Abstract
Green technology, also known as environmental technology or clean technology, refers to developing and implementing products, processes, and systems that are environmentally friendly and contribute to resource conservation while reducing negative environmental impact. The present study identified the effects of ultrasound-assisted extraction (UAE) conditions over total phenolic content, total flavonoid content, and antioxidant activity of pecan nutshell extracts of Western, Wichita, and Mahan cultivars. The analyzed UAE conditions were extraction time, amplitude, and sample-solvent ratio. Antioxidant activity was evaluated through ABTS, DPPH, and FRAP assays. Optimal extraction conditions were used to obtain an extract for the determination of medium inhibitory concentration (IC50) and FTIR analysis. Results indicate that an extraction time of 25 min favored the extraction of phenolic compounds and flavonoids; in addition, extract with an extraction time of 1 min exhibits antioxidant activity by ABTS, while an extraction time of 5 min presents higher DPPH and FRAP activity. The highest phenolic compounds and flavonoids concentration was obtained with a 50% amplitude. Antioxidant activity by ABTS was higher for an extract with 80% amplitude, while an extract with 30% amplitude had higher antioxidant activity by DPPH and FRAP. A sample-solvent ratio of 1/50 had the highest content of phenolic compounds, while flavonoid content was higher in the extract with a 1/30 sample-solvent ratio. The sample-solvent ratio using UAE did not have an effect on antioxidant activity by ABTS and DPPH; however, antioxidant activity by FRAP was increased by UAE, where a 1/30 sample-solvent ratio exhibited the highest value. FTIR spectra suggests the presence of phenolic compounds, proteins, and polysaccharides. Also, bioactive compounds can be recovered from pecan nutshell through ultrasound-assisted extraction. Therefore, green technologies, like the UAE, promote sustainable development by minimizing environmental degradation and conserving natural resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The human population has been experiencing continuous growth, leading to an increasing demand for food production [1]. Since the 1970s, agricultural production has tripled, reaching 23.7 million tons of food daily [2]. As a result, the generation of agro-industrial waste has been increasing at the same rate; these residues are the result of physical, chemical, and biological processes involved in the industrialization of animal or plant products, which cannot be used as raw materials for the production chain [3,4,5]. Therefore, agribusiness seeks to reduce its environmental, economic, and social impacts by studying and developing new alternatives for sustainable management of agro-industrial waste [6].
Inadequate management of agro-industrial waste can lead to various environmental and socioeconomic problems [7]. Due to its organic nature and the significant amount of biomass it represents, agro-industrial waste is generally incinerated or deposited in landfills [8, 9]. Consequently, carbon dioxide and methane can be produced, contributing to global warming as they come in contact with the increasing concentration of greenhouse gases in the atmosphere [10, 11]. Furthermore, socioeconomic impacts associated with agro-industrial waste are mainly due to the lack of available technologies and economic resources for its proper management; as a result, companies resort to unsustainable waste management practices, especially in developing countries [12, 13]. Furthermore, the lack of viable alternatives to profit from agro-industrial waste leads to the squandering of low-cost raw materials; in addition, agro-industrial waste can cause a public health crisis and affect the population's quality of life [14, 15]. Therefore, among agribusiness’ most important strategies to improve waste management is identifying techniques for the recovery and valorization of waste [16].
Composting and animal feed production are the main alternatives to improve agro-industrial waste management; however, new technologies have expanded how agro-industrial waste can be reused [17, 18]. Among them is the extraction of bioactive compounds that interest the food, cosmetic, and pharmaceutical industries [19, 20]. Currently, industrial extraction methods require long extraction times and large volumes of solvents that can pose a risk to human health [21]. A more sustainable option for the extraction of bioactive compounds is using non-conventional techniques, as they require less organic solvents and operational times and provide high-quality extracts, classified as green technologies [22, 23]. Green technologies for the extraction of bioactive compounds are revolutionizing the fields of natural product chemistry and pharmaceuticals. Techniques such as supercritical fluid extraction, microwave-assisted extraction, and ultrasound-assisted extraction offer efficient and environmentally friendly alternatives to traditional solvent-based methods. These green extraction technologies utilize renewable resources, minimize waste generation, and reduce energy consumption, making them sustainable options for obtaining valuable bioactive compounds from botanical sources.
Ultrasound-assisted extraction (UAE) is a green technology extraction technique that allows obtaining good-quality extracts by reducing the time, energy, and power usage required by conventional techniques [24]. UAE offers several advantages over traditional extraction methods. Firstly, UAE reduces the need for harsh solvents, minimizing the environmental impact and ensuring the safety of operators. Additionally, UAE operates at lower temperatures and shorter extraction times than conventional methods, preserving the integrity of heat-sensitive compounds and minimizing energy consumption. Furthermore, UAE enhances extraction efficiency by promoting mass transfer and disrupting cell structures, resulting in higher yields of bioactive compounds from botanical sources. Moreover, UAE is a versatile technique adaptable to various scales, from laboratory research to industrial production, making it suitable for diverse applications in pharmaceuticals, nutraceuticals, and food industries. Overall, UAE represents a sustainable and efficient approach to extracting bioactive compounds while reducing environmental footprint and maximizing product quality. In this technique, sound waves in the range of 20 kHz to 100 MHz produce a phenomenon called cavitation, which allows the recovery of bioactive compounds from a matrix through the formation, growth, and collapse of microbubbles as shown in Fig. 1A [25]. After the collapse of the microbubbles, enough energy to disrupt the cell wall of the matrix is generated, causing the release of the bioactive compounds of interest as shown in Fig. 1B [26]. Therefore, when designing a UAE process, factors such as solvent, frequency, temperature, time, and amplitude must be considered as they affect the extraction of bioactive compounds [27].
Extraction of compounds by UAE. A The cavitation effect and B the mechanism extraction in cell. a Indicates that ultrasonic will act on intact cell. b Indicates cell rupture after ultrasonic treatment. c Indicates that the bioactive components are released. Shen et al. [54]
Pecan nutshell has become of interest for the extraction of bioactive compounds since de la Rosa et al. [28] identified the presence of higher concentrations of phenolic compounds and flavonoids than those in the pecan nut itself. This agro-industrial waste comes from pecan nuts manual or mechanized processing [29]. México's pecan nut production represents 40.1% of world production [30]. Around 80% of the national production is destined for exportation to China and the USA, where the latter receives an average of 28.5 million tons annually [31]. Pecan nutshell represents 40 to 50% of the nut; therefore, yearly waste ranges from 22.8 to 28.5 million tons [29]. For this reason, it is crucial to develop research that provides this agro-industrial waste an added value [6].
Studies have highlighted the potential of pecan nutshell as a source of bioactive compounds with various applications. Pecan nutshell has been identified as rich sources of bioactive compounds with antioxidant and antimicrobial properties [32]. Furthermore, the extraction of phenolic compounds from pecan nutshells has shown promise in generating bioactive molecules or extracts that could have industrial applications [33]. Additionally, pecan nutshell has been explored for its potential in producing activated carbons for supercapacitors, showcasing a sustainable approach to waste utilization [34]. The utilization of pecan nutshell waste presents a multifaceted opportunity for generating value-added products. Through innovative research and sustainable practices, pecan nutshell waste can be transformed into a valuable resource with diverse industrial applications.
