Abstract
Purpose
Chronic myeloid leukemia (CML) is a myeloproliferative disease derived from hematopoietic stem cells (HSCs) that harbor Philadelphia chromosome (Ph chromosome). In clinic, leukemia stem cells (LSCs) in CML are insensitive to the treatment with tyrosine kinase inhibitors, and are responsible for disease relapse. However, the molecular mechanisms for maintaining LSCs survival remain elusive.
Methods
CML patient-derived cell lines and BCR-ABL-induced CML mouse model were used to explore the role of YBX1 in regulating the survival of CML LSCs. Bone marrow transduction and transplantation, and colony-forming unit assay were used to investigate LSC function. The underlying mechanism of how YBX1 regulates LSCs survival were assessed using flow cytometry, RNA sequencing, western blot, RNA decay assay, co-immunoprecipitation and RNA immunoprecipitation.
Results
Here we show that RNA-binding protein YBX1 plays an important role in regulating survival of CML LSCs. We find that YBX1 expression is significantly increased in CML cells, and confirm that YBX1 is required for maintaining survival of LSCs. Deletion of YBX1 impairs the propagation of CML through blocking cell proliferation and inducing apoptosis of LSCs. Mechanistically, we find that YBX1 regulates expression of apoptotic associated genes. YBX1 cooperates with RNA m6A reader IGF2BPs to stabilize YWHAZ transcript in an m6A-dependent manner, and loss of YBX1 decreases YWHAZ expression by accelerating mRNA decay. Restoration of YWHAZ efficiently rescues the defects of YBX1-deficient CML cells.
Conclusion
Our findings reveal a critical role of YBX1 in maintaining survival of CML LSCs, which provides a rationale for targeting YBX1 in CML treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell (HSC) disorder caused by the Philadelphia chromosome due to a reciprocal translocation between chromosomes 9 and 22 [1, 2]. This chromosomal translocation results in the formation of BCR-ABL oncoprotein, which has a constitutive tyrosine kinase activity and promotes the proliferation of leukemia cells via various signaling pathways (e.g. JAK-STAT, MAPK/ERK, and PI3K/Akt/mTOR) [2]. Tyrosine kinase inhibitors (TKIs) can efficiently inhibit BCR-ABL kinase activity, and have become very successful in treating patients with CML in clinic [3]. However, increasing evidence indicates that leukemia stem cells (LSCs) in CML are insensitive to TKIs treatment [4, 5]. Thus, it is generally accepted that eradication of LSCs is required for curing CML [6]. Although previous studies have uncovered some key regulators for CML LSCs maintenance [7,8,9,10,11,12], identifying the underlying mechanisms for regulating LSCs survival remains a challenge in this field.
RNA-binding proteins (RBPs) play important roles in both transcriptional and post-transcriptional processing of RNA, such as fine-tuning gene expression, RNA splicing, polyadenylation, localization, stability, and translation [13, 14]. Dysregulation of some RBPs have been implicated in various human diseases including cancers. Recently studies also reveal the essential roles of RBPs in normal hematopoiesis and hematopoietic malignancies [15,16,17]. For instance, frequent mutations in genes encoding RNA splicing factors have been identified in myeloid dysplasia [18, 19]. N6-methyladenosine (m6A) is the most prevalent mammalian mRNA modification, and plays critical role in determining RNA fates [20, 21]. The writers, erasers, and readers of RNA m6A have emerged as critical players in normal and malignant hematopoiesis [22,23,24,25,26,27,28]. Our recent findings uncover the key role of RNA m6A reader, IGF2BP2, in sustaining HSCs function [29]. We also reveal that the RNA m6A demethylase ALKBH5 is selectively required for maintaining the function of acute myeloid leukemia (AML) stem cells but not normal hematopoietic stem cells [30]. Despite of these recent advances, it is still necessary to explore the roles of different RBPs under various physiological and pathological hematological conditions.
YBX1 belongs to the RBP family and is a multifunctional protein containing the evolutionarily conserved cold-shock domain, which could serve as an oncoprotein functioning in cell proliferation, survival, drug resistance, and chromatin instability in human cancers. YBX1 has been implicated in various biological processes including cell proliferation, survival, drug resistance, and chromatin destabilization [31,32,33]. Interestingly, our recent study demonstrates that YBX1 is selectively required for maintaining AML cell survival, and deletion of YBX1 does not obviously affect normal hematopoiesis [34]. YWHAZ (also called 14–3-3ζ), an isoform of the 14–3-3 family, is a tyrosine 3-monooxygenase/tryptophan 5-monooxygenase, and involves in many oncogenic processes. YWHAZ is a molecular target and prognostic marker for many cancers [35, 36]. YWHAZ interacts with target proteins via phosphorylation motif [37] or directly regulating phosphorylation [38], and plays a key role in signaling transduction. Previous studies have discovered overexpression of YWHAZ in many cancers, including AML [39, 40], in which it promotes cancer initiation and progression by activating PI3K/AKT/mTOR signaling pathway [41]. In here, we uncover that YBX1 regulates YWHAZ expression via an m6A-dependent manner and is required for maintaining the survival of LSCs in CML.
2 Material and methods
2.1 Mice
C57BL/6 J (CD45.2) background Ybx1f/f mice were generated by Biocytogen. Mx1-cre mice and B6.SJL (CD45.1) were obtained from The Jackson Laboratory.
2.2 Cell line culture
Human CML cell lines (K562 and LAMA-84) were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin–streptomycin (Sigma). HEK293T were maintained in DMEM (Hyclone) with 10% FBS and 1% penicillin/streptomycin.
2.3 Bone marrow transduction and transplantation
The retroviral construct BCR-ABL-iCre-GFP carrying the BCR-ABL cDNA were used for inducing CML, and expression of iCre was used to induce deletion of Ybx1 in cells from Ybx1f/f mice. Briefly, donor mice (Eight-week-old, Animal Center of Medical Research Institute at Wuhan University) were primed with 5-fluorouracil (5-FU; 200 mg/kg). The bone marrow cells from donor mice were seeded at a density of 2 × 106 /mL in DMEM with 10 ng/mL IL-3 (PeproTech), 10 ng/mL IL-6 (PeproTech), and 100 ng/mL SCF (PeproTech). After incubating at 37 °C for 24 h, the cells were transduced twice with retroviral stocks. Recipient mice were irradiated with 10 Gy and bone marrow cells were transplanted by intravenous injection. For secondary transplantation, recipient mice were irradiated with lethal dose, and then 2 × 105 bone marrow cells from primary transplantation mice were transplanted.
