LM25 (n = 40) or IGL (n = 41). Eighty patients completed the study, and one patient discontinued from the IGL group due to subject decision.
Baseline characteristics
Patient demographics and baseline disease characteristics are shown in Table 1. At baseline, the mean (SD) age was 53.8 years (10.37) and mean (SD) HbA1c levels were 8.6 % (0.73); 40 patients (49.4 %) had baseline HbA1c <8.5 %. Overall, prior to baseline, 80 patients (98.8 %) were receiving concomitant metformin, 14 patients (17.3 %) were receiving concomitant pioglitazone, and 13 patients (16.0 %) were receiving both concomitant metformin and pioglitazone. A total of 51 (63.0 %) patients had a preexisting condition, most commonly vascular (37 patients [45.7 %]) and metabolic and nutritional (20 patients [24.7 %]) disorders, and 65 patients (80.2 %) were receiving concomitant medications for conditions other than diabetes.
Efficacy
For the primary end point, mean (SD) change in HbA1c from baseline to week 24 was −1.2 % (1.11) for the LM25 group and −1.0 % (1.18) for the IGL group for the PP population. The corresponding mean (SD) change in HbA1c for the ITT population was −1.2 % (1.11) for the LM25 group and −1.0 % (1.16) for the IGL group.
The mean (SD) changes in HbA1c from baseline to week 12 were −0.7 % (1.20) and −0.7 % (1.44) in the LM25 and IGL groups, respectively. The observed HbA1c levels throughout the study are presented in Fig. 2a.
Glycemic control throughout the study for the intention-to-treat population receiving insulin lispro low mixture (LM25; insulin lispro protamine suspension 75 % and insulin lispro solution 25 %) twice daily or basal insulin glargine once–daily and prandial insulin lispro once - daily (IGL). HbA 1c glycosylated hemoglobin A1c. a Box plot of observed HbA1c levels (per patient population). b Mean unadjusted 7-point self-monitoring of blood glucose levels at baseline and 24 weeks
Median FBG concentration (IQR) at baseline was 101.80 mg/dL (91.89–107.21 mg/dL) for the LM25 group and 97.30 mg/dL (88.29–106.31 mg/dL) for the IGL group. Median changes in FBG (IQR) from baseline to week 12 were 17.12 mg/dL (3.60–36.94 mg/dL) for the LM25 group and 11.71 mg/dL (−6.31–36.04 mg/dL) for the IGL group. In addition, median changes in FBG (IQR) from baseline to week 24 were 13.51 mg/dL (−12.61–36.04 mg/dL) for the LM25 group and 21.62 mg/dL (−5.41–45.95 mg/dL) for the IGL group.
The mean unadjusted 7-point self-monitoring of blood glucose levels at baseline and 24 weeks are presented in Fig. 2b. The mean (SD) daily average 7-point SMBG profile values were 165.6 mg/dL (26.13) and 162.0 mg/dL (30.81) at baseline and 138.7 mg/dL (21.08) and 135.9 mg/dL (13.33) at week 24 for the LM25 and IGL groups, respectively. At week 24, the total mean (SD) daily doses were 46.9 IU (18.14) for the LM25 group and 41.8 IU (14.07) for the IGL group. Daily basal insulin doses for the LM25 and IGL groups at end point were 35.2 IU (13.60) and 27.4 IU (11.61), respectively. In addition, doses of daily prandial insulin at end point for the LM25 and IGL groups were 11.7 IU (4.53) and 14.5 IU (4.88), respectively.
Safety
At least one TEAE was reported by 20 patients (50.0 %) in the LM25 group and 15 patients (36.6 %) in the IGL group. Two patients (5.0 %) in the LM25 group and one patient (2.4 %) in the IGL group experienced events that were considered possibly related to the study treatments. Two serious TEAEs (myocardial infarction and hypoglycemia) were reported in two patients from the LM25 group. No patient discontinued because of adverse events or died during the study. Eight patients (20.0 %) in the LM25 group and 14 patients (34.1 %) in the IGL group experienced at least one episode of documented symptomatic hypoglycemia. Ten patients (25.0 %) in the LM25 group and 13 patients (31.7 %) in the IGL group experienced at least one episode of asymptomatic hypoglycemia. Six patients (15.0 %) in the LM25 group and five patients (12.2 %) in the IGL group experienced at least one episode of nocturnal hypoglycemia. One patient (2.5 %) in the LM25 group experienced severe hypoglycemia, and no patients reported severe hypoglycemia in the IGL group (Table 2). The mean (SD) change in body weight from baseline to week 24 was 0.0 kg (1.86) in the LM25 group and −0.2 kg (2.08) in the IGL group.