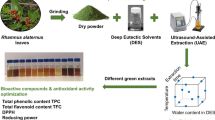
Therefore, the present research project focuses on exploring how ultrasound-assisted extraction (UAE) parameters impact the recovery of polyphenols from pecan nutshell waste biomass and their antioxidant activity. Pecan nut shells, often disregarded as biomass waste, contain valuable polyphenols renowned for their antioxidant properties, beneficial for human health and various industrial applications. UAE, leveraging cavitation and microstreaming effects, offers a promising method to enhance the extraction efficiency of these bioactive compounds. We will investigate the influence of UAE parameters such as time, amplitude and sample-solvent ratios on both the quantity and quality of extracted polyphenols. Furthermore, assessing the antioxidant activity of these polyphenols post-extraction will provide insights into their potential applications in functional foods, pharmaceuticals, and cosmetics. By optimizing UAE conditions tailored to pecan nutshell biomass, focuses on contributing to sustainable practices in agriculture and waste utilization, thereby advancing the valorization of agricultural by-products. Hence, this study aims to extract polyphenolic compounds using ultrasound-assisted extraction and evaluate the effect of ultrasound conditions on total phenolic content, total flavonoid content, and antioxidant activity.
2 Materials and methods
2.1 Reagents
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), Folin ciocalteu’s phenol 2 N, Quercetin, gallic acid, and all HPLC grade standards were from Sigma-Aldrich (St. Louis, MO, USA). Chemicals and solvents were of commercial grade.
2.2 Sample preparation
2.2.1 Raw material
Pecan nutshell [Carya illinoinensis (Wangenh) K.Koch] of a mixture of Wichita, Western, and Mahan varieties were obtained from “Huerta La Capilla” farm located in Baviácora, Sonora, Northwest of México [29°40′21.174 N, 110°08′33.812 W] (Fig. 2). Impurities were removed manually. To reduce particle size, simples were powdered using an Osterizer mixer (Oster® 006812–001-NP0, Mexico City) and sieved with a 60-mesh screen (Seedburo Equipment Company, Des Plaines, IL, USA). Samples were placed in polypropylene bags and stored at -5 °C till further analysis.
Geographic location of the nut collection: “Huerta La Capilla” farm located in Baviácora, Sonora, Northwest of Mexico [29°40′21.174 N, 110°08′33.812 W]. Official maps obtained from the National Institute of Statistics and Geography (INEGI), https://www.inegi.org.mx/default.html
2.3 Ultrasound-Assisted Extraction (UAE) of polyphenolic compounds from pecan nutshell
The UAE was performed using an ultrasound device equipped with a flat tip (Sonifier® S450D, 40 kHz, 400 W, Branson UltrasoundsTM). To determine the optimal extraction conditions, the pecan nutshell samples were extracted varying extraction time, amplitude, and sample-solvent ratio independently in a three-phase experiment. Distilled water was used as a solvent for all extractions. The first phase consisted of varying extraction time from 1 to 60 min with a constant amplitude of 40% and a 1:30 sample-solvent ratio. Amplitude varied from 0 to 100% in the second phase using the chosen time from the first phase and a constant 1:30 sample-solvent ratio. In the third phase, the sample-solvent ratio (g/mL) was varied as follows: 1:20, 1:30, 1:40, and 1:50; while the chosen time and amplitude from previous phases were kept constant. In each phase, a control sample without UAE was used. After the extraction, the samples were centrifuged (Sorvall Legend XTR Centrifuge, Eppendorf, Hamburg, Germany) at 7500 rpm for 15 min at a temperature of 4 °C. Supernatant was recovered and labeled as pecan nutshell extract (PNSE). Samples were stored at 4°c in vials until analysis. The UAE ultrafiltration process and evaluated parameters are shown in Fig. 3.
2.4 Quantification of phenolic compounds
2.4.1 Determination of Total Phenolic Content (TPC)
Total phenolic content (TPC) was determined according to the Folin-Ciocalteu method conducted by Eldeen et al. [35]. 25 μL of Folin-Ciocalteu 1N solution was added to 10 μL of PNSE. After incubation for 5 min at room temperature, 25 μL of 20% (w/v) sodium carbonate (Na2CO3) was added. 140 μL of distilled water was added to yield a 200 μL final volume. The microplate was incubated for 30 min, and absorbance was determined at 760 nm in a microplate.
reader (Thermo Fisher Scientific Inc. Multiskan GO, NY, USA). A calibration curve was made between 0 and 1 mg/mL with gallic acid as standard; calibration was measured by triplicate. Results were expressed in equivalent milligrams of gallic acid by gram of sample (mg EGA/g). All measurements were performed in triplicates. The determination was performed for each sample in the three phases of the experiment.
2.4.2 Determination of Total Flavonoid Content (TFC)
Total flavonoid content was determined using the colorimetric method according to Perla et al. [36]. 80 μL of extract was added to 80 μL of an ethanolic solution of aluminum trichloride (AlCl3) (20 g/L). The microplate was incubated for 1 h in darkness at room temperature. After incubation, the absorbance was read at 415 nm. A calibration curve was made between 0 and 1 mg/mL using quercetin as standard. Calibration and sample determination were performed in triplicates. Results were expressed as equivalent milligrams of quercetin by gram of sample (mg QE/g). The determination was performed for each sample in the three phases of the experiment.
2.5 Antioxidant activity
2.5.1 ABTS radical scavenging assay
This assay was conducted using the method according to Pérez-Perez et al. [37]. ABTS radical was prepared according to the methodology proposed by Re et al. [38]. 19.3 mg of ABTS were dissolved in 5 mL of distilled water; separately, 0.0378 g of K2S2O8 were mixed with 1 mL of distilled water. Subsequently, 88 µL of the K2S2O8 solution was added to the ABTS solution and left in darkness for 12 h. This last solution was adjusted to an absorbance of 0.7 ± 0.01 at a wavelength of 734 nm. 20 µL of the sample were placed in a 96-well microplate in presence of 270 µL of the ABTS radical solution. The absorbance was read at 734 nm after 30 min of reaction. Antioxidant activity was calculated in μmoles equivalent trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) per g of sample (μmol TE/g), % inhibition (Eq. 1).
The determination was performed for each sample in the three phases of the experiment.
2.5.2 DPPH radical scavenging capacity assay
DPPH assay was performed according to Pérez-Perez et al. [37]. 1.5 mg of DPPH• radical were dissolved in 50 mL of methanol; this solution was adjusted to an absorbance of 0.7 ± 0.01 at a wavelength of 515 nm. Subsequently, 20 µL of the sample was added to 200 µL of a radical solution. The microplate was incubated for 30 min in darkness. Absorbance was read at a wavelength of 515 nm. A standard curve of Trolox from 0 to 1 mg/mL was prepared, and the results were reported as µmol of Trolox equivalents per gram of sample (μmol TE/g), and by % of inhibition (Eq. 1). The determination was performed for each sample in the three phases of the experiment.
