Abstract
Background
The CXCL12-CXCR4 chemokine axis plays an important role in cell trafficking as well as in tumor progression. In colorectal cancer (CRC), the chemokine receptor CXCR4 has been shown to be an unfavorable prognostic factor in some studies, however, the role of its activated (phosphorylated) form, pCXCR4, has not yet been evaluated. Here, we aimed to investigate the prognostic value of CXCR4 and pCXCR4 in a large cohort of CRC patients.
Patients and methods
A tissue microarray (TMA) of 684 patient specimens of primary CRCs was analyzed by immunohistochemistry (IHC) for the expression of CXCR4 and pCXCR4 by tumor cells and tumor-infiltrating immune cells (TICs).
Results
The combined high expression of CXCR4 and pCXCR4 showed a favorable 5-year overall survival rate (68%; 95%CI = 59–76%) compared to tumors showing a high expression of CXCR4 only (48%; 95%CI = 41–54%). High expression of pCXCR4 was significantly associated with a favorable prognosis in a test and validation group (p = 0.015 and p = 0.0001). Moreover, we found that CRCs with a high density of pCXCR4+ tumor-infiltrating immune cells (TICs) also showed a favorable prognosis in a test and validation group (p = 0.054 and p = 0.004). Univariate Cox regression analysis for TICs revealed that a high density of pCXCR4+ TICs was a favorable prognostic marker for overall survival (HR = 0.97,95%CI = 0.96–1.00; p = 0.01). In multivariate Cox regression survival analyses a high expression of pCXCR4 in tumor cells lost its association with a better overall survival (HR = 0.99; 95%CI = 0.99–1.00, p = 0.098).
Conclusion
Our results show that high densities of CXCR4 and pCXCR4 positive TICs are favorable prognostic factors in CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Colorectal cancer (CRC) is the second most common cause of cancer mortality in developed countries [1]. While surgical tumor resection remains the cornerstone therapy for localized CRC, adjuvant therapy is recommended for patients with locally advanced stages and palliative systemic therapy for patients with metastatic disease [2]. The selection for adjuvant chemotherapy is largely based on clinical criteria such as tumor node metastasis (TNM) classification and other histopathological factors. However, these clinico-pathological features alone may inadequately predict cancer aggressiveness [3, 4]. Therefore, several prognostic and predictive biomarkers have been studied to improve prognostic information and patient selection for adjuvant treatment [5, 6]. Numerous studies have conclusively shown that microenvironmental factors are crucial for CRC development and progression [7, 8] and also highlight the role of chemokines in tumor invasion and cancer metastasis [9]. As metastatic disease dramatically decreases survival [10], it is pivotal to continue evaluating the prognostic value of chemokines in CRC.
The chemokine CXCL12 and one of its receptors, CXCR4, have been shown to play key roles in the tumor-stromal communication affecting cancer growth, angiogenesis and metastasis formation [11]. CXCR4 expression has been linked to cancer progression and metastasis in hematopoietic as well as in various non-hematopoietic malignancies [9, 12, 13]. It has also been reported as a prognostic marker in cancers of different origin [14,15,16]. Its interaction with CXCL12 is thought to play an important role in tumor proliferation, invasion, lymph node homing and metastatic progression. After binding by the corresponding ligand CXCL12, CXCR4 is phosphorylated [17]. Since the phosphorylated form of CXCR4 (pCXCR4) plays the biologically active role, the analysis of CXCR4 expression alone appears to be insufficient to support its functional role in cancer metastasis.
Literature regarding the prognostic role of CXCR4 is relatively vague. Some studies have shown that high CXCR4 expression in CRC patients correlates with an advanced tumor stage [18], an increased risk for recurrence and distant metastasis [19,20,21] and a poor overall survival [22]. A recent meta-analysis concluded that there is a significant association between CXCR4 expression and poor survival [23]. However, most studies have included small numbers of patients and have presented important methodological differences. On the other hand, there are also studies that failed to observe significant correlations between CXCR4 expression and metastasis [24] and/or overall survival [17, 25].
pCXCR4 expression has been reported to have a prognostic value superior to that of CXCR4 expression in breast cancer [26]. Interestingly, there is a lack of data regarding the prognostic significance of pCXCR4 in CRC. In addition, most studies have only evaluated CXCR4 expression on tumor cells, while its expression on tumor-infiltrating immune cells (TICs) has not been thoroughly investigated.