2.4 Flow cytometry analysis and cell sorting
Bone marrow (BM) and peripheral blood (PB) of CML mice were collected for FACS analysis. Cells were stained in PBS containing 2% FBS with primary antibodies (CD3, CD4, CD8, B220, Gr-1, Mac-1 and Ter119) at 4 °C for 15 min. Following PBS washing, the secondary antibody (APC-Cy7-conjugated streptavidin for recognizing biotin and PE-conjugated c-Kit and APC-conjugated Sca-1) were added at 4 °C for 15 min in the dark. The CML stem cell population (GFP+Lin−c-Kit+Sca-1+) was analyzed by FACS. All of these antibodies were purchased from eBioscience.
2.5 Plasmid constructions and Lentiviruses transduction
shRNAs targeting human YBX1 and IGF2BP1 were designed and inserted into pLKO.1 according to instructions. For the overexpression of YWHAZ, BCL2 and MYC, pCDH-CMV-Blast was used by standard molecular cloning methods. The sequences were listed in Supplemental Table 1.
Lentiviruses were produced in HEK293T cells after transfecting with viral packaging constructs pMD2.G and pSPAX2. Viral supernatants were collected at 48 and 72 h after transfection. Target cells were infected with virus and 8 μg/mL polybrene, and the medium was changed 12 h after infection, and added antibiotics (2 μg/mL puromycin) 48 h after infection.
2.6 Cell proliferation and colony-forming unit assay
For proliferation assay, human leukemia cells (K562 and LAMA-84) were transduced with lentivirus followed by puromycin selection. 20,000 cells were seeded into 24-well plates, counting cells every other day. For colony-forming unit assay, transduced human leukemia cells were cultured in 1.2% methylcellulose medium (100 IU/mL penicillin and 100 μg/mL streptomycin, 10% FBS) at 37 °C. Mouse LSCs (GFP+Lin−Sca-1+c-Kit+) cells were sorted by FACS, and plated in methylcellulose medium (Methocult GF M3434; StemCell Technologies). Colonies were counted at day 7.
2.7 Cell cycle and apoptosis analysis
To analyze cell cycle, cells were cultured with Hoechst 33342 at 37 °C for 90 min, followed by flow cytometric analysis. To analyze apoptosis, cells and Annexin V (eBioscience, 88–8005-72) were incubated at 37 °C for 30 min, and 7AAD was added before flow cytometric analysis. FlowJo software was used to analyze the results.
2.8 Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated using TRIzol reagent (Takara). After DNase I treatment, reverse transcription reactions were performed using ReverTra Ace qPCR RT Kit (TOYOBO) according to the manufacturer’s instructions. Relative gene expression was measured using Fast SybrGreen PCR Master Mix with a Bio-Rad real-time PCR System. All the primer sequences were showed in Supplemental Table 2.
2.9 MeRIP-qPCR
Cells were crosslinked by UV (stratalinker 1800, 400 mJ/cm2). The nucleus was fragmented and pre-cleaned, and then incubated with protein A beads-antibody at 4 °C overnight. After washing with high salt wash buffer and RIP buffer for three times respectively, beads (Thermo Fisher Scientific) were resuspended in PBS, followed by DNA digestion at 37 °C for 15 min and proteinase K (20 mg/mL, Thermo Fisher) digestion at 55 °C for 30 min. RNAs were isolated by TRIzol, extraction and analysed by quantitative PCR. All the primer sequences were showed in Supplemental Table 2.
2.10 m6A modification site prediction
The potential m6A modification sites of YWHAZ were predicted by SRAMP (http://www.cuilab.cn/sramp).
2.11 Co-immunoprecipitation
Cells transfected with plasmids were lysed with lysis buffer (20 mM Tris–HCl pH 7.4, 150 mM NaCl, 2 mM EDTA pH 8.0, 1% NP40, 1 × Protease inhibitor) on ice for 30 min and then fragmented. After centrifugation, the supernatant was incubated with anti-FLAG beads or HA antibody for 4 h at 4 °C. The immune complexes were washed three times and then boiled in 2 × SDS loading buffer for western blot detection.
2.12 Western blot analysis
Cells were lysed by use of RIPA with protease inhibitor cocktail (Roche), the total cell lysates were separated by SDS- PAGE gels. Membranes were blocked and incubated overnight at 4 °C with primary antibody, after which HRP-linked secondary antibodies were incubated for 1 h at room temperature. Antibodies used were as follows: YBX1 (Cat#ab12184, Abcam), IGF2BP1 (IMP1, clone D33A2, Cat#8482, CST), IGF2BP3 (IMP3, Cat#ab177477, Abcam), MYC (Cat#18583, CST), BCL2 (Cat#ab182858, Abcam), and GAPDH (Cat#60004–1, Proteintech) were used as loading control.
2.13 RNA decay assay
LAMA-84 cells were treated with actinomycin D (5 μg/mL) for indicated time and collected. Total RNA was extracted and analyzed by RT-PCR. GAPDH was used as endogenous control. The rate of disappearance of mRNA concentration at a given time (dC/dt) is proportional to both the rate constant for decay (Kdecay) and the cytoplasmic concentration of the mRNA (C). This relation is described by the following equation: dC/dt = -KdecayC. The mRNA degradation rate Kdecay was estimated by: ln(C/C0) = -Kdecay t. To determine the half-life (t1/2), this means 50% of the mRNA is decayed (C/C0 = 1/2). Substituted to the above equation got the following equation: ln (1/2) = -Kdecay t1/2. from where: t1/2 = ln2/Kdecay.
2.14 RNA-seq and data analysis
For RNA-seq, LSCs were sorted from WT and Ybx1cKO CML mice. Total RNA was isolated. Poly(A) mRNA was subsequently purified from 1 μg total RNA using NEBNext Poly (A) mRNA Magnetic Isolation Module. The RNA-seq library was prepared with a TruSeq Sample Prep Kit v2 (Illumina), according to the manufacturer’s protocol. RNA libraries were sequenced on an Illumina Hiseq X Ten platform with paired-end reads (150-bp read length). For RNA-seq analysis, reads were mapped to mouse genome version 38 (GRCm38) with Hisat2 (v 2.1.0), FeatureCounts (v 1.6.4) was used to calculate counts from bam files. DESeq2 (v 1.26.0) was employed for data normalization and differential expression analysis of RNA-seq counts.
2.15 Statistical analysis
The log-rank test was used to compare survival curves, and all the other experiments were analyzed using two-way Student's t test. For the comparison of different specimens, the unpaired t test was used. For the comparison of different treatments within the same specimen, the paired t test was used. P values of less than 0.05 were considered statistically significant. In the figures, asterisks indicate *p < 0.05, **p < 0.01, and ***p < 0.001.