2.5.3 Ferric Reducing Antioxidant Power (FRAP) assay
The reduction antioxidant power of the ferric ion was determined using the methodology described by Del-Toro-Sánchez et al. [39]. Ferric reducing antioxidant power (FRAP) reagent was formed by assimilation of the acetate buffer (300 mmol/L, pH 3.6), 10 mM 2,4,6-tripyridyl-s- triazine (TPTZ) in HCl (40 mM) and FeCl3-6H2O (20 mM) in 10:1:1 ratio. To determine the antioxidant power, 20 μl of the extract was added to 280 μl of the FRAP solution. The samples were read at 638 nm after 30 min of incubation. A standard Trolox curve from 0 to 1 mg/mL was prepared, and the results were expressed as µmol Trolox equivalents per gram of sample (μmol TE/g). The determinations were made in triplicate. The determination was performed for each sample in the three phases of the experiment.
2.6 Extract characterization
2.6.1 Medium inhibitory concentration (IC50)
The medium inhibitory concentration (IC50) for both ABTS and DPPH radicals was determined through a systematic study involving a series of sample concentrations, which ranged from 1.9 to 250 µg/mL. To ascertain the IC50 values, Eq. 1 is utilized, which facilitated the calculation of the percentage inhibition at each concentration level. This method enabled a detailed analysis of how effectively the radicals were inhibited across the specified concentration range, providing insights into the antioxidant potential of the tested substances.
2.6.2 Fourier Transform Infrared (FT-IR) spectroscopy
Infrared spectra were obtained using an FT-IR Spectrometer (Frontier, Perkin Elmer, Waltham, USA). The analyses were conducted for pecan nutshell extract, silver nitrate, and silver nanoparticles. The spectra were recorded using the attenuated total reflectance (ATR) technique in transmittance mode. A spectrum scan from 4000 to 500 cm⁻1 was utilized, covering a broad range of the infrared spectrum to detect various molecular vibrations characteristic of different functional groups in the samples. An average of 32 scans were recorded for each measurement to enhance the signal-to-noise ratio and ensure data reliability. To guarantee reproducibility and accuracy, each determination was performed in triplicate, meaning each sample was analyzed three times and the values averaged to minimize experimental errors and provide more consistent and dependable results.
2.7 Statistical analysis
General linear models (GLMs) were utilized to perform an analysis of variance (ANOVA) with a significance threshold of 0.05 (p < 0.05) to determine the statistical differences among the groups. Post-hoc comparisons between the means were conducted using Tukey’s multiple range test, maintaining a 95% confidence interval to ensure the reliability of the findings. These statistical analyses were carried out using the InfoStat 2008 software, which provided robust and accurate results. Furthermore, for all analyses, the mean and standard deviation (SD) values were meticulously calculated to offer a detailed understanding of the data distribution and variability. This comprehensive approach ensures that the conclusions drawn from the study are both statistically significant and scientifically valid.
3 Results and discussion
3.1 Effect of time in ultrasound-assisted extraction of pecan nutshell
3.1.1 Total phenolic content and total flavonoid content
The first extraction parameter evaluated in this research was extraction time. The total phenol content (TPC) values of the extracts are represented in Fig. 4. Significant differences were identified between the samples. Results do not exhibit a trend in total phenolic content as extraction time increases. The highest concentration was obtained at an extraction time of 25 min with a 158.96 ± 1.70 mg GAE/g sample value. A higher value of 291.05 mg GAE/g sample in aqueous pecan nutshell extracts with an extraction time of 60 min was reported by Hilbig et al. [19]; the difference in values can be due to the application of heat at 60 °C in their experiment as well as variety of the pecan nutshell. Using an extraction time of 30 min, Flores-Estrada et al. [20] reported values of 102.78 ± 4.33 mg GAE/g and 87.611 ± 2.04 for methanolic extracts of Western and Wichita varieties, respectively. This may indicate that phenolic compounds present in pecan nutshell are more soluble in water than methanol since, in the present study, a higher value for an extraction time of 30 min (133.25 ± 6.00 GAE/g) was obtained using water as solvent. In comparison to other non-conventional extraction techniques, in a study conducted by do Prado et al. [40], pecan nut shell extracts obtained by supercritical extraction with CO2 exhibited values between 0.34 and 9.30 mg GAE/g; this suggests that ultrasound-assisted extraction may be a better technique for the obtaining of phenolic compounds. In addition, phenolic content in pecan nutshells from the present study compared to the previously mentioned studies may be affected by the variety, climatological conditions, maturation, production cycle, and cultivation methods in which the samples were collected. This can be observed in a study by Prado et al. [41], where pecan nutshell extracts from samples collected in different years exhibited different phenolic content. For this study, the optimal extraction conditions were selected based on the sample that exhibited the highest phenolic content value; therefore, the optimal extraction time was 25 min.
A Total phenols content (TPC) and B total flavonoid content (TFC) in pecan nutshell [Carya illinoinensis (Wangenh.) K.Koch] extracts obtained at various extraction times. Values represent the mean ± standard deviation of the triplicate. Values with different letters are significantly different (p < 0.05)
Total flavonoid content (TFC) in extracts obtained at different extraction times can be observed in Fig. 4. Samples showed significant differences between each other. Results do not exhibit a trend in total flavonoid content as extraction time increases. The sample corresponding to 25 min of extraction time exhibited the highest value of flavonoid content with 93.87 ± 0.17 mg QE/g sample. Pecan nutshell extract without ultrasound-assisted extraction has a value of 3.40 ± 0.17 mg QE/g sample; therefore, this method favored the extraction of flavonoids. Rodríguez-Roque et al. reported lower flavonoid content values [42] those being 3.8 mg EQ/g sample for an ultrasound-assisted methanolic extract exposed to a 30 min extraction time. In addition, the value reported by the previously mentioned authors is also lower than that obtained at the same extraction time in the present research, 76.09 ± 1.08 mg EQ/g sample. Similar values to those obtained in this study for the extraction times of 25 and 30 min were reported by Flores-Estrada et al. [20], obtaining a concentration of 94.89 ± 1.17 and 79.03 ± 1.25 mg CE/g for methanolic pecan nutshell extracts of Western and Wichita varieties respectively; this can be attributed to the proximity of the location from which each of the samples of both studies were retrieved. Comparison between studies was difficult due to different extraction methods, solvents, and reporting units. For example, de la Rosa, Alvarez-Parrilla, and Shahidi [28] reported a concentration ranging from 26.3 ± 2.6 to 36.1 ± 1.8 mg CE/g sample in pecan nutshell extracts using 80% acetone as a solvent and an extraction time of 30 min.
The effect of time on UAE, reveal a notable influence of extraction time on both the yield of TPC and TFC and their antioxidant potential. As expected, extending the extraction time led to increased extraction efficiency, resulting in higher TPC. This phenomenon can be attributed to the enhanced disruption of plant cell walls and the subsequent release of phenolic compounds into the solvent. However, it's crucial to note that an optimal extraction time exists, beyond which no significant improvement in phenolic content is observed. This suggests a saturation point in the extraction process, possibly due to factors such as degradation or rearrangement of phenolic compounds under prolonged exposure to ultrasonic waves. Moreover, while longer extraction times may enhance the yield of phenolic compounds, they could also lead to increased energy consumption and potential degradation of thermolabile compounds. Therefore, the determination of an optimal extraction time is essential for maximizing phenolic content while minimizing energy input and preserving compound stability. Future studies could delve deeper into the mechanisms underlying the observed saturation effect and explore strategies to mitigate potential degradation pathways during prolonged ultrasonic extraction processes.
