Abstract
Purpose
The role of cytokine-producing B cells in antitumor immunity is mostly overlooked. In the present study, we investigated changes in B cell cytokine profiles in breast tumor-draining lymph nodes (TDLNs) during disease progression, and associations of these changes with prognostic indicators.
Methods
Flow cytometry was used to measure the expression of TNF-α, IL-10, TGF-β, IL-2 and IFN-γ in B cells from 42 axillary lymph nodes. The frequencies of IL-10+ and FoxP3+ T regulatory cells (Tregs) were also determined.
Results
No significant changes in B cell cytokine profiles were observed during breast cancer progression from stage I to III, but the percentage of B cells with high TNF-α expression (TNFhi) showed a negative relationship with lymph node involvement and Her2 expression (p < 0.05). The percentage of IL-10+ B cells was found to be significantly higher in non-metastatic lymph nodes in node-negative compared to node-positive patients (p = 0.001). The frequencies of IL-10+ and TNFhi B cells were found to be negatively correlated with the number of involved lymph nodes. The frequency of TNFhi B cells showed an inverse correlation with the frequency of FoxP3+ Tregs, which in turn was associated with indicators of a poor prognosis.
Conclusions
Our data indicate that the cytokine profiles of B cells in TDLNs of patients with breast cancer show associations with various disease parameters. TNFhi and IL-10+ B cells correlated positively with indicators of a good prognosis. Further functional studies are required to elucidate the role of cytokine production by B cells in immunity against breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tumor immunology studies have shown that immune responses to tumors may play critical roles in cancer outcome. The immune profile of tumor-draining lymph nodes (TDLNs) or tumor-infiltrating lymphocytes has been proposed to be associated with disease parameters, and to be potentially useful as an indicator of a patient’s prognosis or response to therapy [1,2,3,4]. For many years, T cells were the primary focus of tumor immunology research. Recently, however, more attention has been given to the role of B cells in immunity against cancer.
Apart from generating tumor-specific antibodies, B cells can produce different effector molecules and cytokines (e.g. granzyme B, TNF-α and IFN-γ), express co-stimulatory molecules, and present or cross-present antigens, thereby promoting antitumor immunity directly or indirectly through effector CD4+ and CD8+ T cells [5, 6]. In vitro and in vivo studies have suggested a role for activated or engineered B cells in enhancing antitumor immunity [7,8,9]. In this context, B cells in the tumor margins of hepatocellular carcinomas have been found to co-localize with CD8+ T cells, and higher frequencies of these B cells have been reported to be associated with increased survival. Further work showed that these B cells express IFN-γ, granzyme B, TRAIL and high levels of co-stimulatory molecules, which may reflect their role in antitumor immunity [10].
In addition, B cells may be pro-tumorigenic given that they are able to produce suppressor cytokines such as IL-10, TGF-β or IL-35, or express inhibitory molecules such as PD-L1 [11]. A subset of IL-10+PD-L1+ plasma cells has been shown to suppress cytolytic activity against prostate cancer, and several other studies have provided evidence that IL-10+ and TGF-β+ B cells may contribute to immune suppression in various types of cancer [12,13,14,15]. In addition to suppressing T effector cell responses, B cells can negatively influence antitumor immunity by inducing and/or expanding T regulatory (Treg) cells [11]. It has been shown that B cells can induce Treg cells in the breast tumor microenvironment in a TGF-β dependent manner, thereby enhancing tumor metastasis [16]. However, another study reported that B cell depletion resulted in breast cancer progression, whereas the transfer of CpG-stimulated B cells improved antitumor immune responses. These findings underscore the complex role of B cells in breast cancer immunity [17].
Clearly, a more complete insight into the roles of B cells in the tumor microenvironment requires additional research. To shed light on these roles, we tracked changes in B cell phenotypes and cytokine profiles in TDLNs from women with breast cancer during disease progression. In a previous study we reported that the phenotypic profile of B cells in TDLNs undergoes significant changes upon lymph node metastasis [18]. To expand these findings, we assessed the cytokine profiles of B cells and their relationships with disease parameters in the present study. To explore possible connections between B cell subsets and Treg cells, we also determined the frequencies of IL-10+ and FoxP3+ Treg cells and their correlation with different cytokine-producing B cells.
2 Materials and methods
2.1 Patients
Forty-two women with breast cancer were enrolled in this study, and one axillary lymph node (LN) sample was obtained from each patient. None of the patients received chemotherapy or radiotherapy prior to surgery. The study was approved by the Ethics Committee of Shiraz University of Medical Sciences, and all patients provided their informed consent in writing. A small part of each LN was excised and kept in RPMI-1640 medium containing 10% FBS (both from Gibco, Life Technologies, USA), while the remaining tissue was used for routine pathological examination. The clinical and pathological characteristics of the patients are listed in Table 1. The LNs were categorized into two groups: metastatic lymph nodes (MLNs) with tumor involvement, and non-metastatic lymph nodes (nMLNs), which were tumor free. The patients were also categorized into two groups: node positive (LN+), with at least one involved lymph node, and node negative (LN−), with no involved lymph nodes.
2.2 Isolation of mononuclear cells from lymph node samples
Fresh LN samples were cut into very small pieces in RPMI-1640 medium containing 10% FBS and 1% penicillin/streptomycin, and filtered using a 40-μm cell strainer (SPL Life Sciences, South Korea). To isolate mononuclear cells, cell suspensions were centrifuged over a Ficoll-Hypaque gradient (Lymphodex, Inno-Train Diagnostik, Germany).