3 Results
3.1 High expression of YBX1 in human chronic myeloid leukemia cells
To investigate the role of YBX1 in CML, we first analyzed its expression level by surveying the publicly available gene expression profiling database for human bulk CD34+ cells from CML patients [42]. Interestingly, higher expression of YBX1 was observed in the majority patients with CML, especially in patients in blast crisis phase (Fig. 1A). Using the same database, we also comprehensively assessed the expression levels of the detected 876 RBP mRNAs, and found that YBX1 mRNA level ranked among the top candidates in these RBPs (Fig. 1B). And higher expression of YBX1 was also detected at the protein level in patient-derived CML cell lines (K652 and LAMA-84), compared with healthy control cells (Fig. 1C). These data promoted us to assess YBX1 function in chronic myeloid leukemia.
Elevated expression of YBX1 is required for survival of human myeloid leukemia cells. (A) YBX1 expression in human CML samples. Microarray analysis (GSE4170) showed expression of YBX1 in CD34+ the bone marrow and peripheral blood (PB) cells from 42 chronic-phase (blue), 17 accelerated (purple), and 32 blast-crisis-phase (brown) CML patients compared to normal human CD34+ cells. (B) Expression profiling of 876 RNA-binding protein-encoding genes in CML patients. The public microarray database GSE4170 was used, and 876 RBPs were detected in this database. (C) Western blot analysis of YBX1 expression in various patient-derived myeloid leukemia cell lines K562 and LAMA-84; mononuclear cells derived from cord blood were used as a healthy control, and GAPDH served as the loading control. (D) qPCR analysis of YBX1 expression in LAMA-84 leukemia cells after transduction with shRNA lentiviruses targeting YBX1. (E) Immunoblot showing YBX1-KD efficiency in LAMA-84 leukemia cells. (F) Growth curves of LAMA-84 leukemia cells after transduction with lentiviruses for shYBX1#1, shYBX1#2, or shControl. (G) Colony formation assay of LAMA-84 leukemia cells after transduction with indicated lentiviruses. (H) Cell cycle distribution of leukemia cells after transduction with the indicated lentiviruses. Cells were labeled with Hoechst 33342 and assessed by flow cytometry. (I) Percentages of apoptotic leukemia cells at day 4 after YBX1 knockdown. Error bars denote mean ± SD. Two-tailed Student’s t test: *P < 0.05; **P < 0.01; ***P < 0.001
3.2 YBX1 is essential for maintaining human chronic myeloid leukemia cell survival
To explore the role of YBX1 in CML cells, we knocked down YBX1 using short hairpin RNAs (shRNA). Both shRNAs markedly deleted YBX1 expression (Fig. 1D, E, Fig. S1A-B). We found that knockdown of YBX1 obviously inhibited proliferation and clonogenicity of both LAMA-84 and K562 CML cells (Fig. 1F-G, Fig. S1C-E). In addition, deletion of YBX1 moderately blocked cell cycle, and significantly promoted apoptosis of CML cells (Fig. 1H-I). Together, these results indicate that YBX1 is required to maintain human CML cell survival.
3.3 Ybx1 is essential for murine CML development
To determine the role of YBX1 in CML development in vivo, we used conditional Ybx1 knockout mice we generated previously [34]. To simultaneously express BCR-ABL and delete Ybx1 in the same cell, we used BCR-ABL-iCre-GFP retroviral construct that carry BCR-ABL and iCre (improved Cre). BM cells from 5-FU-treated WT and Ybx1fl/fl mice were transduced with BCR-ABL-iCre-GFP retrovirus, and transplanted into lethally irradiated recipient mice to induce CML (Fig. 2A-B). Then we sorted CML stem cell population (GFP+Lin−c-Kit+Sca-1+) from BM and confirmed Ybx1 deletion in LSCs (Fig. 2C). In primary transplantation, recipients of BCR-ABL-transduced WT or Ybx1cKO BM cells developed and succumbed to CML within 4 weeks after BMT, and no significant difference in survival was observed between WT and Ybx1cKO CML mice (Fig. 2D). In addition, there was no significant difference in the degree of splenomegaly or lung hemorrhage between these two groups of CML mice (Fig. 2E-F). We next performed secondary transplantation assay to assess the effect of Ybx1 on LSCs. As expected, LSCs from WT CML mice efficiently induced CML and all secondary recipient mice died within 4 weeks post-BMT, however, Ybx1cKO LSCs failed to induce CML in the secondary recipient mice (Fig. 2G). In addition, recipients of Ybx1cKO LSCs displayed much less severe splenomegaly and lung hemorrhage than recipients of WT LSCs (Fig. 2H-I). The defective CML phenotype in the absence of Ybx1 was consistent with a gradual decrease of the percentages of leukemia cells in peripheral blood (Fig. 2J). Thus, these results indicate that Ybx1 is required for CML development.
Ybx1 is essential for CML development. (A) Scheme for generating Ybx1 conditional knockout mouse. (B) Experimental scheme for establishing CML mouse model. Bone marrow cells from 5-FU (200 mg/kg) pretreated WT or Ybx1cKO donor mice are transduced with BCR-ABL-iCre-GFP retrovirus twice and transplanted into lethally irradiated recipients for induction of CML. (C) qRT-PCR analysis of Ybx1 expression in sorted LSCs (GFP+Lin−Sca-1+c-Kit+) from CML mice. (D) Kaplan–Meier survival curves for primary recipients of BCR-ABL-iCre-GFP-transduced BM cells from WT or Ybx1cKO donor mice (n = 5). BMT, bone marrow transplantation. (E) Gross appearance of the lung and spleen from primary recipients 14 days after BMT (n = 5). (F) The spleen weight of primary recipients 14 days after BMT (n = 5). (G) Kaplan–Meier survival curves for secondary recipients of BCR-ABL-iCre-GFP-transduced BM cells from WT or Ybx1fl/fl donor mice (n = 5). (H) Gross appearance of the lung and spleen of secondary recipients 15 days after BMT (n = 5). (I) The spleen weight of secondary recipients 15 days after BMT (n = 5). (J) FACS analysis of GFP+Gr-1+ cells in PB of primary and secondary recipients of BCR-ABL-iCre-GFP-transduced BM cells from WT or Ybx1fl/fl donor mice (n = 5). (C-J) Shown is 1 representative of 3 independent experiments. Error bars denote mean ± SD. Two-tailed Student’s t test: *P < 0.05; **P < 0.01; ***P < 0.001
3.4 Ybx1 is essential for survival maintenance of LSCs
Given that CML is a stem cell disease, we focused on LSCs and hypothesized that the defect of CML development due to Ybx1 deficiency is caused by its inhibitory effect of on LSCs. To verify this idea, we first examined LSCs (BCR-ABL-expressing LSK cells) in primary CML mice, and did not observe significant differences in the percentages and total numbers of LSCs between WT and Ybx1cKO CML mice (Fig. 3A-B), consistent with the similar survival of WT and Ybx1cKO primary CML mice. We further analyzed LSCs in the secondary recipient mice receiving WT or Ybx1cKO LSCs from primary CML mice. Two weeks after the secondary BMT, the percentages and numbers of LSCs in the BM were dramatically lower in the absence of Ybx1 (Fig. 3C-D), indicating that Ybx1 is required for maintenance of the LSC function. To understand the mechanism by which Ybx1 maintains the function of LSCs, we analyzed the effect of Ybx1 on cell cycle and apoptosis of LSCs. We found that deletion of Ybx1 resulted in an accumulation of LSCs in the G0-G1 phase, accompanied with obvious reduction in the S-G2-M phase of cell cycle (Fig. 3E-F), suggesting that Ybx1 deficiency impaired cell cycle progression of LSCs. Furthermore, we found that Ybx1cKO LSCs showed marked higher apoptosis than WT LSCs (Fig. 3G-H). To investigate whether Ybx1 affects self-renewal of LSCs, we carried out an in vitro colony-forming unit assay. Sorted LSCs from mice receiving BCR-ABL-transduced WT and Ybx1cKO BM cells were plated in vitro, and as expected, Ybx1cKO LSCs gave rise to less colonies than did WT LSCs, indicating that loss of Ybx1 impairs self-renewal ability of LSCs (Fig. 3I-J). Together, these results suggest that Ybx1 is required for regulating the function of CML LSCs.