3.1.2 Antioxidant activity by ABTS, DPPH, FRAP assays
Antioxidant activity by ABTS assay of aqueous extracts of pecan nutshell obtained at different times can be observed in Fig. 5 and Table 1. Significant differences were observed among all the samples and no trend was observed as extraction time increased. An extraction time of 1 min exhibited higher antioxidant activity with a value of 99.91 ± 0.08% of inhibition and 3518.22 ± 168.80 μmol TE/g sample. Hilbig et al. [19] obtained a value of 2574.32 ± 9.90 μmol TEAC/g sample using an extraction time of 60 min, which is similar to the value obtained for the same extraction time in the present study, it being 2251.27 ± 292.37 μmol TE/g. In the same study, Hilbig et al. [19] obtained a similar value for an ethanolic extract, it being 2573.00a ± 2.27 μmol TEAC/g sample. In methanolic pecan nutshell extracts such as those analyzed by Flores-Estrada et al. [20], values were lower than those obtained in this study for a 30 min extraction time, 91.09 ± 0.31 and 91.55 ± 0.25 μmol TEAC/g for Western and Wichita varieties respectively. Antioxidant activity can be attributed to the presence of phenolic compounds and flavonoids since these phytochemicals are recognized as having powerful antioxidant activity; these compounds reduce oxidation by scavenging free radicals to form stable ones [43].
Comparison with other studies was difficult due to extraction methods, solvents, and reporting units. For example, do Prado et al. [40] reported 1809.01 ± 27.18 μmol TEAC/g in an aqueous extract obtained through infusion for 10 min. In addition, de la Rosa, Alvarez-Parrilla, and Shahidi [28] reported values between 518.4 ± 80.7 to 644.2 ± 62.2 μmol TEAC/g sample in extracts obtained through an infusion with 80% acetone. Regarding non-conventional techniques, Dunford et al. [44] reported higher values of antioxidant activity using microwave and subcritical water extraction, those being 4069.5 ± 2.5 to 5288.1 ± 0.9 μmol TEAC/g for the first and 4920.6 ± 0.9 to 5390.4 ± 2.3 μmol TEAC/g for the last. A higher antioxidant activity in the previously mentioned research can be due to the studied pecan nutshell varieties and climatological conditions since they differed from those studied in this work.
Values corresponding to antioxidant activity by DPPH assay can be observed in Fig. 5 and Table 1. Significant differences between the samples were observed. As extraction time increased no trend was observed in the antioxidant activity. The highest antioxidant activity corresponds to an extraction time of 5 min with a value of 525.81 ± 0.99 μmol TE/g and 86.83 ± 0.14% of inhibition. A higher value was obtained by Hilbig et al. [19] for an extraction time of 60 min; it is 1268.03 ± 0.18 μmol TEAC/g sample; however this value is higher than the one obtained in this study (513.08 ± 5.24 μmol TE/g sample). The same author obtained a similar value of 1287.08 ± 0.24 μmol TEAC/g sample for an ethanolic extract. These values could be influenced by a higher extraction of phenolic compounds and flavonoids due to extraction at higher temperatures, 60 °C for the aqueous extract and 80 °C for the ethanolic extract. In methanolic extracts, Flores-Estrada et al. [20] reported a value of 955.69 ± 7.49 and 945.78 ± 7.49 μmol TE/g sample for Western and Wichita varieties respectively, which are also higher values than those obtained in this research. The DPPH● method interacts mainly with hydrophobic molecules such as flavonoids. However, values in flavonoid content are similar between the previously mentioned study and the present research; therefore, differences in values could be attributed to the type of flavonoids present in methanolic extracts [20, 39].
Various pecan nutshell studies have antioxidant activity for extracts obtained through other methods; in addition, other solvents and reporting units have been used; therefore, comparison with this study was difficult. Such is the case for the research conducted by Engler Ribeiro et al. [45], who reported a value of 293 ± 1 mg TEAC/g sample for an extract obtained by infusion in water at 98 °C; Do Prado et al. [46] obtained a value of 385 ± 94 μmol TEAC/g for an extraction using the same conditions. In another study using non-conventional extraction, Dunford, Gumus, and Gur [44] reported values from 33.7 ± 1.3 to 86.8 ± 0.8% inhibition with subcritical water extraction; in addition, microwave extraction exhibited values of 78.3 ± 1.3 and 83.5 ± 0.8% inhibition in two different varieties than those analyzed in this study.
Values corresponding to the antioxidant activity by FRAP assay of pecan nutshell extracts obtained at different extraction times can be observed in Fig. 5. The increase in extraction time did not exhibit a trend in antioxidant activity. An extraction time of 5 min exhibited the highest value of antioxidant activity, 1278.88 ± 38.39 µmol TE/g sample. Few articles have reported the antioxidant activity of pecan nutshell extracts by FRAP assay; therefore, comparisons between results were difficult due to different extraction methods, solvents, and reporting units. Dunford et al. [44] reported higher values of 4267.2 ± 2.2 and 4880.6 ± 0.9 µmol TE/g sample for aqueous extracts from two different varieties than those analyzed in this research; the difference in values can be attributed to the climatological conditions in which each of the varieties are cultivated. In methanolic extracts, FRAP antioxidant activity values were reported by Rodríguez-Roque et al. [42] for pecan nutshell extracts from Western variety cultivated in two different years, those being 3156.1a ± 0.02 and 2577.6b ± 0.12 mmol TE/g.
The relationship between extraction time and antioxidant activity can be explained as follows: antioxidant compounds present in nutshells are often released more rapidly at the beginning of the extraction process. Therefore, antioxidant activity may be high initially due to the immediate presence of these compounds. Over time, extraction may reach a saturation point where the amount of released antioxidant compounds no longer significantly increases. At this point, antioxidant activity may stabilize or even decrease. The polarity of compounds may influence the extraction rate. More hydrophilic compounds may be extracted more quickly initially, while lipophilic compounds may be released more slowly, which could explain why antioxidant activity may be higher initially and decrease with extraction time. Also, Furthermore, in the ABTS assay it gave greater antioxidant activity than DPPH and FRAP since this assay is particularly sensitive to hydrophilic and lipophilic compounds. In conclusion, the relationship between extraction time and antioxidant activity of nutshell extract may be influenced by the rate at which antioxidant compounds are released, as well as the polarity of these compounds. The ABTS, DPPH, and FRAP assays provide different perspectives on antioxidant activity, each highlighting specific aspects of the antioxidant mechanisms present in the extract.
3.2 Effect of amplitude in ultrasound-assisted extraction of pecan nutshell
3.2.1 Total phenolic content and total flavonoid content
TPC and TFC obtained in this study can be observed in Fig. 6, respectively. Significant differences were observed among values for both determinations. To our knowledge there are no studies that have reported the effect of amplitude in pecan nutshell extracts; however, a study conducted by Dunford et al. [44] has reported values for a 5% amplitude using a Sonic Dismembrator, Model 550, 500 V, 20 kHz, Fisher Scientific, Pittsburg, PA, USA. The previous authors analyzed the total phenolic content in two pecan nutshell varieties from Oklahoma, USA; values of 310.4 ± 0.2 and 333.7 ± 3.2 mg GAE/g were obtained. In this research, the highest value for phenolic content corresponded to an amplitude of 50%; it is 119.15 mg GAE/g sample; this value is lower than that reported by Dunford et al. [44]. Differences in values can be attributed to the pecan nutshell varieties and their cultivation climatological conditions used in each study; in addition, different ultrasound frequencies were used in each experiment. No studies have reported the effect of amplitude on total flavonoid content. This study exhibited the highest total flavonoid content using an amplitude of 50%, with a 72.17 mg EQ/g sample value. As mentioned before, the optimal amplitude in this study was chosen considering the highest phenolic content value; therefore, the optimal extraction amplitude was 50%.