2.3 B cell enrichment and activation
After the isolation of mononuclear cells, B cells were enriched by depleting CD3+ T cells using anti-CD3 micro-beads and MACS columns (Miltenyi Biotec, Germany) according to the manufacturer’s instructions. Through this method, B cells were enriched to more than 80%. Next, cells were resuspended in complete medium (2 × 106 cells/ml) and stimulated with CpG (ODN 2006, 10 μg/ml; Invivogen, USA) and recombinant CD40L (200 ng/ml, R&D Systems, USA) for 10 h. In the final 6 h of stimulation, PMA (50 ng/ml, Sigma-Aldrich, Germany), Ionomycin (1 μg/ml, Sigma-Aldrich) and Brefeldin A (1 μl, BD Biosciences, USA) were added to the culture.
2.4 Flow cytometry
2.4.1 Antibodies
The anti-human antibodies used for flow cytometry include: [B cell associated markers: PerCP–Cy5.5–conjugated anti-CD19 (Clone HIB19), FITC–conjugated anti-CD24 (Clone ML5), FITC–conjugated anti-CD23 (Clone M-L233), FITC–conjugated anti-CD25 (Clone M-A251), PE–conjugated anti-CD27 (Clone M-T271), PE–conjugated anti-CD38 (Clone HIT2), PE–conjugated anti-CD1d (Clone CD1d42), PE–conjugated anti-CD11c (Clone B-ly6), PE–conjugated anti-IgD (Clone IA6–2), FITC–conjugated anti-CD43 (Clone 1G10)], PerCP–Cy5.5–conjugated anti-CD4 (Clone RPA-T4), PE–conjugated anti-FoxP3 (Clone 259D/C7), APC–conjugated anti-IL-10 (Clone JES3-19F1), PE–conjugated anti-TGF-β (Clone TW4-9E7), PE–conjugated anti-TNF-α (Clone MAB11), APC–conjugated anti-IL-2 (Clone MQ1-17H12) and their isotype-matched antibodies, all purchased from BD Biosciences. PE–conjugated anti-IFN-γ (Clone 25,723) and its isotype control were obtained from R&D Systems.
2.4.2 Surface and intracellular staining for B cell cytokine profiling and B10 cell phenotyping
After activation, B cells were harvested, washed with staining buffer (PBS + 2% FBS), stained with anti-CD19 antibody, fixed with 1% paraformaldehyde, permeabilized with perm/wash buffer (BD Biosciences) and stained with anti-IL-10, anti-IFN-γ, anti-TGF-β, anti-IL-2 and anti-TNF-α, or their respective isotype antibodies. For B10 cell phenotyping, B cells were first stained for surface CD19 and other B cell markers (as noted above) and, after fixation and permeabilization, stained with anti-IL-10 antibody.
2.4.3 Treg cell detection
Lymphocytes were stimulated for 5 h with PMA (20 ng/ml) and Ionomycin (1 μg/ml) in the presence of Brefeldin A (1 μl/ml). Once activated, lymphocytes were washed and stained with anti-CD25 antibody, fixed and permeabilized using FoxP3 buffer set (BD Biosciences) and subsequently stained with anti-CD4, anti-FoxP3 and anti-IL-10 antibodies.
2.4.4 Flow cytometry data analysis
All flow cytometry data were acquired using a FACSCalibur cytometer (BD Biosciences) and analyzed with FlowJo software (version 7.6.2, USA). Lymphocytes were gated and dead cells were excluded according to their forward and side scatter. The geometric mean fluorescent intensity (MFI) of each cytokine was regarded as the criterion for its expression. MFIs were normalized to the isotype of each test.
2.5 Statistical analysis
To compare the frequencies of B cell and T cell subpopulations in two or more groups, the nonparametric Mann-Whitney U test and Kruskal-Wallis H test were used, respectively. Spearman’s rank correlation was used to determine the correlation between the frequency of each B or T cell subset with each other or with tumor size or the number of involved LNs. SPSS software (version 13.5; SPSS Inc., USA) was used for all statistical analyses, and p values < 0.05 (two-tailed) were considered statistically significant. GraphPad Prism 5 software (GraphPad Software, Inc., USA) was used to produce the graphs.
3 Results
3.1 B cell cytokine profiles and IL-10+/FoxP3+ Treg cells in MLNs and nMLNs of patients with breast cancer
The frequencies of B cells that produced IL-10, IL-2, TGF-β, IFN-γ and TNF-α were determined in axillary LNs of patients with breast cancer (Fig. 1, Table 2), and compared between metastatic and non-metastatic LNs (MLNs and nMLNs; Fig. 2a). We found that MLNs and nMLNs did not differ in terms of frequencies of TGF-β+, IFN-γ+ and IL-2+ B cells. The percentage of IL-10 producing B cells was slightly lower in MLNs than in nMLNs, but the difference was not statistically significant (p = 0.1). The frequency of TNF-α producing B cells was found to be lower (although not significantly so) in MLNs than in nMLNs (p = 0.065). In almost all LNs, we detected two distinct populations of TNF-α producing B cells: those with a higher expression of TNF-α (TNFhi) and those with a lower expression of this cytokine (TNFlow). The frequency of TNFhi B cells was significantly lower in MLNs (p = 0.026), whereas the percentage of TNFlow B cells did not differ significantly between MLNs and nMLNs. In line with this finding, the geometric MFI of TNF-α production was found to be significantly lower in B cells from MLNs than from nMLNs (p = 0.025).