The purpose of our study was to comparatively investigate the prognostic value of CXCR4 and pCXCR4 expression in a large cohort of CRC patients. In addition to CXCR4/pCXCR4 expression by the tumor cells, we also assessed the relevance of CXCR4/pCXCR4 positive TICs.
2 Materials and methods
2.1 Tissue microarray construction
684 unselected, non-consecutive, clinically annotated, primary CRC specimens were included in a tissue microarray (TMA) following approval by the local ethics committee. Formalin-fixed, paraffin-embedded (FFPE) tissue blocks were prepared according to standard procedures. Tissue cylinders with a diameter of 0.6 mm were punched from morphologically representative areas of each donor block and brought into one recipient paraffin block (30 × 25mm), using a semi-automated tissue arrayer. Each punch was made from the center of the tumor to enable each TMA spot to include at least 50% tumor cells.
2.2 Clinico-pathological features
Clinico-pathological data were collected retrospectively in a non-stratified and non-matched manner. Annotation included patient age and gender, tumor diameter, location, pT/pN stage, grade, histologic subtype, vascular invasion, border configuration, presence of peritumoral lymphocytic inflammation at the invasive tumor front and disease-specific survival. Tumor border configuration and peritumoral lymphocytic inflammation were evaluated using the original H&E slides of the resection specimens corresponding to each TMA punch.
2.3 Immunohistochemistry
Staining protocols for the primary antibodies directed against CXCR4 (Abcam, ab2074; 1:50) and pCXCR4 (Abcam, ab74012; 1:200) were performed as recommended by the manufacturers, including positive control tissue samples exactly as previously described [27]. Immunohistochemistry was performed using the automated staining system Benchmark XT (Roche/Ventana Medical Systems, Tuscon, AZ).
2.4 Evaluation of immunohistochemistry
Immunohistochemical readings were performed by two trained research fellows [F.R. and I.F] and data were independently validated by an additional investigator [L.T.]. Histoscores for tumor cells were obtained by multiplying percentages positive cells by stain intensities (0 = negative, 1 = weak, 2 = moderate, 3 = strong). TICs were counted for each punch (approximately one high power [20×] field).
2.5 Statistical analysis
All statistical analyses were performed using STATA software version 13 (StataCorp, College Station, TX, USA). Associations with survival were explored using the Cox proportional hazards regression model. Cut-off values used to classify CRC with low or high immune cell infiltrations were obtained using median values. Therefore, threshold values for CXCR4 and pCXCR4 positivity in TICs were 23 and 2 cells/TMA-punch and 100 and 0 for CXCR4 and pCXCR4 for histoscores, related to tumor expression, respectively. Chi-square, Fisher’s exact, and Kruskal-Wallis tests were used to determine associations between CXCR4 and pCXCR4 positivity and clinico-pathological features.
For survival analysis, the study population was randomly assigned to test and validation groups. Univariate survival analysis was performed by Kaplan-Meier and log rank tests. Further analysis included four combinations of CXCR4 and pCXCR4 positivity: CXCR4−/pCXCR4-, CXCR4+/pCXCR4-, CXCR4−/pCXCR4+ and CXCR4+/pCXCR4 + .
The assumption of proportional hazards was verified for all markers by analyzing correlation of Schoenfeld residuals and ranks of individual failure times. Any missing clinico-pathological information was assumed to be missing at random. Subsequently, CXCR4 and pCXCR4 data were entered into multivariate Cox regression analysis and hazard ratios (HR), and 95% confidence intervals (CI) were used to determine prognostic effects on survival time. P-values < 0.05 were considered statistically significant.
3 Results
3.1 Patient and tumor characteristics
Tissue samples from a total of 684 CRC patients were analyzed. The median age was 70 years (range: 30–95) and 53.2% of the patients were female. In 69.3% of the patients CRC was located in the left hemicolon and in the remaining 30.4% in the right hemicolon. The TMA included 600 mismatch repair (MMR)-proficient CRC specimens and 84 MMR-deficient CRC specimens (12.3%), as identified by MLH1, MSH2 and MSH6 expression analysis [28]. The median overall survival was 55 months (range 0–151) and the 5-year overall survival rate was 53.7 (95% CI = 49.8–57.4). Losses due to missing information or miscarried TMA punches usually represented about 15% of the data (Table 1).