Ybx1 is required for maintaining LSC function. (A-B) The percentages (A) and total numbers (B) of LSCs (GFP+ Lin-Sca-1+c-Kit+ cells) in BM of primary CML recipients (n = 5). (C-D) The percentages (C) and total numbers (D) of LSCs (GFP+ Lin-Sca-1+c-Kit+ cells) in BM of secondary CML recipient mice (n = 5). (E–F) The cell-cycle analysis of LSCs from BM of CML mice (n = 5). (G-H) The apoptosis analysis of LSCs from BM of CML mice (n = 5). (I-J) Colony formation assay. LSCs were sorted and cultured in MethoCult M3434 for 7 days. Data show results from two representative experiments. Two-tailed Student’s t test: *P < 0.05; **P < 0.01; ***P < 0.001
3.5 Ybx1 regulates expression of apoptosis related genes in CML LSCs
To comprehensively understand the underlying mechanism of Ybx1 in leukemogenesis, we performed RNA-seq assay and profiled gene expression alteration of LSCs sorted from WT and Ybx1cKO CML mice. A total of 1306 genes (717 downregulated and 589 upregulated) were significantly altered upon Ybx1 deletion (Fig. 4A). In line with previous studies [34, 43], GO analysis showed that the downregulated genes in Ybx1cKO LSCs were enriched in mRNA processing, RNA splicing regulation, positive regulation of translation, cell cycle regulation, and regulation of the intrinsic apoptotic signaling pathway (Fig. 4B). GSEA also showed also similar findings (Fig. 4C-H). Consistent with increased apoptosis in Ybx1cKO CML cells, the expression of prosurvival genes including Myc, Ddx3x, Bcl2, Ddias, Birc6, Celf4, Bard1, Gemin5, Cdk1, Cdk4, and Knl1 were substantially decreased in Ybx1cKO CML cells (Fig. 4I). In contrast, deletion of Ybx1 upregulated the expression of proapoptotic genes, such as Bik, Dapk3, Anxa1, Bax, Siva1, Dap, Gpx4, Mxd4 and Mxi (Fig. 4J). Together, our data indicate that Ybx1 is essential for regulating the survival of CML LSCs.
Loss of Ybx1 changes the expression of cell cycle and apoptotic associated genes in CML LSCs. (A) Heatmap showing differential expression of Ybx1 targets in LSCs from WT and Ybx1cKO CML mice. (B) GO enrichment analysis showing the enriched terms for significantly downregulated and upregulated genes in Ybx1cKO LSCs. (C-H) Gene set enrichment analysis (GSEA) plots showing enrichment of mTOR signaling pathway, MYC targets, genes for oxidative phosphorylation, G2/M checkpoint, mRNA processing, and RNA splicing in WT LSCs comparing to Ybx1cKO LSCs. FDR, false discovery rate; NES, normalized enrichment score. (I) qRT-PCR analysis validating the change of Myc, Ddx3x, Bcl2, Ddias, Birc6, Cefl4, Bard1, Gemin5, Cdk1, Cdk4, and Knl1 in Ybx1cKO LSCs comparing to WT LSCs (J) qRT-PCR analysis showing the expression levels of pro-apoptotic genes (Bik, Dapk3, Anxa1, Bax, Siva1, Dap, and Gpx4), Myc antagonist genes (Mxd4 and Mxi) in WT and Ybx1cKO LSCs. Two-tailed Student’s t test: *P < 0.05; **P < 0.01; ***P < 0.001
3.6 YWHAZ mediates the function of YBX1 in CML cells
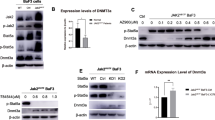
Next, we investigated the molecular mechanism of how YBX1 regulates the survival of CML cells. Given that CML cell line LAMA-84 was established from the blood of a patient with CML in acute phase, and has morphological features of undifferentiated blast cells, we mainly used LAMA-84 to explore the mechanisms in the following experiments. Our previous study indicates that c-MYC and BCL2 are the functional downstream targets of YBX1 in AML, we also found the significant enrichment of G2/M checkpoint, MYC targets in YBX1high LSCs (Fig. S2A-C). Interestingly, we found that knockdown of YBX1 in LAMA-84 cells obviously downregulated the expression of MYC and BCL2 at both mRNA and protein levels (Fig. S2D-F). Consistent with our previous findings in AML cells, YBX1 deletion did not significantly change global m6A level in LAMA-84 cells (Fig. S2G), and found that YBX1 regulated the expression of BCL2 and MYC by modulating their mRNA stability in CML cells in an m6A-dependent manner (Fig. S2H-L). We restored c-MYC and BCL2 and found that ectopic expression of c-MYC and BCL2 partially rescued the clonogenic and proliferative defects of YBX1-deficient CML cells (Fig. S3A-D). In addition, restoration of c-MYC and BCL2 also prevented the induction of apoptosis and rescued cell cycle in YBX1-deficient CML cells (Fig. S3E-G). Overall, these data indicate that c-MYC and BCL2 partially mediates the function of YBX1 in maintaining LSCs survival in CML.