A Total phenols content (TPC) and B total flavonoid content (TFC) in pecan nutshell [Carya illinoinensis (Wangenh.) K.Koch] extracts obtained at various extraction amplitude. Values represent the mean ± standard deviation of the triplicate. Values with different letters are significantly different (p < 0.05)
In an ultrasound-assisted extraction, the microbubbles that produce cavitation are generated by the different amplitudes of ultrasound waves [47]. As the amplitude increases, more power is delivered to the sample; therefore, solvent penetration to the cell walls and cavitation is increased; this can favor the release of phenolic compounds [48]. However, the breakage of bonds produced by higher amplitudes can lead to a degradation of the extracts due to the generation of radicals [49]. This can explain the decrease in phenolic and flavonoid content values reported in this study, which correspond to amplitudes higher than 50%. A similar trend in total phenolic content in potato peel extracts was reported by Wang et al. [48]. Oroian et al. [50] reported a different trend in the content of phenolic compounds and flavonoids from propolis, since these values increased as amplitude increased. Differences in their trend could be due to the use of an ultrasound bath in which ultrasound waves need to travel through the water before reaching the sample, reducing the power to promote a higher extraction of compounds [51].
The amplitude in ultrasound-assisted extraction (UAE) plays a crucial role in the extraction efficiency of bioactive compounds such as phenols and flavonoids from walnut shell extract. Amplitude refers to the intensity of the ultrasonic waves applied during the extraction process. The results indicate that the extraction of TPC and TFP was highest at an amplitude of 50%. This suggests that moderate levels of ultrasonic energy are optimal for extracting these compounds from walnut shells. At lower amplitudes, the energy might not be sufficient to disrupt the plant cell walls effectively, limiting the release of phenolic and flavonoid compounds. Conversely, at higher amplitudes, there's a risk of degradation of these compounds due to excessive energy input or cavitation effects. Mechanisms involved are: the ultrasonic waves in UAE induce cavitation, which creates microbubbles in the solvent. When these bubbles implode near the plant material, they generate localized high temperatures and pressures, leading to the disruption of cell walls and increased mass transfer. This facilitates the release of phenolic and flavonoid compounds into the solvent, enhancing extraction efficiency. Also, the choice of amplitude in UAE should be optimized based on factors such as the type of plant material, solvent, and target compounds. In the case of nutshells, an amplitude of 50% appears to be optimal for maximizing the extraction of phenols and flavonoids.
3.2.2 Antioxidant activity by ABTS, DPPH, FRAP assays
The effect of amplitude in antioxidant activity by ABTS, DPPH, and FRAP assays can be observed in Fig. 7 and Table 2. Significant differences were observed between all the samples. As mentioned in the previous section, to our knowledge, the effect of amplitude on pecan nutshell extracts has not been analyzed by previous authors. For the ABTS assay, the highest antioxidant activity corresponded to an amplitude of 80% with a value of 2836.87 µmolTE/g and 100% of inhibition. As for DPPH, an extraction amplitude of 30% exhibited the highest value, 528.78 µmolTE/g, and 87.25% of inhibition. Antioxidant activity by FRAP assay exhibited a value of 883.29 µmolTE/g for an amplitude of 30%, with the second highest value of 875.84 µmolTE/g for an amplitude of 50%. This suggests that phenolic compounds and flavonoids present in the extract obtained with an amplitude of 50% work through a mechanism of action by electron transfer. Comparison between studies was difficult due to differences in the ultrasound method, equipment used, and varieties analyzed. For example, in the research conducted by Dunford et al. [44], a Sonic Dismembrator, Model 550, 500 V, 20 kHz, Fisher Scientific, Pittsburg, PA, USA was used for the UAE with an amplitude of 5. The authors reported values of 4880.6 ± 0.9 and 4267.2 ± 2.2 µmol/g extract for to varieties retrieved from Oklahoma, USA.
Based on the effect of amplitude on antioxidant activity of nutshell compounds, where ABTS showed higher activity than DPPH and FRAP: the higher antioxidant activity observed with the ABTS assay compared to DPPH and FRAP suggests that the compounds extracted from walnut shells exhibit greater effectiveness against the ABTS radical cation. This could be attributed to the specific chemical composition of the extract, which may contain compounds with stronger affinity for the ABTS radical. The amplitude in UAE can influence the extraction of various antioxidant compounds from walnut shells. At an optimal amplitude (e.g., 50%), the extraction process likely yields a higher concentration of these bioactive compounds, resulting in increased antioxidant activity. However, excessively high amplitudes may lead to the degradation of certain antioxidants or the formation of undesirable by-products, potentially reducing overall antioxidant activity. Differences in Assay Sensitivity: The ABTS assay is known to be more sensitive to certain types of antioxidants compared to DPPH and FRAP assays. Therefore, even slight variations in the concentration of specific antioxidant compounds extracted at different amplitudes can significantly affect the observed antioxidant activity, leading to discrepancies between the assays.
3.3 Effect of sample-solvent ratio in ultrasound-assisted extraction of pecan nutshell
3.3.1 Total phenolic content and total flavonoid content
TPC and TFC for extracts with variable sample-solvent ratios can be observed in Fig. 8, respectively. This determination used extracts of all sample-solvent ratios without UAE as a control. Significant differences were observed between the samples and controls. The UAE favored the extraction of phenolic compounds and flavonoids in all the sample-solvent ratio samples. The highest value for total phenolic content was for a sample-solvent ratio of 1/50, it being 132.72 mGAE/g sample. To our knowledge, only Hilbig et al. [19] have reported the effect of sample-solvent ratio in aqueous pecan nutshell extracts. In their study a value of 291.05 mg mGAE/g was obtained for a 1/30 ratio being their highest value; differences in values could be influenced by varieties of the samples as well as temperature extraction. In the same study, Hilbig et al. [19] reported a 394.39 mg GAE/g sample value for ethanolic extracts using a 1/30 sample-solvent ratio. In another study, Flores-Estrada et al. [20] reported values of 102.78 ± 4.33 mg GAE/g and 87.611 ± 2.04 for methanolic extracts of Western and Wichita varieties respectively using a sample-solvent ratio of 1/10. As mentioned before, the optimal sample-solvent ratio was chosen in relation to the sample with the highest phenolic content; therefore, the optimal sample-solvent ratio for pecan nutshell extracts is 1/50.