(a) Comparison of the B cell cytokine profiles in MLNs and nMLNs of breast cancer patients. CD19+ cells were gated as B cells, and the percentages of cells expressing each cytokine in the B cell gate was determined. Geometric MFI of TNF-α was regarded as a criterion for its expression in B cells. (b) Comparison of the frequencies of CD4+ T cells, CD25+/CD25− FoxP3+ CD4+, and IL-10+ CD4+ T cells in MLNs and nMLNs of breast cancer patients. The percentage of CD4+ cells was determined in the lymphocyte population. The percentage of each subset was subsequently determined in the CD4+ gate. Data are shown as the mean ± SEM. * p < 0.05. SEM: standard error of the mean, MFI: mean fluorescent intensity
Next, to investigate the relationship between changes in different cytokine-producing B cell subpopulations and IL-10+ or FoxP3+ Treg cells, the percentages of CD4+CD25+FoxP3+, CD4+CD25−FoxP3+ and CD4+IL-10+ T cells were determined in 10 MLNs and 19 nMLNs of patients with breast cancer (Fig. 3, Table 2). The percentage of CD4+ T cells in the lymphocyte gate was found to be significantly lower in MLNs than in nMLNs (p = 0.048, Fig. 2b). The percentage of CD25+FoxP3+CD4+ T cells was slightly higher in MLNs, whereas IL-10+CD4+ T cells were non-significantly less frequent in MLNs (p = 0.085).
Further analysis revealed a significant inverse correlation between the percentage of TNFhi B cells and the number of involved LNs, whereas the percentage of TNFlow B cells correlated directly with the number of involved LNs (Table 3). Similarly, the geometric MFI of TNF-α in B cells exhibited a significant negative correlation with the number of involved LNs (Table 3). In addition, we found that the percentage of IL-10+ B cells in nMLNs correlated negatively with the number of involved LNs. On the other hand, a significant positive correlation was noted between the frequency of CD25+FoxP3+CD4+ T cells and the number of metastatic LNs (Table 3).
3.2 B cell cytokine profiles and Treg cells in nMLNs of LN+ and LN− patients
To investigate the effect of lymphatic involvement on the B cell cytokine profile in nMLNs, the percentages of cytokine-producing B cells were compared in nMLNs of LN+ and LN− patients (Fig. 4a). In nMLNs of LN+ patients, we observed significantly lower frequencies of IL-10+ and TNFhi B cells (p = 0.001 and p = 0.034, respectively) and a significantly higher frequency of TNFlow B cells (p = 0.008). No significant differences were found in the frequencies of TGF-β-, IFN-γ-, IL-2- and TNF-α-producing B cells between LN+ and LN− patients. Similarly, IL-10+ and FoxP3+ Treg cells showed no significant differences between the two groups (Fig. 4b).
(a) Comparison of B cell cytokine profiles in nMLNs of LN+ and LN− breast cancer patients. CD19+ cells were gated as B cells, and the percentages of cells expressing each cytokine were determined in the B cell gate. (b) Comparison of the frequencies of CD4+ T cells, CD25+/CD25− FoxP3+ CD4+, and IL-10+ CD4+ T cells in nMLNs of LN+ and LN− breast cancer patients. The percentage of CD4+ cells was determined in the lymphocyte population, after which the percentage of each subset was determined in the CD4+ gate. Data are shown as the mean ± SEM. * p < 0.05, ** p < 0.01. SEM: standard error of the mean
Comparisons of the frequencies of cytokine-producing B cells or Treg cells in MLNs and nMLNs of LN+ patients revealed no significant differences, but both TNF-α+ and TNFhi B cells, as well as IL-10+ T cells, were found to be less frequent (although not significantly so) in MLNs than in nMLNs.
3.3 Relationship between the frequency of cytokine-producing B cells or Treg cells and tumor size or disease stage
Patients with breast cancer were categorized into two groups according to their tumor size (greatest dimension): patients with tumors ≤ 2 cm (Ts1) and those with tumors ≥ 2 cm (Ts2). Subsequent analysis of the frequencies of different cytokine-producing B cell subsets revealed no significant differences between Ts1 and Ts2 (Fig. 5a). However, the percentages of FoxP3+ and CD25+FoxP3+CD4+ T cells were significantly higher in Ts2 (p = 0.020 and p = 0.015, respectively, Fig. 5b). In line with these findings, significant positive correlations were seen between the frequencies of FoxP3+ and CD25+FoxP3+CD4+ T cells and the greatest dimension of the tumor (Table 3).
(a) Comparison of the frequencies of different cytokine-producing B cell subsets in TDLNs of breast cancer patients according to their tumor size. CD19+ cells were gated as B cells, after which the percentages of cells expressing each cytokine were assessed in the B cell gate. (b) Comparison of the frequencies of CD4+ T cells, CD25+/CD25− FoxP3+ CD4+, and IL-10+ CD4+ T cells in TDLNs of breast cancer patients according to their tumor size. The percentage of CD4+ cells was determined in the lymphocyte population and the percentage of each subset was determined in the CD4+ gate. Data are shown as the mean ± SEM. * p < 0.05. TS1: patients with tumor size ≤ 2 cm, Ts2: patients with tumor size > 2 cm, SEM: standard error of the mean
Neither the cytokine profile of B cells nor the frequency of Treg cells showed significant associations with breast cancer stages, but in nMLNs the frequencies of both IL-10+ and TNFhi B cells showed non-significant decreasing trends from stage I to stage III.