We further sought to identify potential key regulators which expression is affected by YBX1. We integrated RNA-seq data for Ybx1-knockdown CML cells. Interestingly, we identified 10 differentially expressed genes upon Ybx1 deficiency (Fig. 5A). By interrogating publicly available gene expression datasets of CML patients and performing correlation analysis, we found that, among these 10 potential candidates, expression of YWHAZ and EIF4B consistently showed significant correlation with YBX1 level in two datasets for CML patients (Fig. 5B). As the role of YWHAZ in CML remains unclear, we focused on YWHAZ for further investigation. Knockdown of YBX1 obviously downregulated YWHAZ expression in human CML cells (Fig. 5C). Furthermore, we observed a higher expression of YWHAZ in CML patients (Fig. 5D). Thus, these data suggest that YWHAZ is one of downstream targets of YBX1 in CML.
YBX1 regulate YWHAZ expression in a m6A-dependent manner in CML leukemia cells. (A) Dot plot showing the relative expression of differential genes in WT and Ybx1cKO LSCs. (B) Expression correlation analysis of potential downstream targets versus YBX1 in CML patients. The correlation values (R) for YBX1 vs. individual candidate were shown (y axis for GSE14671 and x axis for GSE4170). Each dot represents the R value for each paired analysis. (C) qRT-PCR analysis showing the downregulation of YWHAZ in CML leukemia cells upon Ybx1 knockdown. (D) The expression level of YWHAZ in CML patients. The public database GSE5550 including 9 CML paitents and 8 healthy controls were used. (E) The transcription rates of YWHAZ mRNA in WT or Ybx1cKO leukemia cells. The public database GSE159152 was used. (F) The potential m6A modification sites of YWHAZ were predicted by SRAMP. (G) IGV tracks showing the distribution of m6A peaks in YWHAZ transcripts by analyzing the public database GSE98623. (H) MeRIP-qPCR analysis of m6A enrichment of YWHAZ in LAMA-84 cells. (I) The mRNA half-life of YWHAZ in control and YBX1-KD LAMA-84 cells. (J) Co-IP and western blot detection of the interaction of YBX1 with IGF2BP1 and IGF2BP3. (K) Immunoblot for IGF2BP1 expression in LAMA-84 cells 4 days after transduction with the indicated lentiviruses (GAPDH was used as a loading control). (L) YBX1 RIP-qPCR analysis showing YBX1 binding to YWHAZ mRNA in shCon or shIGF2BP1 leukemia cells. Two-tailed Student’s t test: *P < 0.05; **P < 0.01; ***P < 0.001
Next, we explored how YBX1 regulates the expression of YWHAZ. Interestingly, we did not detect obvious difference in the transcriptional rates of YWHAZ between WT and Ybx1cKO leukemia cells utilizing a previously published SLAM-seq dataset (Fig. 5E), suggesting that YBX1 does not affect the transcription of YWHAZ. Our previous study demonstrates that YBX1 plays a key role in regulating gene expression in an m6A-dependent manner[34], thus we investigated whether YBX1 regulates m6A-dependent mRNA stability of YWHAZ. SRAMP predictive analysis result exhibited that m6A modification sites were abundant in YWHAZ, indicating that YWHAZ was highly possible to be modified via m6A methylation (Fig. 5F). The integrative genomics viewer (IGV) displayed high enrichment of m6A peaks in YWHAZ (Fig. 5G). MeRIP-qPCR also showed higher m6A enrichment in YWHAZ transcripts (Fig. 5H). As expected, we found that loss of YBX1 caused an obvious decrease in the half-life of YWHAZ mRNA in LAMA-84 cells (Fig. 5I). These findings suggest that YBX1 regulated m6A-mediated YWHAZ mRNA stability. We observed an interaction of YBX1 with IGF2BP1/3 in LAMA84 cells (Fig. 5J). YBX1 RIP-PCR showed that YBX1 directly binds YWHAZ transcripts in LAMA-84 cells, which was impaired with knockdown of IGF2BP1 (Fig. 5K-L). Taken together, our data indicate that YBX1 stabilizes m6A-tagged YWHAZ mRNA by cooperating with IGF2BPs in CML cells. Next, we assessed whether YWHAZ mediates the function of YBX1 in CML. As expected, YWHAZ restoration in YBX1-deficient CML cells significantly rescued the clonogenic and proliferative defect (Fig. 6A-D). Moreover, reintroduction of YWHAZ also partially rescued the cell cycle and apoptotic defects of YBX1-deficient CML cells (Fig. 6E-H). Overall, these data indicate that YWHAZ is a functional downstream target of YBX1 in CML cells.
YWHAZ mediates the function of YBX1 in CML cells. (A) RT-PCR showing the expression of YWHAZ in control and YBX1-KD LAMA-84 leukemia cells with or without restoration of YWHAZ. (B) Growth curve of LAMA-84 leukemia cells after transduction with indicated lentiviruses. (C) Representative images from colony formation assay for YBX1-KD LAMA-84 leukemia cells with or without YWHAZ restoration. (D) Colony formation assay of LAMA-84 leukemia cells after transduction with indicated lentiviruses. (E–F) Cell cycle distribution of leukemia cells after knocking down YBX1 with or without YWHAZ restoration. (G-H) Percentages of apoptotic leukemia cells after knocking down YBX1 with or without tYWHAZ restoration at day 4. (I) Working model. Two-tailed Student’s t test: *P < 0.05; **P < 0.01; ***P < 0.001
4 Discussion
Studies on CML have brought great achievements in clinic, as it could serve as a paradigm for cancer research and therapy [1,2,3]. One of the major issues in current CML biology remains understanding the biology of LSCs. In here, our study reveals that RNA-binding protein YBX1 is critical for maintaining LSCs survival in CML by cooperating with IGF2BPs and regulating YWHAZ stability in m6A-dependent manner (Fig. 6I). This study extend our knowledge about the role of YBX1 in hematologic malignancies.
Recent findings from both our and another group have shown that YBX1 is required for the development and maintenance of human and murine AML in vitro and in vivo [34, 43]. Meanwhile, genetic deletion of YBX1 had no obvious deleterious effects on normal hematopoiesis, which make it a potentially therapeutic target for myeloid leukemia therapy. However, it remains unknown whether YBX1 plays a role in CML. In this work, firstly provide the clear evidence for the role of YBX1 in CML LSCs. We demonstrated that YBX1 is upregulated in CML cells and is required for CML development through specifically regulating LSCs survival. Thus, the present study strengthens the feasibility of specifically targeting YBX1 in CML treatment.