A) Total phenols content (TPC) and B) Total flavonoid content (TFC) in pecan nutshell [Carya illinoinensis (Wangenh.) K.Koch] extracts obtained at various sample-solvent ratio. Values represent the mean ± standard deviation of the triplicate. Values with different letters are significantly different (p < 0.05)
As for flavonoid content, the highest content was exhibited by a 1/30 sample-solvent ratio, its value being 58.40 mg EQ/g sample; this value was followed by a value of 57.94 mg EQ/g sample corresponding to a 1/50 ratio with no significant differences between them. In methanolic extracts, a value of 3.8 mg EQ/g sample was reported by Rodríguez-Roque et al. [42] for a 1/5 ratio. In addition, in a research by Flores-Estrada et al. [20], extraction with a 1/10 ratio exhibited a value of 94.89 ± 1.17 and 79.03 ± 1.25 mg CE/g for methanolic pecan nutshell extracts of Western and Wichita varieties, respectively. In comparison with other extraction techniques, Engler Ribeiro et al. [45] reported a value of 93 ± 2 mg GAE/g for pecan nutshell extract of Barton variety using a 1/50 ratio with infusion. In another study, de la Rosa et al. [28] reported values between 65.3 ± 6.9 mg GAE/g and 92.5 ± 9.0 mg GAE/g for Wichita and Western pecan nutshell extracts obtained by infusion with 80% acetone with a 1/100 ratio.
The sample-solvent ratio plays a crucial role in the extraction of bioactive compounds such as TPC and TFC from natural sources like nutshell. In this study, varying sample-solvent ratios (1/20, 1/30, 1/40, and 1/50) were investigated, with the highest yield of phenols and flavonoids observed at a sample-solvent ratio of 1/50 using ultrasound-assisted extraction. The observed trend of higher TPC and TFP at a lower sample-solvent ratio could be attributed to several factors. Firstly, at a lower sample-solvent ratio, there is a higher concentration of the sample in the solvent, facilitating better interaction between the solvent and the bioactive compounds present in the nutshell. This increased concentration gradient may enhance the mass transfer of phenols and flavonoids from the solid matrix to the solvent phase, thereby improving extraction efficiency. Additionally, a lower sample-solvent ratio might lead to reduced solubility of interfering substances present in the nutshell, which could otherwise hinder the extraction of phenolic and flavonoid compounds. Moreover, the efficiency of ultrasound-assisted extraction could be maximized at lower sample-solvent ratios, as higher concentrations of the sample may enhance cavitation effects, facilitating the disruption of plant cell walls and releasing more bioactive compounds into the solvent. However, it is essential to consider that beyond a certain point, further reduction in the sample-solvent ratio may not necessarily result in increased extraction efficiency. The optimal ratio depends on various factors such as the nature of the sample, solvent polarity, extraction technique, and target compounds. Therefore, while a lower sample-solvent ratio showed favorable results in this study, optimization experiments may be required to determine the most efficient ratio for phenol and flavonoid extraction from nutshell under specific conditions.
3.3.2 Antioxidant activity by ABTS, DPPH, FRAP assays
The effect of sample-solvent ratio in pecan nutshell extracts antioxidant activity by ABTS, DPPH, and FRAP assays can be observed in Fig. 9; in addition, % of inhibition of ABTS and DPPH radicals is presented in Table 3. As for ABTS, UAE did not affect the antioxidant activity of the samples since no significant differences were found between the sample and the control sample. In addition, no significant differences between a 1/50 ratio and the other samples were observed; the highest value of antioxidant activity by ABTS corresponded to this sample, 3748.00 ± 843.08 µmolTE/g. For a lower sample-solvent ratio (1/30), Hilbig et al. [19] reported a value of 2574.32 ± 9.90 µmolTE/g; however, differences in values can be attributed to different varieties used by their study and ours. Using a 1/10 sample-solvent ratio, Flores-Estrada et al. [20] reported 91.09 ± 0.31 and 91.55 ± 0.25 μmol TEAC/g for methanolic extracts of Western and Wichita varieties respectively. To our knowledge, % of inhibition of ABTS has not been reported for pecan nutshell extracts. In this study, a 1/20 sample-solvent ratio exhibited a 99.86 ± 0.00% of inhibition. Comparison between studies was difficult due to differences in extraction techniques, pecan nutshell varieties, and reporting units. For example, in an extraction by infusion, Do Prado et al. [46] reported a value of 1404 ± 330 µmolTE/g for other pecan nutshell varieties.
Values for antioxidant activity by DPPH did not exhibit significant differences between the samples and their control samples; however, significant differences were observed between the samples with UAE. The sample-solvent ratio that exhibited the highest antioxidant activity by DPPH was 1/50 with a value of 881.14 ± 0.95 μmol TE/g sample; in addition, the highest % of inhibition was observed in the 1/40 sample-solvent ratio with a value of 87.62 ± 1.48. An increase in solvent favored the extraction of compounds that could inhibit the DPPH radical. This study had difficulty compared with other studies due to differences in pecan nutshell varieties, solvents, extraction methods, and reporting units. Using a lower ultrasound frequency, Dunford, Gumus, and Gur [44] reported 74.1 ± 4.1 and 82.4 ± 1.1% inhibition for pecan nutshell extracts with a 1/20 ratio from two varieties from Oklahoma. Aqueous extracts of Barton pecan nutshell with a sample-solvent ratio of 1/30 presented a value of antioxidant activity by DPPH of 1268.03 ± 0.18 μmol TEAC/g sample [19]. In methanolic extracts, Flores-Estrada et al. [20] reported values of 955.69 ± 7.49 and 945.78 ± 7.49 μmol TEAC/g sample for Western and Wichita varieties respectively using a 1/10 sample-solvent ratio. In a study by do Prado et al. [40], extraction by infusion exhibited antioxidant activity values of 612.24 ± 26.73 μmol TEAC/g sample for Barton pecan nutshell extract with a 1/50 sample-solvent ratio.
UAE favored the increase of antioxidant activity by FRAP in the samples corresponding to a 1/20 and 1/30 sample-solvent ratio. Significant differences between the samples with UAE were observed. The highest antioxidant activity by FRAP was exhibited in the pecan nutshell extract with a 1/30 ratio with a value of 806.77 ± 4.42 μmol TEAC/g sample. In the study by Dunford, Gumus, and Gur [44], a ferric reducing power of 4880.6 ± 0.9 and 4267.2 ± 2.2 μmol TEAC/g sample was reported for pecan nutshell extracts of two varieties using a 1/20 ratio. In methanolic extracts, Rodríguez-Roque et al. [42] reported values of 3156.1 ± 0.02 and 2577.6 ± 0.12 mmol ET/g for pecan nutshell extracts with a 1/5 ratio from Western variety cultivated in two different years.
The antioxidant activity of the nutshell extract was evaluated using three different assays: ABTS, DPPH, and FRAP. Interestingly, the highest antioxidant activity was observed using the ABTS assay, and this activity was particularly pronounced at a sample-solvent ratio of 1/50. The observed variation in antioxidant activity across different sample-solvent ratios could be attributed to the differential solubility and concentration of bioactive compounds extracted under varying conditions. The ABTS assay, which measures the ability of antioxidants to scavenge ABTS radicals, showed higher activity compared to the DPPH and FRAP assays. This discrepancy might be due to the different mechanisms and kinetics involved in these assays. At a lower sample-solvent ratio, where the extraction efficiency of phenols and flavonoids was maximized, it is plausible that a higher concentration of these compounds contributed to enhanced antioxidant activity. Phenols and flavonoids are well-known antioxidants capable of scavenging free radicals and preventing oxidative damage. Therefore, the increased concentration of these compounds in the extract obtained at a sample-solvent ratio of 1/50 likely led to the observed higher antioxidant activity in the ABTS assay.