3.4 Cytokine profiles of B cells and IL-10+/FoxP3+ Treg cells in TDLNs of breast cancer patients according to Her2, ER and PR expression
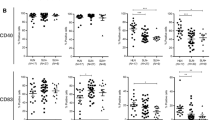
No significant differences were found in the percentages of IL-10+, IL-2+, TGF-β+ or IFN-γ+ B cells in TDLNs of Her2+ compared to Her2– patients (Fig. 6a). TNF-α+ B cells showed a decreasing trend in Her2+ compared to Her2– patients (p = 0.053), and the frequency of TNFhi B cells was found to be significantly lower in Her2+ patients (p = 0.030). On the other hand, we found that the frequencies of different cytokine-producing B cell subsets in MLNs did not differ significantly between Her2+ and Her2– patients. In contrast, we found that the frequency of TNF-α+ B cells along with that of TNFhi B cells was significantly lower in nMLNs of Her2+ compared to Her2– patients (p = 0.008 and p = 0.014, respectively). Analysis of the association between the frequency of Treg cells and Her2 expression revealed that the frequency of CD4+FoxP3+ T cells was significantly higher in Her2+ patients (p = 0.024, Fig. 6b). The percentage of CD4+CD25+FoxP3+ T cells was higher in Her2+ patients, but this difference was not statistically significant (p = 0.052).
(a) Comparison of B cell cytokine profiles in TDLNs of Her2+ and Her2– breast cancer patients. CD19+ cells were gated as B cells after which the percentages of cells expressing each cytokine were assessed in the B cell gate. (b) Comparison of the frequencies of CD4+ T cells, CD25+/CD25− FoxP3+, and IL-10+ CD4+ T cells in TDLNs of breast cancer patients according to Her2 status. The percentage of CD4+ cells was determined in the lymphocyte population, after which the percentage of each subset was determined in the CD4+ gate and compared in Her2+ versus Her2– patients. Data are shown as the mean ± SEM. * p < 0.05, ** p < 0.01. SEM: standard error of the mean
The cytokine profiles of B cells showed no associations with ER expression. However, the frequency of TNFhi B cells showed a decreasing trend, whereas the percentage of TNFlow B cells showed an increasing trend, in ER– patients compared to ER+ patients. Similarly, we found that the frequency of TNFhi B cells was significantly lower in PR– patients (p = 0.034, Fig. 7a), whereas TNFlow B cells showed an increasing trend in TDLNs from these patients (p = 0.050). T cell analyses revealed that the percentage of CD25+FoxP3+ T cells in TDLNs from ER–/PR– patients was significantly higher than that in ER+/PR+ patients (p = 0.028 and p = 0.015, respectively, Fig. 7b).
(a) Comparison of B cell cytokine profiles in TDLNs of PR+ and PR– breast cancer patients. CD19+ cells were gated as B cell and the percentages of cells expressing each cytokine were assessed in the B cell gate. (b) Comparison of the frequencies of CD4+ T cells, CD25+/CD25− FoxP3+ CD4+, and IL-10+ CD4+ T cells in TDLNs of breast cancer patients according to PR expression. The percentage of CD4+ cells was determined in the lymphocyte population and the percentage of each subset was determined in the CD4+ gate and compared in PR+ versus PR– patients. (The results for the association with ER expression were the same as for PR; the only difference was that TNFhi B cells showed a decreasing trend (which was not siginificant) in ER– patients). Data are shown as the mean ± SEM. * p < 0.05. SEM: standard error of the mean
3.5 Relationship between the frequencies of IL-10+CD4+ or FoxP3+ Treg cells and different cytokine-producing B cells
We found that the percentages of CD4+IL-10+ and CD25+FoxP3+CD4+ T cells did not correlate significantly with that of IL-10- or TGF-β-producing B cells in TDLNs from breast cancer patients. Significant inverse correlations, however, were observed between the frequencies of TNF-α+ or TNFhi B cells and CD25+FoxP3+CD4+ T cells (Table 3). Similarly, we found that the geometric MFI of TNF-α in B cells correlated inversely with the frequency of CD25+FoxP3+CD4+ Treg cells. Nevertheless, when LNs were categorized into MLNs and nMLNs, the correlations were significant only in the latter.
3.6 Surface phenotype of IL-10 producing B cells in TDLNs of breast cancer patients
The surface phenotype of IL-10-competent B cells (B10 cells) was determined in 9 TDLNs (4 MLNs and 5 nMLNs) from patients with breast cancer. Flow cytometric analysis showed that most IL-10+CD19+ cells were CD23−, CD43−, CD5− and CD11c−. The frequencies of IL-10+ B cells with a CD1dlow/int or CD38int/hi phenotype were significantly higher than those with a CD1dhi or CD38low/− phenotype. In addition, we found that 83.4 ± 6% of B10 cells were CD25−. Most (82.2 ± 6.2%) IL-10 producing B cells exhibited a CD24hiCD27+ active/memory rather than a CD24lowCD27− naïve phenotype. Moreover, our data showed that B10 cells could have both a CD27+IgM− switched memory or a CD27+IgM+ unswitched memory phenotype. Of note is that a population of B cells with the CD27−IgM− phenotype (CD27− memory B cells) accounted for 7.4 ± 3.4% of the B cells in axillary LNs. A small fraction of B10 cells (2.2 ± 0.7%) was found to have a CD27−IgM− phenotype.
4 Discussion
It is well accepted that the immune profile of axillary LNs in patients with breast cancer changes during cancer progression, and that these changes are related to disease parameters [19, 20]. We determined the cytokine profiles of B cells in TDLNs from patients with breast cancer, and tracked how they change during LN involvement and disease progression.