As an RNA-binding protein, YBX1 plays essential roles in determining RNA fates, such as pre-mRNA splicing, mRNA packaging, translational regulation. In line with our previous study[34], our data indicate that YBX1 also cooperates with m6A readers IGF2BPs in stabilizing m6A-tagged RNA. In addition, many studies have revealed the role of RNA m6A modifiers in AML, rather than CML. It should be further investigated the role of m6A-tagged RNA in maintaining LSCs. Our study reveals the cross-talk between YBX1 and YWHAZ. YWHAZ overexpression has been found in multiple cancers, including AML and CML, associating with high risk of tumorigenesis and worse prognosis overall [39, 40, 44, 45]. It is reported that YWHAZ could regulate the activation of the mTOR signaling axes via interacting with TSC2 [46]. Interestingly, our findings show that Ybx1 deletion leads to the alteration of the mTOR signaling pathway in LSCs (Fig. 4C), suggesting that Ybx1 might regulated YWHAZ and then altered the activation of mTOR signaling axes in the survival of CML LSCs. Our study found that m6A modification is essential in maintaining YWHAZ mRNA stability in CML cells. The exact mechanism of how YBX1 works in regulating m6A-tagged RNA may be context dependent. In conclusion, this work uncovers a conserved mechanism of YBX1 in regulating survival of CML stem cells.
Data availability
The datasets generated, RNA-seq is being deposited in GSE199304. This paper does not report original code. Any additional information required to access and analyze the data reported in this paper is available from the lead contact upon request.
References
H. Zhang, S. Li, Molecular mechanisms for survival regulation of chronic myeloid leukemia stem cells. Protein Cell 4, 186–196 (2013)
S. Wong, O.N. Witte, The BCR-ABL story: bench to bedside and back. Annu. Rev. Immunol. 22, 247–306 (2004)
B.J. Druker, F. Guilhot, S.G. O’Brien, I. Gathmann, H. Kantarjian, N. Gattermann, M.W. Deininger, R.T. Silver, J.M. Goldman, R.M. Stone, F. Cervantes, A. Hochhaus, B.L. Powell, J.L. Gabrilove, P. Rousselot, J. Reiffers, J.J. Cornelissen, T. Hughes, H. Agis, T. Fischer, G. Verhoef, J. Shepherd, G. Saglio, A. Gratwohl, J.L. Nielsen, J.P. Radich, B. Simonsson, K. Taylor, M. Baccarani, C. So, L. Letvak, R.A. Larson, I. Investigators, Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 355, 2408–2417 (2006)
Y. Hu, S. Swerdlow, T.M. Duffy, R. Weinmann, F.Y. Lee, S. Li, Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc. Natl. Acad. Sci. U. S. A. 103, 16870–16875 (2006)
S.M. Graham, H.G. Jorgensen, E. Allan, C. Pearson, M.J. Alcorn, L. Richmond, T.L. Holyoake, Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 99, 319–325 (2002)
A. Giustacchini, S. Thongjuea, N. Barkas, P.S. Woll, B.J. Povinelli, C.A.G. Booth, P. Sopp, R. Norfo, A. Rodriguez-Meira, N. Ashley, L. Jamieson, P. Vyas, K. Anderson, A. Segerstolpe, H. Qian, U. Olsson-Stromberg, S. Mustjoki, R. Sandberg, S.E.W. Jacobsen, A.J. Mead, Single-cell transcriptomics uncovers distinct molecular signatures of stem cells in chronic myeloid leukemia. Nat. Med. 23, 692–702 (2017)
H. Zhang, C. Peng, Y. Hu, H. Li, Z. Sheng, Y. Chen, C. Sullivan, J. Cerny, L. Hutchinson, A. Higgins, P. Miron, X. Zhang, M.A. Brehm, D. Li, M.R. Green, S. Li, The Blk pathway functions as a tumor suppressor in chronic myeloid leukemia stem cells. Nat. Genet. 44, 861–871 (2012)
H. Zhang, H. Li, N. Ho, D. Li, S. Li, Scd1 plays a tumor-suppressive role in survival of leukemia stem cells and the development of chronic myeloid leukemia. Mol. Cell Biol. 32, 1776–1787 (2012)
H. Zhang, H. Li, H.S. Xi, S. Li, HIF1alpha is required for survival maintenance of chronic myeloid leukemia stem cells. Blood 119, 2595–2607 (2012)
K. Rothe, A. Babaian, N. Nakamichi, M. Chen, S.C. Chafe, A. Watanabe, D.L. Forrest, D.L. Mager, C.J. Eaves, S. Dedhar, X. Jiang, Integrin-linked kinase mediates therapeutic resistance of quiescent CML stem cells to tyrosine kinase inhibitors. Cell Stem Cell 27, 110-124 e119 (2020)
E.M. Kuntz, P. Baquero, A.M. Michie, K. Dunn, S. Tardito, T.L. Holyoake, G.V. Helgason, E. Gottlieb, Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nat. Med. 23, 1234–1240 (2017)
K. Naka, T. Hoshii, T. Muraguchi, Y. Tadokoro, T. Ooshio, Y. Kondo, S. Nakao, N. Motoyama, A. Hirao, TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature 463, 676–680 (2010)
X.D. Fu, M. Ares Jr., Context-dependent control of alternative splicing by RNA-binding proteins. Nat. Rev. Genet. 15, 689–701 (2014)
M.W. Hentze, A. Castello, T. Schwarzl, T. Preiss, A brave new world of RNA-binding proteins. Nat. Rev. Mol. Cell Biol. 19, 327–341 (2018)
M.G. Kharas, C.J. Lengner, F. Al-Shahrour, L. Bullinger, B. Ball, S. Zaidi, K. Morgan, W. Tam, M. Paktinat, R. Okabe, M. Gozo, W. Einhorn, S.W. Lane, C. Scholl, S. Frohling, M. Fleming, B.L. Ebert, D.G. Gilliland, R. Jaenisch, G.Q. Daley, Musashi-2 regulates normal hematopoiesis and promotes aggressive myeloid leukemia. Nat. Med. 16, 903–908 (2010)