Furthermore, the choice of solvent and extraction technique also influences the composition and activity of the extract. Ultrasound-assisted extraction might facilitate the extraction of a broader spectrum of bioactive compounds with antioxidant properties, contributing to the overall antioxidant capacity of the extract. In conclusion, the choice of sample-solvent ratio significantly influences both the extraction efficiency of phenols and flavonoids and the antioxidant activity of the nutshell extract. Optimizing this parameter is crucial for maximizing the yield of bioactive compounds with potent antioxidant properties, which could have implications for the development of nutraceuticals or functional foods with enhanced health benefits.
3.4 Extract characterization
3.4.1 Determination of medium inhibitory concentration (IC50)
The determination of the medium inhibitory concentration (IC50) holds paramount importance in assessing the antioxidant activity of natural compounds. In the context provided, the IC50 values for both ABTS and DPPH radicals were investigated utilizing the optimal extraction conditions, which included a 25-min extraction time, 50% amplitude, and a 1/50 sample-solvent ratio. Figure 10A shows the kinetics of concentrations ranging from 1.9 µg/mL to 250 µg/mL of the pecan nutshell extract, where concentrations above 58.93 µg/mL inhibited 50% of the ABTS radical. Figure 10B shows the kinetics DPPH radical, concentrations ranging from 3.9 µg/mL to 250 µg/mL were evaluated; in this case, 50% of the DPPH radical was inhibited by concentrations above 124.73 µg/mL. To our knowledge, no other study has reported IC50 neither for the ABTS radical nor for the DPPH radical. Figure 10A illustrates the kinetics of concentrations ranging from 1.9 µg/mL to 250 µg/mL of pecan nutshell extract concerning the ABTS radical. Notably, concentrations exceeding 58.93 µg/mL were found to inhibit 50% of the ABTS radical. This observation underscores the potency of the pecan nutshell extract in scavenging the ABTS radical, highlighting its potential as an antioxidant agent.
Similarly, Fig. 10B portrays the kinetics of the DPPH radical, with concentrations ranging from 3.9 µg/mL to 250 µg/mL being evaluated. In this instance, it was revealed that concentrations surpassing 124.73 µg/mL were required to inhibit 50% of the DPPH radical. This finding further emphasizes the significant antioxidant capacity of the pecan nutshell extract against the DPPH radical. It is noteworthy to mention that, to the best of our knowledge, no prior study has reported IC50 values for either the ABTS or DPPH radicals concerning the pecan nutshell extract. This underscores the novelty and significance of the current study's findings, providing valuable insights into the antioxidant potential of this natural extract. In conclusion, the determination of IC50 values for both ABTS and DPPH radicals underlines the efficacy of the pecan nutshell extract as a potent antioxidant agent.
The difference in IC50 values between ABTS and DPPH radicals can be attributed to several factors, including their chemical properties and reaction mechanisms. Firstly, ABTS and DPPH assays differ in their chemical structures and reactivity. ABTS radical is a blue-colored radical cation that is relatively stable and soluble in water, while DPPH radical is a purple-colored stable free radical soluble in organic solvents. These differences can affect how each radical interacts with antioxidant compounds. Secondly, the reaction mechanisms of antioxidants with ABTS and DPPH radicals vary. ABTS assay involves the reduction of the blue ABTS radical cation to its colorless form by antioxidants, while DPPH assay involves the reduction of the purple DPPH radical to its corresponding hydrazine by antioxidants. These different reaction mechanisms may result in varying degrees of antioxidant activity observed for the same compound in ABTS and DPPH assays. Additionally, the kinetic parameters of the reactions, such as the rate of reaction and affinity of the antioxidant for each radical, can influence the IC50 values. Some antioxidants may have higher affinity or react more readily with one radical compared to the other, leading to differences in IC50 values.
Furthermore, the concentrations of radicals used in the assays and the experimental conditions (such as pH, temperature, and solvent) can also affect the IC50 values. Variations in these factors may result in differences in the observed antioxidant activity between ABTS and DPPH assays. Also, pecan nutshell extract may contain a variety of antioxidant compounds such as polyphenols, flavonoids, and phenolic acids. The composition and concentration of these compounds can differ, influencing their ability to neutralize different types. Each radical may require different action mechanisms from antioxidants to neutralize their activity. For instance, some antioxidants may be more effective in donating electrons to reduce the ABTS radical, while others may have higher affinity for the DPPH radical. This can result in a lower IC [50] for one of the radicals. Different extract components may have varying affinities toward ABTS and DPPH radicals due to their unique chemical properties. Some extract compounds may be more effective in neutralizing one of the radicals compared to the other, influencing the observed differences in IC50 values. Overall, the differences in IC50 values between ABTS and DPPH assays can be attributed to the unique properties and reaction mechanisms of each radical, as well as the experimental conditions used in the assays.
3.4.2 Partial elucidation of compounds in the extracts by FTIR
The infrared spectra for the aqueous extract obtained with the optimal extraction conditions of 25 min extraction time, 50% amplitude and 1/50 sample-solvent ratio is shown in Fig. 11. A band at a wavenumber of 3254.4 cm−1 could be assigned to a –OH stretching in phenolic compounds or carbohydrates; this band could also correspond to a stretching of an N–H bond in an amino group. At 2918.4 and 2851.2 cm−1 wavelengths, two bands can be assigned to a C-H stretching of an alkane. A C = O bond from an aldehyde could exhibit the band at 1742.4 cm−1. The band observed at 1603.2 cm−1 could correspond to an N–H bond belonging to an amine. At 1334.4 cm−1 a band corresponding to a possible -OH bending from an alcohol can be observed. Bands at 1192, 1142.4, 1097.6, and 1030.4 cm−1 a band are presented for a C-N bond for an amine. Bands at 825.6 and 721.6 cm−1 could correspond to a C = C bond in alkenes. The obtained spectra suggest the presence of structures such as phenolic compounds, proteins, and polysaccharides in the aqueous pecan nutshell extract; examples of these structures are shown in Fig. 11. Several studies have reported the presence of polysaccharides such as holocellulose, and lignin, as well as phenolic compounds like catechin and gallic acid present in pecan nutshell of Western and Wichita varieties; therefore, pecan nutshell can be a source of bioactive compounds [52, 53].
The partial characterization of the pecan nutshell extract reveals a complex composition rich in various organic compounds. The infrared spectra obtained under optimal extraction conditions depict prominent bands indicative of specific functional groups. Notably, the presence of phenolic compounds or carbohydrates is suggested by the prominent band at 3254.4 cm−1, attributed to -OH stretching. The spectra (Fig. 11A) show the presence of diverse compounds including phenolic compounds, proteins, and polysaccharides. Studies have reported the presence of phenolic compounds such as gallic acid, ellagic acid, protocatechuic acid, p-hydroxybenzoic acid, catechin, and catechol in pecan nutshell extracts [28, 54,55,56]. These phenolic compounds contribute to the antioxidant activity of pecan nutshell extracts, making them valuable sources of bioactive molecules with potential health benefits. Furthermore, pecan nutshell extracts have been found to contain condensed tannins, which are known for their high antioxidant capacity [33, 57]. The presence of these compounds enhances the overall antioxidant potential of pecan nutshell extracts, making them effective in combating oxidative stress and providing health-promoting effects.