3.2 CXCR4 and pCXCR4 expression in CRC surgical specimens
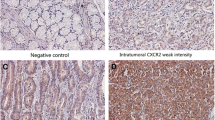
The expression of CXCR4 and pCXCR4 in tumor cells and in TICs was highly variable. Representative examples of negative and positive CXCR4/pCXCR4 tumor cells and TICs are presented in Fig. 1. Expectably, only a fraction of CXCR4+ cells showed evidence of receptor phosphorylation. The mean values for CXCR4 and pCXCR4 histoscores were 154.6 (± 98.7) and 31.7 (± 62.4), respectively. For TIC density, the corresponding numbers were 76.3 (±153.2) and 5.1 (±10.2), respectively.
Positive (high) CXCR4/pCXCR4 expression in CRC cells and CRC infiltration by CXCR4/pCXCR4-positive TICs compared to negative samples. CRC samples were stained with CXCR4 and pCXCR4-specific reagents. Tumor punches are representative of absence of infiltration (panel a and b), positive/high expression on tumor cells (panel c and d), and positive/high infiltration of CXCR4/pCXCR4-positive TICs (panels e and f), respectively. Magnification: 60×
High correlation coefficients were observed between CXCR4+ and pCXCR4+ immune cell infiltration (rs = 0.363, p < 0.001) and CXCR4 and pCXCR4 histoscores (rs = 0.264, p < 0.001), thus confirming the integrity of our measurements. More importantly, pCXCR4 histoscore and pCXCR4+ immune cell infiltration were also significantly correlated (rs = 0.244, p < 0.001), suggesting that the CXCL12 ligand may be active in the tumor microenvironment on both tumor and infiltrating immune cells. Instead, we found that CXCR4+ histoscore and CXCR4+ immune cell infiltration were poorly correlated (rs = 0.121).
3.3 Association of clinico-pathological features according to CXCR4 and pCXCR4 expression
Clinico-pathological features under investigation and their relation to the four subgroups identified using median values of CXCR4+ and pCXCR4+ expression (CXCR4low/pCXCR4low, CXCR4high/pCXCR4low, CXCR4low/pCXCR4high and CXCR4high/pCXCR4high) are listed in Tables 2 and 3 for histoscores and immune cell density infiltration, respectively. CRC tissues with a high expression of pCXCR4, alone or in combination with CXCR4 in the tumor cells, were significantly more frequently located in the left-sided colon (p = 0.006) and were characterized by a significantly lower T- (p = 0.001) and N-stage (p < 0.001), absence of vascular invasion (p = 0.004) and absence of an infiltrative tumor border (p = 0.001) (Table 2). CRCs with a high density of CXCR4/pCXCR4+ or pCXCR4+ TICs were also characterized by a significantly lower N-stage (p < 0.001) (Table 3).
In order to directly evaluate the relevance of receptor phosphorylation, we next differentially analyzed CRC clinico-pathological features in tissues with a high CXCR4 but a different pCXCR4 expression. Most interestingly, we found that CRCs with a high CXCR4+ histoscore were characterized by a significantly more frequent pT1-2 and pN0 stage in pCXCR4+ compared to pCXCR4- cases (p = 0.011 and p = 0.002, respectively) and by a significantly more frequent detection of a “pushing” tumor border (p = 0.028).
Regarding TICs in the presence of a high CXCR4+ infiltration, we found that CRCs displaying pCXCR4+ “high” infiltrates were significantly more frequently characterized by a pN0 stage compared to tumors with pCXCR4+ immune cell infiltration (p = 0.0004). Taken together, these data suggest that receptor phosphorylation, rather than mere receptor expression, is associated with important clinico-pathological characteristics.
3.4 Prognostic significance of CXCR4 and pCXCR4 expression by tumor cells and by tumor-infiltrating immune cells
Kaplan-Meier curves related to histoscore data revealed that pCXCR4 expression was significantly associated with a favorable prognosis in both test and validation groups (p = 0.0001 and p = 0.015; Fig. 2a and b). In sharp contrast, we found that expression of CXCR4 in the absence of pCXCR4 had no impact on overall survival, even considering the whole cohort (p = 0.132; data not shown). Indeed, CRCs with a high expression of CXCR4 and pCXCR4 on tumor cells showed a favorable 5-year overall survival rate (68%, 95%CI 59–76%) (p < 0.001; Table 2), whereas no difference was observed between tumors with a high CXCR4 expression only (48%, 95%CI = 39–56%) or low CXCR4/pCXCR4 expression (48%, 95%CI = 41–54%).