E. Wang, S.X. Lu, A. Pastore, X. Chen, J. Imig, S. Chun-Wei Lee, K. Hockemeyer, Y.E. Ghebrechristos, A. Yoshimi, D. Inoue, M. Ki, H. Cho, L. Bitner, A. Kloetgen, K.T. Lin, T. Uehara, T. Owa, R. Tibes, A.R. Krainer, O. Abdel-Wahab, I. Aifantis, Targeting an RNA-binding protein network in acute myeloid leukemia. Cancer Cell 35, 369-384 e367 (2019)
D.J. Hodson, M. Screen, M. Turner, RNA-binding proteins in hematopoiesis and hematological malignancy. Blood 133, 2365–2373 (2019)
T.A. Graubert, D. Shen, L. Ding, T. Okeyo-Owuor, C.L. Lunn, J. Shao, K. Krysiak, C.C. Harris, D.C. Koboldt, D.E. Larson, M.D. McLellan, D.J. Dooling, R.M. Abbott, R.S. Fulton, H. Schmidt, J. Kalicki-Veizer, M. O’Laughlin, M. Grillot, J. Baty, S. Heath, J.L. Frater, T. Nasim, D.C. Link, M.H. Tomasson, P. Westervelt, J.F. DiPersio, E.R. Mardis, T.J. Ley, R.K. Wilson, M.J. Walter, Recurrent mutations in the U2AF1 splicing factor in myelodysplastic syndromes. Nat. Genet. 44, 53–57 (2011)
K. Yoshida, M. Sanada, Y. Shiraishi, D. Nowak, Y. Nagata, R. Yamamoto, Y. Sato, A. Sato-Otsubo, A. Kon, M. Nagasaki, G. Chalkidis, Y. Suzuki, M. Shiosaka, R. Kawahata, T. Yamaguchi, M. Otsu, N. Obara, M. Sakata-Yanagimoto, K. Ishiyama, H. Mori, F. Nolte, W.K. Hofmann, S. Miyawaki, S. Sugano, C. Haferlach, H.P. Koeffler, L.Y. Shih, T. Haferlach, S. Chiba, H. Nakauchi, S. Miyano, S. Ogawa, Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 478, 64–69 (2011)
R. Desrosiers, K. Friderici, F. Rottman, Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl. Acad. Sci. U. S. A. 71, 3971–3975 (1974)
K.D. Meyer, S.R. Jaffrey, The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 15, 313–326 (2014)
I. Barbieri, K. Tzelepis, L. Pandolfini, J. Shi, G. Millan-Zambrano, S.C. Robson, D. Aspris, V. Migliori, A.J. Bannister, N. Han, E. De Braekeleer, H. Ponstingl, A. Hendrick, C.R. Vakoc, G.S. Vassiliou, T. Kouzarides, Promoter-bound METTL3 maintains myeloid leukaemia by m(6)A-dependent translation control. Nature 552, 126–131 (2017)
L.P. Vu, B.F. Pickering, Y. Cheng, S. Zaccara, D. Nguyen, G. Minuesa, T. Chou, A. Chow, Y. Saletore, M. MacKay, J. Schulman, C. Famulare, M. Patel, V.M. Klimek, F.E. Garrett-Bakelman, A. Melnick, M. Carroll, C.E. Mason, S.R. Jaffrey, M.G. Kharas, The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med. 23, 1369–1376 (2017)
J. Paris, M. Morgan, J. Campos, G.J. Spencer, A. Shmakova, I. Ivanova, C. Mapperley, H. Lawson, D.A. Wotherspoon, C. Sepulveda, M. Vukovic, L. Allen, A. Sarapuu, A. Tavosanis, A.V. Guitart, A. Villacreces, C. Much, J. Choe, A. Azar, L.N. van de Lagemaat, D. Vernimmen, A. Nehme, F. Mazurier, T.C.P. Somervaille, R.I. Gregory, D. O’Carroll, K.R. Kranc, Targeting the RNA m(6)A Reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell 25, 137-148 e136 (2019)
Z. Li, P. Qian, W. Shao, H. Shi, X.C. He, M. Gogol, Z. Yu, Y. Wang, M. Qi, Y. Zhu, J.M. Perry, K. Zhang, F. Tao, K. Zhou, D. Hu, Y. Han, C. Zhao, R. Alexander, H. Xu, S. Chen, A. Peak, K. Hall, M. Peterson, A. Perera, J.S. Haug, T. Parmely, H. Li, B. Shen, J. Zeitlinger, C. He, L. Li, Suppression of m(6)A reader Ythdf2 promotes hematopoietic stem cell expansion. Cell Res. 28, 904–917 (2018)
H. Weng, H. Huang, H. Wu, X. Qin, B.S. Zhao, L. Dong, H. Shi, J. Skibbe, C. Shen, C. Hu, Y. Sheng, Y. Wang, M. Wunderlich, B. Zhang, L.C. Dore, R. Su, X. Deng, K. Ferchen, C. Li, M. Sun, Z. Lu, X. Jiang, G. Marcucci, J.C. Mulloy, J. Yang, Z. Qian, M. Wei, C. He, J. Chen, METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)A modification. Cell Stem Cell 22, 191-205 e199 (2018)
C. Zhang, Y. Chen, B. Sun, L. Wang, Y. Yang, D. Ma, J. Lv, J. Heng, Y. Ding, Y. Xue, X. Lu, W. Xiao, Y.G. Yang, F. Liu, m(6)A modulates haematopoietic stem and progenitor cell specification. Nature 549, 273–276 (2017)
H. Lee, S. Bao, Y. Qian, S. Geula, J. Leslie, C. Zhang, J.H. Hanna, L. Ding, Stage-specific requirement for Mettl3-dependent m(6)A mRNA methylation during haematopoietic stem cell differentiation. Nat. Cell Biol. 21, 700–709 (2019)
R. Yin, J. Chang, Y. Li, Z. Gao, Q. Qiu, Q. Wang, G. Han, J. Chai, M. Feng, P. Wang, T. Zhang, X. Xie, J. Hu, Y. Cheng, C. Guo, J. Wang, K. Gao, M. Cui, S. Li, Y. Zheng, W. Jiang, Y. Hu, Q.Y. Yang, H. Zhang, Differential m(6)A RNA landscapes across hematopoiesis reveal a role for IGF2BP2 in preserving hematopoietic stem cell function. Cell Stem Cell 29, 149-159 e147 (2022)
J. Wang, Y. Li, P. Wang, G. Han, T. Zhang, J. Chang, R. Yin, Y. Shan, J. Wen, X. Xie, M. Feng, Q. Wang, J. Hu, Y. Cheng, T. Zhang, Y. Li, Z. Gao, C. Guo, J. Wang, J. Liang, M. Cui, K. Gao, J. Chai, W. Liu, H. Cheng, L. Li, F. Zhou, L. Liu, Y. Luo, S. Li, H. Zhang, Leukemogenic chromatin alterations promote AML leukemia stem cells via a KDM4C-ALKBH5-AXL signaling axis. Cell Stem Cell 27, 81-97 e88 (2020)
V. Evdokimova, P. Ruzanov, H. Imataka, B. Raught, Y. Svitkin, L.P. Ovchinnikov, N. Sonenberg, The major mRNA-associated protein YB-1 is a potent 5’ cap-dependent mRNA stabilizer. EMBO J. 20, 5491–5502 (2001)
V. Evdokimova, C. Tognon, T. Ng, P. Ruzanov, N. Melnyk, D. Fink, A. Sorokin, L.P. Ovchinnikov, E. Davicioni, T.J. Triche, P.H. Sorensen, Translational activation of snail1 and other developmentally regulated transcription factors by YB-1 promotes an epithelial-mesenchymal transition. Cancer Cell 15, 402–415 (2009)