We found that B cells expressed IL-10, TGF-β, IL-2, IFN-γ and TNF-α at different frequencies. Between 13% and 92% of B cells produced TNF-α, with a declining trend in MLNs compared to nMLNs. Moreover, we found that B cell production of TNF-α was associated with breast cancer parameters such as LN involvement and Her2 expression. TNF-α is a pleiotropic cytokine produced by multiple immune and nonimmune cells, including tumor cells [21]. TNF-α plays a major role in mediating and orchestrating inflammatory responses, but evidence also points to the notion that this cytokine may exert suppressive effects on inflammation and immunity [22]. Similarly ample, but controversial, evidence exists on the effects of TNF-α on tumor immunity or tumor growth [22]. Multiple studies have demonstrated that TNF-α induces tumor growth, vascularization and metastasis via several mechanisms such as those mediating chronic inflammation, endothelial cell differentiation and angiogenesis, epithelial-to-mesenchymal transition and Treg cell expansion [22, 23]. On the other hand, TNF-α has been found to inhibit tumor growth and to enhance tumor immunity by mediating hemorrhagic necrosis in certain types of tumors, recruiting effector T and NK cells to the site of the tumor, and providing costimulatory signals for CD4+ and CD8+ T cells [21,22,23]. It has been suggested that the role played by TNF in inflammation and immunity is complicated, and depends on a variety of factors including its dose, form (soluble versus membrane-bound), receptor usage (TNFR1 versus TNFR2), duration of exposure (short versus chronic) and concomitant stimulation (e.g. by other cytokines) [24]. Tumor cells secrete low amounts of TNF-α, and low doses of this cytokine have been reported to support tumor growth, but it has also been reported that TNF-α causes dose-dependent cell death in prostate cancer cells [21, 25]. Several studies have shown that angiogenesis is induced by lower doses of TNF-α, whereas higher doses inhibit endothelial cell proliferation, capillary structure formation and vascularization [26,27,28]. According to our data, the MFI of TNF-α expression and the frequency of B cells with high levels of TNF-α expression are significantly lower in breast cancer MLNs compared to nMLNs, whereas the percentage of B cells expressing lower levels of TNF-α remained unchanged. This TNF profile of B cells may favor tumor growth in MLNs, because lower doses of TNF-α can induce tumor growth, vascularization and possibly metastasis, whereas these effects are inhibited by higher doses.
It has been proposed that the immune profile of TDLNs is different in LN– compared to LN+ breast cancer patients. In TDLNs from LN– patients, “immune-related pathways” have been found to be upregulated, whereas in TDLNs from LN+ patients, upregulation of “tumor-promoting pathways” has been observed even in nMLNs [29]. In line with this, we previously reported an increase in the percentage of B cells with a regulatory phenotype in nMLNs from node-positive compared to node-negative breast cancer patients [18]. Here, we found that the percentage of TNFhi B cells was significantly lower, whereas the frequency of TNFlow B cells was higher, in nMLNs from LN+ compared to LN– patients. As noted above, low TNF production can maintain chronic inflammation and vessel formation, thereby preparing a safe microenvironment for metastatic tumor cell homing. Therefore, in comparison to TNFhi B cells, B cells with a lower TNF-α expression may exhibit more potential to support further nodal invasion. The significant inverse correlations observed between the percentage of TNFhi B cells or the MFI of TNF-α in B cells and the number of involved LNs in breast cancer patients may support this idea.
It has been shown that B cells may induce and expand Treg cells by producing TGF-β, IL-10 and IL-35 or via contact-dependent mechanisms [11]. In the present study, we found that IL-10- or TGF-β-producing B cells exhibited no correlation with Treg cell frequencies. Instead, we observed a negative correlation between the percentage of TNFhi B cells or the MFI of TNF in B cells and the frequency of CD25+FoxP3+ Treg cells, which in turn showed a positive correlation with poor prognostic indicators such as tumor size and the number of involved LNs. Accumulating data suggest that TNF can be critical in the balance between Treg and T effector cells under inflammatory conditions [24]. High levels of TNFR2, which mostly binds to membrane-bound TNF (mTNF), are expressed by mouse and human Treg cells, and TNFR2 signaling has been reported to play a pivotal role in Treg cell survival, functional stability and expansion [30,31,32,33,34]. However, other studies have shown that TNF can downregulate FoxP3 expression and Treg cell activity in vitro, and reduce TGF-β-induced Treg cell differentiation [35,36,37]. On the other hand, TNF has been reported to have a critical co-stimulatory effect on CD4+ and CD8+ T effector cells and to promote their proliferation, inflammatory cytokine production, and resistance to Treg cell or TGF-β suppression [38,39,40,41]. Most costimulatory effects of TNF have been attributed to TNFR2 which, in contrast to Treg cells, is expressed at low levels in resting T cells but becomes upregulated shortly after activation [24].
Our knowledge of how TNF can play such contradictory roles is still incomplete. One possibility, however, is that chronic exposure to TNF decreases T effector cell activation while augmenting Treg cell expansion and activation [24, 42, 43]. Furthermore, the negative effect of TNF on Treg cells has been seen at higher rather than lower doses [24]. Hence, B cells with a high TNF expression may facilitate the co-stimulatory signal for T effector cells with a lower TNF receptor expression to a greater extent than those for Treg cells, allowing the former to compete with Treg cells in TNF consumption, thereby hindering Treg cell expansion as suggested by Chen and Oppenheim [23]. This hypothesis can be tested by investigating the expression of TNF receptors on T effector and T regulatory cells, and by determining the association between TNFhi B cells and T cell polarization. Because TNFR2 binds mostly to membrane-bound TNF, it might be informative to determine the expression of mTNF on B cells and its role in T cell polarization.