Effects of pCXCR4+ tumor cells and pCXCR4+ TIC infiltration on overall survival in patients with CRC. Kaplan-Meier curves were created according to the expression of pCXCR4 on tumor cells (curves a-c) and pCXCR4+ TICs (curves d-f) in patients with CRC (n = 684). Kaplan-Meier overall survival curves for the histoscore show that high expression of pCXCR4 was significantly associated with a favorable prognosis in a test (a, p = 0.0001) and validation (b, p = 0.015) group. Overall survival was also evaluated for CRC showing evidence of a CXCR4/pCXCR4 high histoscore, as compared to tumors with CXCR4 high pCXCR4 low values on the whole cohort (c, p = 0.005). Kaplan-Meier curves for infiltration by TICs showed at least a trend towards a favorable prognosis for CRCs with a high density of pCXCR4+ TICs in a test (c, p = 0.054) and validation (d, p = 0.004) group. Kaplan-Meier curves were also analyzed for CRC showing evidence of CXCR4/pCXCR4 high TIC infiltrates, as compared to tumors with CXCR4 high pCXCR4 low TIC densities on the whole cohort (f, p = 0.06)
CRC with a high density of CXCR4/pCXCR4+ infiltrating TICs also showed a more favorable 5-year overall survival rate (63%, 95%CI = 54%-70%) compared to tumors with a low CXCR4/pCXCR4+ TIC density (p = 0.004) (Table 3). Kaplan-Meier curves for TICs revealed a trend to a more favorable prognosis for CRC with a high density of pCXCR4+ TICs compared to tumors with a low pCXCR4+ TIC density in a test group (p = 0.054; Fig. 2d) and a significant association with a more favorable prognosis in a validation group (p = 0.004; Fig. 2e). Kaplan-Meier curves for tumors with CXCR4/pCXCR4 high TIC infiltrates compared to tumors with CXCR4 high pCXCR4 low TIC densities showed a trend to a better prognosis for the CXCR4 high/pCXCR4 high group (p = 0.06, Fig. 2f).
3.5 Univariate and multivariate analysis of CXCR4 and pCXCR4 expression by tumor cells and tumor-infiltrating immune cells
Univariate Cox regression analysis of the histoscores revealed that CXCR4 and pCXCR4 positive staining is significantly associated with an increased overall survival (HR 0.99; 95%CI = 0.99–1.0; p = 0.035 and p < 0.001, respectively). Age, male gender, T-stage, tumor grading, N-stage, invasive margin and vascular invasion were all significantly associated with a poor prognosis in univariate analyses (Table 4). Univariate Cox regression analysis for TICs showed that a high density of pCXCR4+ TICs also serves as a favorable prognostic marker for overall survival (HR = 0.98; 95%CI = 0.97–1.00; p = 0.02). Instead, we found that the density of CXCR4+ TICs had no impact on overall survival (Table 5).
Through multivariate hazard Cox regression survival analysis, however, we found that high expression of pCXCR4+ on tumor cells (histoscore) failed to retain its role as an independent prognostic factor for overall survival (HR = 0.99; 95%CI = 0.99–1.0; p = 0.098; Table 4). Similarly, multivariate Hazard Cox regression survival analysis of TICs showed the absence of an independent prognostic significance for CXCR4+ (HR = 1.0; 95%CI = 0.99–1.0; p = 0.920) and pCXCR4+ (HR = 0.99; 95%CI = 0.97–1.01; p = 0.200) immune cell infiltration on overall survival (Table 5). On the other hand, we found that an increased age (HR = 1.04; 95%CI = 1.03–1.05; p < 0.001), male gender (HR = 1.53; 95%CI = 1.2–1.9; p < 0.001), a higher T-stage (HR = 2.02; 95%CI = 1.33–3.08; p = 0.001) and N-stage (HR = 2.38; 95%CI = 1.85–3.07; p < 0.001) were independently associated with a poor prognosis (Tables 4 and 5).