S.P. Somasekharan, A. El-Naggar, G. Leprivier, H. Cheng, S. Hajee, T.G. Grunewald, F. Zhang, T. Ng, O. Delattre, V. Evdokimova, Y. Wang, M. Gleave, P.H. Sorensen, YB-1 regulates stress granule formation and tumor progression by translationally activating G3BP1. J. Cell Biol. 208, 913–929 (2015)
M. Feng, X. Xie, G. Han, T. Zhang, Y. Li, Y. Li, R. Yin, Q. Wang, T. Zhang, P. Wang, J. Hu, Y. Cheng, Z. Gao, J. Wang, J. Chang, M. Cui, K. Gao, J. Chai, W. Liu, C. Guo, S. Li, L. Liu, F. Zhou, J. Chen, H. Zhang, YBX1 is required for maintaining myeloid leukemia cell survival by regulating BCL2 stability in an m6A-dependent manner. Blood 138, 71–85 (2021)
A. Matta, K.M. Siu, R. Ralhan, 14-3-3 zeta as novel molecular target for cancer therapy. Expert Opin. Ther. Targets 16, 515–523 (2012)
C.L. Neal, D. Yu, 14-3-3ζ as a prognostic marker and therapeutic target for cancer. Expert Opin. Ther. Targets 14, 1343–1354 (2010)
X. Han, Y. Han, H. Jiao, Y. Jie, 14-3-3ζ regulates immune response through Stat3 signaling in oral squamous cell carcinoma. Mol. Cells 38, 112–121 (2015)
C.L. Neal, J. Xu, P. Li, S. Mori, J. Yang, N.N. Neal, X. Zhou, S.L. Wyszomierski, D. Yu, Overexpression of 14-3-3ζ in cancer cells activates PI3K via binding the p85 regulatory subunit. Oncogene 31, 897–906 (2012)
R. Su, J.N. Gong, M.T. Chen, L. Song, J.W. Zhang, c-Myc suppresses miR-451⊣YWTAZ/AKT axis via recruiting HDAC3 in acute myeloid leukemia. Oncotarget 7, 77430–77443 (2016)
R. Liang, X.Q. Chen, Q.X. Bai, Z. Wang, T. Zhang, L. Yang, B.X. Dong, G.X. Gao, H.T. Gu, H.F. Zhu, Increased 14-3-3ζ expression in the multidrug-resistant leukemia cell line HL-60/VCR as compared to the parental line mediates cell growth and apoptosis in part through modification of gene expression. Acta Haematol. 132, 177 (2014)
F. Guo, D. Jiao, G.Q. Sui, L.N. Sun, Y.J. Gao, Q.F. Fu, C.X. Jin, Anticancer effect of YWHAZ silencing via inducing apoptosis and autophagy in gastric cancer cells. Neoplasma 65, 693–700 (2018)
J.P. Radich, H. Dai, M. Mao, V. Oehler, J. Schelter, B. Druker, C. Sawyers, N. Shah, W. Stock, C.L. Willman, S. Friend, P.S. Linsley, Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc. Natl. Acad. Sci. U. S. A. 103, 2794–2799 (2006)
F. Perner, T.M. Schnoeder, Y. Xiong, A.K. Jayavelu, N. Mashamba, N.T. Santamaria, N. Huber, K. Todorova, C. Hatton, B. Perner, T. Eifert, C. Murphy, M. Hartmann, J.I. Hoell, N. Schroder, S. Brandt, A. Hochhaus, P.R. Mertens, M. Mann, S.A. Armstrong, A. Mandinova, F.H. Heidel, YBX1 mediates translation of oncogenic transcripts to control cell competition in AML. Leukemia 36, 426–437 (2022)
C.L. Neal, D. Yu, 14-3-3ζ as a prognostic marker and therapeutic target for cancer. Expert Opin Ther Targets 14, 1343–1354 (2010)
J.J. Janssen, S.M. Klaver, Q. Waisfisz, G. Pasterkamp, D.P. de Kleijn, G.J. Schuurhuis, G.J. Ossenkoppele, Identification of genes potentially involved in disease transformation of CML. Leukemia 19, 998–1004 (2005)
B. Tian, J. Liu, N. Zhang, Y. Song, Y. Xu, M. Xie, B. Wang, H. Hua, Y. Shen, Y. Li, M. Yang, Oncogenic SNORD12B activates the AKT-mTOR-4EBP1 signaling in esophageal squamous cell carcinoma via nucleus partitioning of PP-1α. Oncogene 40, 3734–3747 (2021)
Funding
This work is supported by grant to H.Z. from the National Natural Science Foundation of China [82230007], and by the Fundamental Research Funds for the Central Universities [2042021kf0225]. This work was also supported by grant from the Hubei Provincial Natural Science Fund for Creative Research Groups [2021CFA003]. We also thank all the staff in the core facility of the Medical Research Institute at Wuhan University for their technical support.
Author information
Authors and Affiliations
Contributions
Contribution: J.C., Q.W., and H.Z. designed the experiments; J.C. and Q.W. contributed to the experimental plan and data interpretation; G.H. performed bioinformatic analyses with conceptual input from H.Z.; Q.W. and J.C. performed mouse experiments with help from Q.Q.; Q.W. performed growth curve and colony formation assays, qPCR, and western blotting on primary samples or cell lines with help from Y.C. and W.L.; J.C. and Y.C. performed plasmid constructions; Q.W. constructed the RNA-sequencing library; J.C. and Q.W. performed statistical analysis and wrote the manuscript. H.Z. wrote the manuscript and supervised this study.
Corresponding author
Ethics declarations
Ethical approval
All mice were bred and maintained in Animal Center of Medical Research Institute at Wuhan University. All animal experiments were performed according to protocols approved by the Animal Care and Use Committee of Medical Research Institute, Wuhan University. This study did not use primary human subjects, thus ethical approval for human subjects is not applicable.
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chai, J., Wang, Q., Qiu, Q. et al. YBX1 regulates the survival of chronic myeloid leukemia stem cells by modulating m6A-mediated YWHAZ stability. Cell Oncol. 46, 451–464 (2023). https://doi.org/10.1007/s13402-022-00762-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-022-00762-w