Health outcomes
Mean baseline and end point subscale scores on the ITSQ and PAM-D21 are summarized in Table 3. Total scores on the ITSQ range from 0 to 100, where 100 indicates complete satisfaction with insulin treatment. Mean (SD) total scores on the ITSQ were 79.6 (12.67) in the LM25 group and 80.0 (14.14) in the IGL group at baseline, and 84.2 (10.55) in the LM25 group and 84.6 (11.53) in the IGL group at week 24. The mean (SD) change from baseline to week 24 was 4.5 (9.93) in the LM25 group and 4.3 (11.32) in the IGL group.
Subscale scores on the PAM-D21 range from 0 to 100, where higher scores indicate better perceptions about diabetes medications. There were no obvious differences in mean scores between treatment groups at baseline in the four subscales (convenience/flexibility, perceived effectiveness, emotional effects, and physical effects).
Discussion
When combination therapy with OAM and basal insulin fails to achieve adequate glycemic control, intensification of insulin therapy is recommended. Per guidelines, different approaches exist for intensifying insulin therapy in patients with T2DM, including adding a prandial insulin or switching patients from their basal insulin regimen to a premixed insulin regimen. In India, the preferable prescribing pattern of OAMs is either metformin alone or metformin in combination with glimepiride [12–14]. Both LM25 and IGL regimens are currently used in clinical practice in India. For patients with T2DM failing to reach glycemic targets on basal insulin, National Guidelines on Initiation and Intensification of Insulin Therapy recommend intensification with premixed insulin analogs (grade A, evidence level 2). Premixed insulin preparations are simple and convenient regimens that provide coverage for postprandial plasma glucose in addition to FBG with the same insulin resulting in effective glycemic control. Physicians strive to provide the best treatment to their patients by practicing evidence-based medicine. However, there is little head-to-head data comparing premixed insulin analogs with the addition of prandial insulin in patients inadequately controlled on a basal-only insulin plus OAM regimen. Data from studies involving the use of multiple doses of prandial insulin lispro have been reported [15, 16].
Single-arm or observational studies have shown improvement in glycemic control when treatment was intensified with a premixed insulin regimen from basal insulin regimen with or without OAMs. This improvement in glycemic control did not lead to risk of hypoglycemia or increased weight [17]. Similarly, open-label, randomized crossover studies have shown significant improvement in glycemic control when intensified with LM25 twice daily plus metformin compared to once-daily insulin glargine in combination with metformin [18]. These studies did not show consistency in the definition of failure to previous therapy. This study did not include a run-in period to optimize the insulin glargine dose as it appears that the patients recruited were on optimal doses of basal insulin, as shown by the FPG concentration ≤121 mg/dL. In Indian patients, fasting glucose was well controlled; however, postprandial glucose contributed to the high HbA1c values. Therefore, the optimal strategy would be to add a prandial component rather than increase the basal dose. Despite the increase observed in FBG in the study, overall glycemic control improved with both strategies, all of which indicate that the prandial component was responsible for the improvement.
The data in the present study report on an Indian subgroup post hoc analysis from a recently completed multiregional clinical trial [10]. In this patient population recruited in India, we observed mean reduction in HbA1c in both groups. These results are consistent with the trial-level results, which showed a decrease in HbA1c after 6 months in patients with T2DM treated with LM25 or IGL. The results from the Indian subpopulations also showed a decrease in mean changes in daily average blood glucose values from 7-point SMBG profile values and a decrease in mean changes in glycemic variability in both treatment groups at weeks 12 and 24, which were consistent with the trial-level results. Mean weight changes in the Indian subpopulation were not clinically relevant, and the study did not demonstrate any obvious differences in overall satisfaction with insulin treatment, although no statistical analysis was done in the Indian subgroup. The overall safety profile was similar between the two groups.
The overall trial-level results of the present study showed glycemic control with LM25 to be noninferior (margin of 0.4 %), and subsequently superior to glycemic control with IGL as measured by change in HbA1c after 24 weeks of treatment. This study did not show any statistically significant differences in mean average daily glucose or glycemic variability. There were no significant treatment differences for the secondary efficacy variables evaluated such as proportion of patients achieving a target HbA1c <7.0 or ≤6.5 % at 24 weeks, change in FPG concentration from baseline to 12 and 24 weeks, and insulin dose (total, basal, and prandial) at 12 and 24 weeks. The mean observed changes in body weight from baseline to week 24 were not clinically relevant [10].
Limitations of this clinical trial included the open-label trial design, dictated by the two regimens’, use of insulin with different appearances, dosing requirements, and injection devices. Prandial insulin lispro was administered before the meal that had the highest 2-h postprandial blood glucose concentration and was given with the same meal throughout the study. Flexibility in dose scheduling was not allowed; however, we believe that this would not have affected study results, as the bolus was given with the largest daily meal, irrespective of the country, diet, or time of day. The study was not powered to assess potential differences between the two insulin regimens depending on the timing of the main meal. Therefore, any impact on glycemic profile, glycemic variability, or incidence of hypoglycemia may be further assessed in future studies. Another limitation was study duration, as longer term effects of two insulin regimens were not explored; however, the 24-week duration has been used in previous studies that examined the efficacy of first insulin intensification with premixed insulin or prandial insulin added to basal insulin [10, 15].