4 Discussion
In the past, several studies have been carried out to evaluate the prognostic significance of CXCR4 expression in CRC. However, methodological differences, inappropriate immunohistochemical protocols and limited sample sizes have hampered the drawing of definite conclusions. Most importantly, none of these studies has analyzed the expression of the phosphorylated, activated form of this receptor, pCXCR4. To the best of our knowledge, this is the first study analyzing the prognostic significance of pCXCR4 in a large cohort of CRC patients. Our data indicate that CXCR4 expression in the absence of phosphorylation on tumor cells has no prognostic significance, whereas expression of its phosphorylated form pCXCR4 is associated with a favorable clinical outcome.
These data are conflicting with those of most previous studies, showing a negative prognostic impact of CXCR4 expression in CRC cells on survival. One study reported a negative prognostic impact of CXCR4 expression in stage I, II and IV CRCs [29]. Another study reported that high CXCR4 expression is associated with higher TNM stages, rectal cancer, metastases and a decreased survival [19]. However, very small sample collections (n = 92 and n = 97, respectively) question the significance of these analyses. High nuclear expression of CXCR4 has also been proposed to be associated with a poor survival in stage III CRC [30] and according to Spetjens et al. [31] nuclear, but not cytoplasmic, location of CXCR4 staining represents an independent negative prognostic factor. However, we failed to observe nuclear CXCR4 staining in our study cohort, and it has been reported that nuclear staining of CXCR4 seems to represent an artifact [32] related either to excessive antigen retrieval by pressure cooking or the application of poorly working primary antibodies, since the outer membrane receptor CXCR4 has no linked nuclear functions [33].
CXCL12, the only known ligand for CXCR4, is produced by a limited number of cell types including endothelial and bone marrow cells, mucosal epithelial cells, tumor cells and T-lymphocytes [34, 35]. CXCL12 expression is increased in tissues characterized by neo-angiogenesis and inflammation, supporting chemotactic gradients attracting CXCR4+ immune cells, mainly CD4+ and dendritic cells. As yet, the effects of CXCR4+ immune cells on tumor progression have not been studied in detail [13, 35]. Our study shows for the first time that activation of CXCR4, as suggested by the presence of its pCXCR4 form, in CRC tumors and infiltrating immune cells is significantly associated with a favorable prognosis. Current understanding of the CXCL12-CXCR4 axis postulates that CXCR4 expression by cancer cells guides them to migrate to ectopic sites with a high CXCL12 expression. However, our data indicate that the presence of pCXCR4 on tumor cells in CRC patients is associated with a lack of evidence of node metastases.
Consistent with our data, Stanisavljevic et al. [30] have shown that CXCL12 expression represents a positive prognostic factor for disease-free survival in CRC. Furthermore, Wendt et al. [36] showed that re-establishment of CXCL12 expression reduced metastasis in in vivo CRC experimental models. Remarkably, Roy et al. [37] also showed in an experimental model of pancreatic cancer that CXCL12 expression inhibited tumor growth and cancer cell metastasis formation through cell-cycle arrest, resulting in an increased overall survival.
From a mechanistic point of view, it is tempting to speculate that local CXCL12 production, resulting in CXCR4 phosphorylation, may facilitate the retention of CXCR4+ tumor cells within primary tumor tissues, thus preventing their migration towards potential metastatic sites. On the other hand, local CXCL12 production may favor the recruitment of immune cells associated with an improved prognosis, including CD8+ T-cells [3, 4, 38]. More intriguingly, a recent study has suggested that CXCL12 produced by neutrophils may guide activated CD8+ T-cells within mucosal tissues [39]. Previously, our group has observed that, indeed, neutrophil infiltration is also associated with a favorable prognosis in CRC [40]. However, CXCL12 production by these cells in CRC patients was not addressed. Further research is warranted to clarify the mechanistic aspects associated with the prognostic significance of pCXCR4 expression in CRC tissues.