One unexpected finding in the present study was the significantly higher frequency of IL-10 producing B cells in the nMLNs of node-negative compared to node-positive patients. IL-10 producing B cells comprise an important subgroup of B regulatory cells, and their capacity to suppress immune responses has been reported in autoimmune disorders, infectious diseases and cancer [44]. Recent studies have shown that the frequency of B10 cells is increased in peripheral blood, tumor tissues or draining lymph nodes in several types of cancer including esophageal, gastric and ovarian cancer, and have assessed the relationship between B10 cells and immune suppression or disease progression [13,14,15]. Whether produced by B or T cells, IL-10 is largely known for its immune-regulatory properties related to the inhibition of T or antigen-presenting cells [45, 46]. However, several reports have suggested that IL-10 can positively influence antitumor immunity and surveillance [47,48,49]. IL-10 deficiency curtails tumor immune surveillance, whereas overexpression in transgenic mice or treatment with pegylated IL-10 (PEG-IL-10) has been found to inhibit carcinogenesis or to offer long-lasting tumor immunity [47, 49,50,51]. This cytokine has been shown to induce tumor rejection via the activation and expansion of tumor-infiltrating CD8+ T cells in a mouse tumor model [47]. It has also been reported that IL-10 augments IFN-γ production by tumor antigen-specific CD8+ T cells, and enhances granzyme B and perforin expression by intra-tumoral CD8+ T cells [49]. Another study has shown that IL-10 inhibited FoxP3+ Treg cell induction and increased IFN-γ+CD4+ T cells, and that treatment with PEG-IL-10 decreased the frequency of tumor-infiltrating FoxP3+ Treg cells in 4T1 tumor-bearing mice [52]. Treatment with PEG-IL-10 has already undergone a phase I clinical trial in advanced stages of several types of solid malignancies, and the preliminary data show that recombinant IL-10 induces effector CD8+ T cell responses [51, 53]. Therefore, currently available evidence shows that IL-10 can enhance cellular immunity against tumors. Accordingly, a higher percentage of IL-10 producing B cells in nMLNs from node-negative patients with breast cancer may positively influence antitumor immunity. This idea, however, awaits further functional studies. In the present study, neither the frequency of IL-10-producing B cells nor the percentage of IL-10+ T cells were related to poor prognostic indicators. Accordingly, additional mechanistic studies are required to fully elucidate the role of B10 cells or IL-10 in immunity against breast cancer.
As a final point regarding potential limitations of this work, we note that because of ethical and medical standards and considerations, obtaining fresh axillary LN samples was difficult. Thus the most important limitation of this study is that the sample sizes in some subgroups were small, so the results of some subgroup analyses should be validated using larger sample sizes. In addition, the main aim of this study was to provide descriptive data on the frequency of cytokine-producing B cells and their relationships with breast cancer parameters. This study may serve as a basis for future functional studies designed to elucidate the role of cytokine-producing B cells in T cell polarization and breast cancer immunity.
5 Conclusion
Our study provides further evidence that the immune profile of TDLNs changes during breast cancer progression and is related to prognostic markers. To our knowledge, this is the first report of an association between the B cell cytokine profile in TDLNs from patients with breast cancer and disease parameters. The frequencies of TNFhi B and B10 cells showed a negative relationship with lymph node involvement. The percentage of TNFhi B cells correlated inversely with that of Treg cells. In turn, higher percentages of Treg cells were found to be associated with poor prognostic indicators such as tumor size and the number of involved lymph nodes. Our findings suggest that TNFhi B cells and B10 cells may play a positive role in immunity against breast cancer. Although this study sheds additional light on the significance of cytokine-producing B cells in TDLNs from patients with breast cancer, more functional studies are needed to further elucidate the interplay between cytokine-producing B cells and tumor or Treg cells.
References
N.A. Giraldo, E. Becht, R. Remark, D. Damotte, C. Sautes-Fridman, W.H. Fridman, The immune contexture of primary and metastatic human tumours. Curr. Opin. Immunol. 27, 8–15 (2014)
T.L. Whiteside, Immune responses to cancer: Are they potential biomarkers of prognosis? Front. Oncol. 3, 107 (2013)
J. Mansfield, P. Lee, Immune-cancer interactions in tumors and tumor-draining lymph nodes: Novel prognostic indicators for breast cancer. J. Immunother. Cancer 2, 255 (2014)
Y. Vahidi, Z. Faghih, A.R. Talei, M. Doroudchi, A. Ghaderi, Memory CD4(+) T cell subsets in tumor draining lymph nodes of breast cancer patients: A focus on T stem cell memory cells. Cell Oncol. 41, 1–11 (2018)