This post hoc subgroup analysis adds new information to the consideration of LM25 as a treatment option in Indian patients inadequately controlled with basal-only insulin plus OAM regimens. Further studies may be required to interpret the best regimen for any specific population/country.
References
Unger J. Insulin initiation and intensification in patients with T2DM for the primary care physician. Diabetes Metab Syndr Obes. 2011;4:253–61.
International Diabetes Federation. Diabetes surge hits every nation. 2013. http://www.idf.org/sites/default/files/Global_WDD_Final.pdf. Accessed 07 Apr 2014.
World Health Organization, International Diabetes Federation. Diabetes action now: an initiative of the World Health Organization and the International Diabetes Federation. 2004. http://www.who.int/diabetes/actionnow/en/DANbooklet.pdf. Accessed 23 Mar 2014.
Diabetes.co.uk. Diabetes in India. 2014. http://www.diabetes.co.uk/global-diabetes/diabetes-in-india.html. Accessed 15 Jul 2014.
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–79.
Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes management—exploring the concept of the basal-plus approach in clinical practice. Diabet Med. 2013;30:276–88.
Mosenzon O, Raz I. Intensification of insulin therapy for type 2 diabetic patients in primary care: basal-bolus regimen versus premix insulin analogs: when and for whom? Diabetes Care. 2013;36 Suppl 2:S212–8.
Das AK, Sahay BK, Seshiah V, Mohan V, Muruganathan A, Kumar A, et al. Chapter 51: Indian National Consensus Group: National guidelines on initiation and intensification of insulin therapy with premixed insulin analogs. In: Muruganathan A, editor. Medicine update. The Association of Physicians of India; 2013. pp. 227–36.
Tinahones FJ, Gross JL, Onaca A, Cleall S, Rodríguez A. Insulin lispro low mixture twice daily versus basal insulin glargine once daily and prandial insulin lispro once daily in patients with type 2 diabetes requiring insulin intensification: a randomized phase IV trial. Diabetes Obes Metab. 2014. doi:10.1111/dom.12303.
World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications, report of a WHO Consultation, part 1: diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 1999.
Vengurlekar S, Shukla P, Patidar P, Bafna R, Jain S. Prescribing pattern of antidiabetic drugs in Indore city hospital. Indian J Pharm Sci. 2008;70:637–40.
Kumar R, Kohli K, Kajal HL. A study of drug prescribing pattern and cost analysis among diabetic patients in a tertiary care teaching institute in north India. J Drug Deliv Ther. 2013;3:56–61.
Patel B, Oza B, Patel KP, Malhotra SD, Patel VJ. Pattern of antidiabetic drugs use in type-2 diabetic patients in a medicine outpatient clinic of a tertiary care teaching hospital. Int J Basic Clin Pharmacol. 2013;2:485–91.
Miser WF, Arakaki R, Jiang H, Scism-Bacon J, Anderson PW, Fahrbach JL. Randomized, open-label, parallel-group evaluations of basal-bolus therapy versus insulin lispro premixed therapy in patients with type 2 diabetes mellitus failing to achieve control with starter insulin treatment and continuing oral antihyperglycemic drugs: a noninferiority intensification substudy of the DURABLE trial. Clin Ther. 2010;32:896–908.
Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care. 2008;31:20–5.
Malone JK, Kerr LF, Campaigne BN, Sachson RA, Holcombe JH, Lispro Mixture-Glargine Study Group. Combined therapy with insulin lispro mix 75/25 plus metformin or insulin glargine plus metformin: a 16-week, randomized, open-label, crossover study in patients with type 2 diabetes beginning insulin therapy. Clin Ther. 2004;26:2034–44.
Malone JK, Bai S, Campaigne BN, Reviriego J, Augendre-Ferrante B. Twice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycaemic control in patients with type 2 diabetes. Diabet Med. 2005;22:374–81.
Acknowledgments
The study was funded by Eli Lilly and Company. The authors would like to thank Angel Rodríguez of Eli Lilly and Company for reviewing this manuscript and Amber Burns of Eli Lilly and Company and Shivanand Jigajinni of inVentiv Health Clinical for the medical writing support.
Conflict of interest
Simon Cleall and Shweta Uppal are employees of Eli Lilly and Company, and Steve Chen was an employee of Eli Lilly and Company during the writing of this manuscript. The authors report no conflicts of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prasanna Kumar, K.M., Shah, S., Shah, P. et al. Insulin lispro low mixture twice daily versus basal insulin glargine once daily and prandial insulin lispro once daily in patients with type 2 diabetes mellitus requiring insulin intensification—a randomized phase IV trial: Indian subpopulation analyses. Int J Diabetes Dev Ctries 37, 116–123 (2017). https://doi.org/10.1007/s13410-015-0387-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-015-0387-z