To date, very few studies have been reported on the prognostic significance of pCXCR4. We could identify only two studies, one in B-acute lymphoblastic leukemia and another one in non-small cell lung cancer (NSCLC) [32, 41]. The study on NSCLC failed to detect any prognostic significance either for CXCR4 or pCXCR4 expression [32], whereas Konoplev et al. [41] reported a negative prognostic significance for pCXCR4 expression, but not CXCR4 expression, in B-acute lymphoblastic leukemia. However, consistent with our findings on total CXCR4 expression, Spano et al. [42] reported better disease outcomes for a higher CXCR4 expression in early-stage NSCLC. Importantly, the CXCL12-CXCR4 axis is considered a potential therapeutic target, not only in hematologic, but also in metastatic solid tumors [43] and clinical trials are now ongoing. Our data suggest an essential need of including the evaluation of both the presence of CXCR4 and its active form, pCXCR4, into the translational design of such studies. Another significant focus of the present study is the evaluation of CXCR4+ and pCXCR4+ TICs. Although there are many reports on the potential function of CXCR4 in different cancer types, there is a lack of data on its expression on such TICs. In this regard, our data may contribute to the definition of immune contexture features and their prognostic significance in CRC [4, 7, 38].
Our study suffers from a number of limitations. Firstly, the TMA technology may fail to represent tumor tissue heterogeneity. However, the punches included in this TMA were derived from tumor centers and included at least 50% cancer cells. Furthermore, the number of individual CRC specimens (> 600) compensates, at least in part, for the heterogeneity of the immune contexture in different tumor areas. Second, this is a retrospective cohort study. However, data emerging from large retrospective analyses may help in the development of prospective and mechanistic studies, currently planned by our group. Finally, the cohort investigated in this study includes CRC patients surgically treated between 1985 and 1998, prior to a widespread use of neoadjuvant treatment regimens for rectal cancer. Thus, while these results may not be fully representative of current clinical treatments, they are more likely to mirror CRC immunobiology. On the other hand, our results would urge the analysis of CXCL12 expression in the samples under investigation. However, detection of cytokines or, generally, soluble factors by immunohistochemistry is highly problematic and gene expression studies, although potentially suggestive, fail to provide evidence of specific protein production.
In summary, our data show for the first time that expression of activated pCXCR4 in tumor cells and a high density of CXCR4 and pCXCR4 positive immune cell infiltration in CRC represent favorable prognostic factors, thereby shedding a new light on the biological role of CXCR4 in CRC progression.
References
J. Ferlay, I. Soerjomataram, R. Dikshit, S. Eser, C. Mathers, M. Rebelo, D.M. Parkin, D. Forman, F. Bray, Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136, E359–E386 (2015)
H. Brenner, M. Kloor, C.P. Pox, Colorectal cancer. Lancet 383, 1490–1502 (2014)
I. Zlobec, A. Lugli, Prognostic and predictive factors in colorectal cancer. Postgrad Med J 84, 403–411 (2008)
F. Pagès, J. Galon, M.-C. Dieu-Nosjean, E. Tartour, C. Sautès-Fridman, W.-H. Fridman, Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 29, 1093–1102 (2010)
T. Tanaka, M. Tanaka, T. Tanaka, R. Ishigamori, Biomarkers for colorectal cancer. Int J Mol Sci 11, 3209–3225 (2010)
G. Lech, R. Słotwiński, M. Słodkowski, I.W. Krasnodębski, Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J Gastroenterol 22, 1745–1755 (2016)
L.M. Coussens, Z. Werb, Inflammation and cancer. Nature 420, 860–867 (2002)