N.J. Flynn, R. Somasundaram, K.M. Arnold, J. Sims-Mourtada, The multifaceted roles of B cells in solid tumors: Emerging treatment opportunities. Target. Oncol. 12, 139–152 (2017)
P. Tsou, H. Katayama, E.J. Ostrin, S.M. Hanash, The emerging role of B cells in tumor immunity. Cancer Res. 76, 5597–5601 (2016)
Q. Li, X. Lao, Q. Pan, N. Ning, J. Yet, Y. Xu, S. Li, A.E. Chang, Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin. Cancer Res. 17, 4987–4995 (2011)
J. Deng, J. Galipeau, B cells for cancer immunotherapy. OncoImmunology 3, e955702 (2014)
C.-A. Shin, H.-W. Cho, A.-R. Shin, H.-J. Sohn, H.-I. Cho, T.-G. Kim, Co-expression of CD40L with CD70 or OX40L increases B-cell viability and antitumor efficacy. Oncotarget 7, 46173 (2016)
J.Y. Shi, Q. Gao, Z.C. Wang, J. Zhou, X.Y. Wang, Z.H. Min, Y.H. Shi, G.M. Shi, Z.B. Ding, A.W. Ke, Z. Dai, S.J. Qiu, K. Song, J. Fan, Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin. Cancer Res. 19, 5994–6005 (2013)
M. Schwartz, Y. Zhang, J.D. Rosenblatt, B cell regulation of the anti-tumor response and role in carcinogenesis. J. Immunother. Cancer 4, 40 (2016)
S. Shalapour, J. Font-Burgada, G. Di Caro, Z. Zhong, E. Sanchez-Lopez, D. Dhar, G. Willimsky, M. Ammirante, A. Strasner, D.E. Hansel, C. Jamieson, C.J. Kane, T. Klatte, P. Birner, L. Kenner, M. Karin, Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 521, 94–98 (2015)
L. Qian, G.R. Bian, Y. Zhou, Y. Wang, J. Hu, X. Liu, Y. Xu, Clinical significance of regulatory B cells in the peripheral blood of patients with oesophageal cancer. Cent. Eur. J. Immunol. 40, 263–265 (2015)
W.W. Wang, X.L. Yuan, H. Chen, G.H. Xie, Y.H. Ma, Y.X. Zheng, Y.L. Zhou, L.S. Shen, CD19+CD24hiCD38hiBregs involved in downregulate helper T cells and upregulate regulatory T cells in gastric cancer. Oncotarget 6, 33486–33499 (2015)
X. Wei, Y. Jin, Y. Tian, H. Zhang, J. Wu, W. Lu, X. Lu, Regulatory B cells contribute to the impaired antitumor immunity in ovarian cancer patients. Tumor Biol. 37, 6581–6588 (2016)
P.B. Olkhanud, B. Damdinsuren, M. Bodogai, R.E. Gress, R. Sen, K. Wejksza, E. Malchinkhuu, R.P. Wersto, A. Biragyn, Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 71, 3505–3515 (2011)
M. Bodogai, C. Lee Chang, K. Wejksza, J. Lai, M. Merino, R.P. Wersto, R.E. Gress, A.C. Chan, C. Hesdorffer, A. Biragyn, Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res. 73, 2127–2138 (2013)
F. Mehdipour, M. Razmkhah, A. Hosseini, M. Bagheri, A. Safaei, A.R. Talei, A. Ghaderi, Increased B regulatory phenotype in non-metastatic lymph nodes of node-positive breast cancer patients. Scand. J. Immunol. 83, 195–202 (2016)
H.E. Kohrt, N. Nouri, K. Nowels, D. Johnson, S. Holmes, P.P. Lee, Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2, e284 (2005)
Z. Faghih, N. Erfani, M.R. Haghshenas, A. Safaei, A.R. Talei, A. Ghaderi, Immune profiles of CD4+ lymphocyte subsets in breast cancer tumor draining lymph nodes. Immunol. Lett. 158, 57–65 (2014)
F. Balkwill, Tumour necrosis factor and cancer. Nat. Rev. Cancer 9, 361–371 (2009)
G.D. Kalliolias, L.B. Ivashkiv, TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 12, 49–62 (2016)
B.W. Tse, K.F. Scott, P.J. Russell, Paradoxical roles of tumour necrosis factor-alpha in prostate cancer biology. Prostate Cancer 2012, 128965 (2012)
X. Chen, J.J. Oppenheim, Contrasting effects of TNF and anti-TNF on the activation of effector T cells and regulatory T cells in autoimmunity. FEBS Lett. 585, 3611–3618 (2011)
E.C. Lee, P. Zhan, R. Schallhom, K. Packman, M. Tenniswood, Antiandrogen-induced cell death in LNCaP human prostate cancer cells. Cell Death Differ. 10, 761–771 (2003)
L.F. Fajardo, H.H. Kwan, J. Kowalski, S.D. Prionas, A.C. Allison, Dual role of tumor necrosis factor-alpha in angiogenesis. Am. J. Pathol. 140, 539–544 (1992)
S.J. Leibovich, P.J. Polverini, H.M. Shepard, D.M. Wiseman, V. Shively, N. Nuseir, Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature 329, 630–632 (1987)
L. Schweigerer, B. Malerstein, D. Gospodarowicz, Tumor necrosis factor inhibits the proliferation of cultured capillary endothelial cells. Biochem. Biophys. Res. Commun. 143, 997–1004 (1987)
N.S. Zuckerman, H. Yu, D.L. Simons, N. Bhattacharya, V. Carcamo-Cavazos, N. Yan, F.M. Dirbas, D.L. Johnson, E.J. Schwartz, P.P. Lee, Altered local and systemic immune profiles underlie lymph node metastasis in breast cancer patients. Int. J. Cancer 132, 2537–2547 (2013)
F. Annunziato, L. Cosmi, F. Liotta, E. Lazzeri, R. Manetti, V. Vanini, P. Romagnani, E. Maggi, S. Romagnani, Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J. Exp. Med. 196, 379–387 (2002)
D. Mougiakakos, C.C. Johansson, R. Jitschin, M. Bottcher, R. Kiessling, Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood 117, 857–861 (2011)
X. Chen, X. Wu, Q. Zhou, O.M. Howard, M.G. Netea, J.J. Oppenheim, TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J. Immunol. 190, 1076–1084 (2013)
X. He, S. Landman, S.C. Bauland, J. van den Dolder, H.J. Koenen, I. Joosten, A TNFR2-agonist facilitates high purity expansion of human low purity Treg cells. PLoS One 11, e0156311 (2016)
M. Leclerc, S. Naserian, C. Pilon, A. Thiolat, G.H. Martin, C. Pouchy, C. Dominique, Y. Belkacemi, F. Charlotte, S. Maury, B.L. Salomon, J.L. Cohen, Control of GVHD by regulatory T cells depends on TNF produced by T cells and TNFR2 expressed by regulatory T cells. Blood 128, 1651–1659 (2016)
M. Nagar, J. Jacob-Hirsch, H. Vernitsky, Y. Berkun, S. Ben-Horin, N. Amariglio, I. Bank, Y. Kloog, G. Rechavi, I. Goldstein, TNF activates a NF-kappaB-regulated cellular program in human CD45RA- regulatory T cells that modulates their suppressive function. J. Immunol. 184, 3570–3581 (2010)
X. Valencia, G. Stephens, R. Goldbach-Mansky, M. Wilson, E.M. Shevach, P.E. Lipsky, TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood 108, 253–261 (2006)
Q. Zhang, F. Cui, L. Fang, J. Hong, B. Zheng, J.Z. Zhang, TNF-alpha impairs differentiation and function of TGF-beta-induced Treg cells in autoimmune diseases through Akt and Smad3 signaling pathway. J. Mol. Cell Biol. 5, 85–98 (2013)
X. Chen, Y. Nie, H. Xiao, Z. Bian, A.J. Scarzello, N.-Y. Song, A.L. Trivett, D. Yang, J.J. Oppenheim, TNFR2 expression by CD4 effector T cells is required to induce full-fledged experimental colitis. Sci. Rep. 6, 32834 (2016)
H. Sepulveda, A. Cerwenka, T. Morgan, R.W. Dutton, CD28, IL-2-independent costimulatory pathways for CD8 T lymphocyte activation. J. Immunol. 163, 1133–1142 (1999)
L. Gorelik, Y. Bar-Dagan, M.B. Mokyr, Insight into the mechanism(s) through which TNF promotes the generation of T cell-mediated antitumor cytotoxicity by tumor bearer splenic cells. J. Immunol. 156, 4298–4308 (1996)
X. Chen, R. Hamano, J.J. Subleski, A.A. Hurwitz, O.M. Howard, J.J. Oppenheim, Expression of costimulatory TNFR2 induces resistance of CD4+FoxP3- conventional T cells to suppression by CD4+FoxP3+ regulatory T cells. J. Immunol. 185, 174–182 (2010)
A.P. Cope, M. Londei, N.R. Chu, S.B. Cohen, M.J. Elliott, F.M. Brennan, R.N. Maini, M. Feldmann, Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J. Clin. Invest. 94, 749–760 (1994)
R.M. Aspalter, M.M. Eibl, H.M. Wolf, Regulation of TCR-mediated T cell activation by TNF-RII. J. Leukoc. Biol. 74, 572–582 (2003)
J.M. Lykken, K.M. Candando, T.F. Tedder, Regulatory B10 cell development and function. Int. Immunol. 27, 471–477 (2015)
R. de Waal Malefyt, H. Yssel, J.E. de Vries, Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J. Immunol. 150, 4754–4765 (1993)
A.P. Vicari, C. Chiodoni, C. Vaure, S. Ait-Yahia, C. Dercamp, F. Matsos, O. Reynard, C. Taverne, P. Merle, M.P. Colombo, A. O'Garra, G. Trinchieri, C. Caux, Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J. Exp. Med. 196, 541–549 (2002)
J. Emmerich, J.B. Mumm, I.H. Chan, D. LaFace, H. Truong, T. McClanahan, D.M. Gorman, M. Oft, IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 72, 3570–3581 (2012)
K.L. Dennis, A. Saadalla, N.R. Blatner, S. Wang, V. Venkateswaran, F. Gounari, H. Cheroutre, C.T. Weaver, A. Roers, N.K. Egilmez, K. Khazaie, T-cell expression of IL10 is essential for tumor immune surveillance in the small intestine. Cancer Immunol. Res. 3, 806–814 (2015)
J.B. Mumm, J. Emmerich, X. Zhang, I. Chan, L. Wu, S. Mauze, S. Blaisdell, B. Basham, J. Dai, J. Grein, C. Sheppard, K. Hong, C. Cutler, S. Turner, D. LaFace, M. Kleinschek, M. Judo, G. Ayanoglu, J. Langowski, D. Gu, B. Paporello, E. Murphy, V. Sriram, S. Naravula, B. Desai, S. Medicherla, W. Seghezzi, T. McClanahan, S. Cannon-Carlson, A.M. Beebe, M. Oft, IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell 20, 781–796 (2011)
H. Groux, F. Cottrez, M. Rouleau, S. Mauze, S. Antonenko, S. Hurst, T. McNeil, M. Bigler, M.G. Roncarolo, R.L. Coffman, A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J. Immunol. 162, 1723–1729 (1999)
I.H. Chan, V. Wu, S. McCauley, E.A. Grimm, J.B. Mumm, IL-10: Expanding the immune oncology horizon. Receptors Clin. Investig. 2 (2015)
I.H. Chan, V. Wu, M. Bilardello, B. Jorgenson, H. Bal, S. McCauley, P. Van Vlasselaer, J.B. Mumm, PEG-rIL-10 treatment decreases FoxP3(+) Tregs despite upregulation of intratumoral IDO. OncoImmunology 5, e1197458 (2016)
K.P. Papadopoulos, A. Naing, J.R. Infante, D.J. Wong, K.A. Autio, P.A. Ott, G.S. Falchook, M. Patel, S. Pant, D.W. Rasco. Anti-tumor activity of PEGylated human IL-10 (AM0010) in patients with pancreatic or colorectal cancer. Pain 1, 4.5 (2016)
Acknowledgements
This study was financially supported by grants from the Iran National Science Foundation (Grant No. 91004076), Shiraz Institute for Cancer Research (Grant No. ICR-100-508) and Shiraz University of Medical Sciences (Grant No. 91-6100). We thank K. Shashok (AuthorAID in the Eastern Mediterranean) for improving the text of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethical approval
This study was performed according to the ethical standards of the Ethical Committee of Shiraz University of Medical Sciences and in compliance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehdipour, F., Razmkhah, M., Faghih, Z. et al. The significance of cytokine-producing B cells in breast tumor-draining lymph nodes. Cell Oncol. 42, 381–395 (2019). https://doi.org/10.1007/s13402-019-00433-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-019-00433-3