A. Lasry, A. Zinger, Y. Ben-Neriah, Inflammatory networks underlying colorectal cancer. Nat Immunol 17, 230–240 (2016)
J.A. Burger, CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood 107, 1761–1767 (2006)
R. Siegel, J. Ma, Z. Zou, A. Jemal, Cancer statistics, 2014. CA Cancer J Clin 64, 9–29 (2014)
F. Guo, Y. Wang, J. Liu, S.C. Mok, F. Xue, W. Zhang, CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene 35, 816–826 (2015)
X. Sun, G. Cheng, M. Hao, J. Zheng, X. Zhou, J. Zhang, R.S. Taichman, K.J. Pienta, J. Wang, CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev 29, 709–722 (2010)
L. Lombardi, F. Tavano, F. Morelli, T.P. Latiano, P. Di Sebastiano, E. Maiello, Chemokine receptor CXCR4: role in gastrointestinal cancer. Crit Rev Oncol Hematol 88, 696–705 (2013)
C.C. Schimanski, P.R. Galle, M. Moehler, Chemokine receptor CXCR4-prognostic factor for gastrointestinal tumors. World J Gastroenterol 14, 4721–4724 (2008)
B.E. Lippitz, Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol 14, e218–e228 (2013)
B.A. Teicher, S.P. Fricker, CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res 16, 2927–2931 (2010)
M. Thelen, Dancing to the tune of chemokines. Nat Immunol 2, 129–134 (2001)
S.-C. Wang, J.-K. Lin, H.-S. Wang, S.-H. Yang, A.F.-Y. Li, S.-C. Chang, Nuclear expression of CXCR4 is associated with advanced colorectal cancer. Int J Color Dis 25, 1185–1191 (2010)
C.C. Schimanski, S. Schwald, N. Simiantonaki, C. Jayasinghe, U. Gönner, V. Wilsberg, T. Junginger, M.R. Berger, P.R. Galle, M. Moehler, Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res 11, 1743–1750 (2005)
J. Kim, T. Mori, S.L. Chen, F.F. Amersi, S.R. Martinez, C. Kuo, R.R. Turner, X. Ye, A.J. Bilchik, D.L. Morton, D.S.B. Hoon, Chemokine receptor CXCR4 expression in patients with melanoma and colorectal cancer liver metastases and the association with disease outcome. Ann Surg 244, 113–120 (2006)
N. Yoshitake, H. Fukui, H. Yamagishi, A. Sekikawa, S. Fujii, S. Tomita, K. Ichikawa, J. Imura, H. Hiraishi, T. Fujimori, Expression of SDF-1 alpha and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer. Br J Cancer 98, 1682–1689 (2008)
L.-N. Li, K.-T. Jiang, P. Tan, A.-H. Wang, Q.-Y. Kong, C.-Y. Wang, H.-R. Lu, J. Wang, Prognosis and Clinicopathology of CXCR4 in Colorectal Cancer Patients: a Meta-analysis. Asian Pac J Cancer Prev 16, 4077–4080 (2015)
S. Lv, Y. Yang, S. Kwon, M. Han, F. Zhao, H. Kang, C. Dai, R. Wang, The association of CXCR4 expression with prognosis and clinicopathological indicators in colorectal carcinoma patients: a meta-analysis. Histopathology 64, 701–712 (2014)
F. Xu, F. Wang, M. Di, Q. Huang, M. Wang, H. Hu, Y. Jin, J. Dong, M. Lai, Classification based on the combination of molecular and pathologic predictors is superior to molecular classification on prognosis in colorectal carcinoma. Clin Cancer Res 13, 5082–5088 (2007)
S. Saigusa, Y. Toiyama, K. Tanaka, T. Yokoe, Y. Okugawa, A. Kawamoto, H. Yasuda, Y. Inoue, C. Miki, M. Kusunoki, Stromal CXCR4 and CXCL12 expression is associated with distant recurrence and poor prognosis in rectal cancer after chemoradiotherapy. Ann Surg Oncol 17, 2051–2058 (2010)
S. Hassan, C. Ferrario, U. Saragovi, L. Quenneville, L. Gaboury, A. Baccarelli, O. Salvucci, M. Basik, The influence of tumor-host interactions in the stromal cell-derived factor-1/CXCR4 ligand/receptor axis in determining metastatic risk in breast cancer. Am J Pathol 175, 66–73 (2009)
L. Brault, A. Rovó, S. Decker, C. Dierks, A. Tzankov, J. Schwaller, CXCR4-SERINE339 regulates cellular adhesion, retention and mobilization, and is a marker for poor prognosis in acute myeloid leukemia. Leukemia 28, 566–576 (2014)
H. Hampel, J.A. Stephens, E. Pukkala, R. Sankila, L.A. Aaltonen, J.-P. Mecklin, A. de la Chapelle, Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology 129, 415–421 (2005)
J. Kim, H. Takeuchi, S.T. Lam, R.R. Turner, H.-J. Wang, C. Kuo, L. Foshag, A.J. Bilchik, D.S.B. Hoon, Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol 23, 2744–2753 (2005)
L. Stanisavljević, J. Aßmus, K.E. Storli, S.M. Leh, O. Dahl, M.P. Myklebust, CXCR4, CXCL12 and the relative CXCL12-CXCR4 expression as prognostic factors in colon cancer. Tumor Biol 37, 7441–7452 (2015)
F.M. Speetjens, G.J. Liefers, C.J. Korbee, W.E. Mesker, C.J.H. Van De Velde, R.L. Van Vlierberghe, H. Morreau, R.A. Tollenaar, P.J.K. Kuppen, Nuclear localization of CXCR4 determines prognosis for colorectal cancer patients. Cancer Microenviron 2, 1–7 (2009)
W. Sterlacci, S. Saker, B. Huber, M. Fiegl, A. Tzankov, Expression of the CXCR4 ligand SDF-1/CXCL12 is prognostically important for adenocarcinoma and large cell carcinoma of the lung. Virchows Arch 468, 463–471 (2016)
T. Pozzobon, G. Goldoni, A. Viola, B. Molon, CXCR4 signaling in health and disease. Immunol Lett 177, 6–15 (2016)
R.J. Phillips, M.D. Burdick, M. Lutz, J.A. Belperio, M.P. Keane, R.M. Strieter, The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med 167, 1676–1686 (2003)
O. Wald, U. Izhar, G. Amir, S. Avniel, Y. Bar-Shavit, H. Wald, I.D. Weiss, E. Galun, A. Peled, CD4+CXCR4highCD69+ T cells accumulate in lung adenocarcinoma. J Immunol 177, 6983–6990 (2006)
M.K. Wendt, P.A. Johanesen, N. Kang-Decker, D.G. Binion, V. Shah, M.B. Dwinell, Silencing of epithelial CXCL12 expression by DNA hypermethylation promotes colonic carcinoma metastasis. Oncogene 25, 4986–4997 (2006)
I. Roy, N.P. Zimmerman, A.C. Mackinnon, S. Tsai, D.B. Evans, M.B. Dwinell, CXCL12 chemokine expression suppresses human pancreatic cancer growth and metastasis. PLoS One 9, e90400 (2014)
J. Galon, A. Costes, F. Sanchez-Cabo, A. Kirilovsky, B. Mlecnik, C. Lagorce-Pagès, M. Tosolini, M. Camus, A. Berger, P. Wind, F. Zinzindohoué, P. Bruneval, P.-H. Cugnenc, et al., Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006)
K. Lim, Y.-M. Hyun, K. Lambert-Emo, T. Capece, S. Bae, R. Miller, D.J. Topham, M. Kim, Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science 349, aaa4352 (2015)
V. Governa, E. Trella, V. Mele, L. Tornillo, F. Amicarella, E. Cremonesi, M.G. Muraro, H. Xu, R. Droeser, S.R. Däster, M. Bolli, R. Rosso, D. Oertli, S. Eppenberger-Castori, L.M. Terracciano, G. Iezzi G, G.C. Spagnoli, The interplay between neutrophils and CD8(+) T cells improves survival in human colorectal cancer. Clin Cancer Res 23, 3847–3858 (2017)
S. Konoplev, J.L. Jorgensen, D.A. Thomas, E. Lin, J. Burger, H.M. Kantarjian, M. Andreeff, L.J. Medeiros, M. Konopleva, Phosphorylated CXCR4 is associated with poor survival in adults with B-acute lymphoblastic leukemia. Cancer 117, 4689–4695 (2011)
J.-P. Spano, F. Andre, L. Morat, L. Sabatier, B. Besse, C. Combadiere, P. Deterre, A. Martin, J. Azorin, D. Valeyre, D. Khayat, T. Le Chevalier, J.-C. Soria, Chemokine receptor CXCR4 and early-stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol 15, 613–617 (2004)
A. Dubrovska, M. Cojoc, F. Peitzsch, F. Trautmann, G.D. Polishchuk, A. Telegeev, Emerging targets in cancer management: role of the CXCL12/CXCR4 axis. Onco Targets Ther 6, 1347–1361 (2013)
Acknowledgements
GI was supported by SNF (CH) grant no. PP00P3-133699 and GCS was supported by SNF (CH) grant no. 310030_149745.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All authors have agreed to the submission of this manuscript and have participated in the study to a sufficient extent to be named as authors.
Conflict of interest
The authors have no conflicts of interest to disclose for the present study.
Rights and permissions
About this article
Cite this article
Weixler, B., Renetseder, F., Facile, I. et al. Phosphorylated CXCR4 expression has a positive prognostic impact in colorectal cancer. Cell Oncol. 40, 609–619 (2017). https://doi.org/10.1007/s13402-017-0348-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-017-0348-2