3.5 Contribution of the study to sustainability
Pecans and their derivatives have been studied from several sustainability perspectives. For instance, Alvarez-Chavez et al. [51] noted that pecan nutshells can be an abundant agro-industrial residue in many locations, which may contribute to the economy and the environment as a sustainable option for bioplastic materials. Furthermore, Cambareri et al. [52] carried out research in which pecan was found to have a potential contribution to Sustainable Development Goal (SDG) 2 regarding “Zero Hunger” for being a reliable sink for storing atmospheric carbon and also for having high nutritional density. Lastly, Kyei et al. [53] conducted a review discussing how the valorization of cashew nut wastes, as nutshell liquid, can reach some SDGs, specifically, SDG 6 “clean water and sanitation,” SDG 9 “industry, innovation, and infrastructure,” and SDG3 “good health and well-being,” through pharmaceuticals products, biodiesel, resins, and other uses. Hence, pecans and their derivatives have great potential to contribute to a more sustainable society by paying attention to the practical implications of studies like the one conducted in the present paper.
Furthermore, ultrasound-assisted extraction (UAE) emerges as a promising green technology for enhancing the extraction of bioactive compounds from agri-food waste. By employing ultrasound waves that produce the cavitation effect, UAE facilitates the breakdown of cell walls, leading to increased yields of valuable compounds such as antioxidants, phenolics, and essential oils. This environmentally friendly approach not only enhances extraction efficiency but also reduces the use of chemical solvents, minimizing environmental impact. Moreover, UAE offers versatility, allowing extraction from a wide range of agri-food waste, thereby contributing to waste valorization and sustainable resource management in the food industry. In some cases, CO2 emissions can be reduced by up to 70–90% due to the elimination or reduction of solvent usage and the energy-efficient nature of ultrasound technology. This reduction in CO2 emissions aligns with the goals of sustainability and environmental conservation, making UAE a promising approach for eco-friendly extraction processes.
4 Conclusions
In this study, concluded that green technology plays a vital role in addressing environmental challenges by offering sustainable solutions for waste management, and resource conservation. Ultrasound-assisted extraction conditions were evaluated in relation to their effect on the total phenolic content, total flavonoid content, and antioxidant activity of pecan nutshell extracts. Total phenolic content and total flavonoid content were favored by an extraction time of 25 min; in addition, antioxidant activity by DPPH and FRAP is present in this extract. The highest values of phenolic compounds and flavonoids were present in the extract obtained with an amplitude of 50%; however, this extract presented higher antioxidant activity by FRAP than by ABTS or DPPH. The effect of ultrasound extraction was observed in the increase of total phenolic content in a 1/50 sample-solvent ratio extract. Compounds in the 1/50 sample-solvent ratio extract exhibited the highest values of antioxidant activity by ABTS and DPPH. Therefore, the optimal ultrasound conditions for pecan nutshell extract are 25 min extraction time, 50% amplitude, and 1/50 sample-solvent ratio. According to the resulting IC50, high concentrations of pecan nutshell extract are required to inhibit 50% of both ABTS and DPPH radicals. Furthermore, phenolic compounds, proteins, and polysaccharides may be present in the pecan nutshell extract. Therefore, green technologies such as those in the UAE must be applied to extraction processes to mitigate the environmental impact generated by conventional technologies. Last but not least, the UAE variables must be studied to find the best performance, especially in agricultural waste. Then, the widespread adoption of green technologies, such as ultrasound-assisted extraction, is imperative for advancing toward a more sustainable and resilient future. These innovations not only contribute to mitigating climate change but also play a significant role in preserving natural ecosystems.
References
Ng HS, Kee PE, Yim HS, Chen PT, Wei YH, Chi-Wei Lan J (2020) Recent advances on the sustainable approaches for conversion and reutilization of food wastes to valuable bioproducts. Biores Technol 302:1–8. https://doi.org/10.1016/j.biortech.2020.122889
Duque-Acevedo M, Belmonte-Ureña LJ, Cortés-García FJ, Camacho-Ferre F (2020) Recovery of agricultural waste biomass: a sustainability strategy for moving towards a circular bioeconomy. In: Baskar C, Ramakrishna S, Baskar S, Sharma R, Chinnappan A, Sehrawat R (eds) Handbook of solid waste management: sustainability through circular economy. Springer, Singapore, pp. 1–30. https://doi.org/10.1007/978-981-15-7525-9_25-2
Godoy Padilla DJ, Daza La Plata R, Curi Fernández LM, Layza Mendiola AE, Roque Alcarraz RE, Hidalgo Lozano V, Gamarra Carrillo SG, Gómez Bravo CA (2020) Caracterización del valor nutricional de los residuos agroindustriales para la alimentación de ganado vacuno en la región de San Martín, Perú. Cienc Tecnol Agropecuaria 21(2):1–14. https://doi.org/10.21930/rcta.vol21_num2_art:13741
Ríos-Pérez F, Soto-Simental S, Quintero-Lira A, Piloni-Martini J, Güemes-Vera N (2020) Harina de cáscara de vaina de cacao: Una opción para el aprovechamiento de residuos agroindustriales. Boletín de Ciencias Agropecuarias Del ICAP 6(11):5–7. https://doi.org/10.29057/icap.v6i11
Andrade de Prá M, Piazza D, Poletto M (2021) Pecan nutshell: morphological, chemical and thermal characterization. J Mater Res Technol 13(July–August 2021):2229–2238. https://doi.org/10.1016/j.jmrt.2021.05.106
Cury RK, Aguas MY, Martinez MA, Olivero VR, Chams ChL (2017) Residuos agroindustriales su impacto, manejo y aprovechamiento. Revista Colombiana de Ciencia Animal - RECIA 9(S1):122–132. https://doi.org/10.24188/recia.v9.ns.2017.530
Vargas Corredor YA, Peréz Pérez LI (2018) Aprovechamiento de residuos agroindustriales en el mejoramiento de la calidad del ambiente. Revista Facultad de Ciencias Básicas 1(1):59–72. https://doi.org/10.18359/rfcb.3108
Mejías-Brizuela N, Orozco-Guillen E, Galáan-Hernández N (2016) Aprovechamiento de los residuos agroindustriales y su contribución al desarrollo sostenible de México. Rev Cienc Ambient Recur Nat 2(6):1–10
Riera M, Maldonado S, Palma R (2018) Agro-industrial residues generated in Ecuador for the elaboration of bioplastics. Ingeniería Industrial 17(3):227–246
Chávez Porras Á, Rodríguez González A (2016) Aprovechamiento de residuos orgánicos agrícolas y forestales en Iberoamérica. Academia y Virtualidad 9(2):90–107. https://doi.org/10.18359/ravi.2004
Ortiz García JE, González Morales DE, Mejía Agudelo Y, García-Alzate LS, Cifuentes-Wchima X (2020) Evaluación de la biomasa residual (cereza) de café como sustrato para el cultivo del hongo comestible Pleurotus ostreatus. Rev ION 33(1):93–102. https://doi.org/10.18273/revion.v33n1-2020009
Cabrera Rodríguez E, León Fernández V, Montano A, Dopico D (2016) Caracterización de residuos agroindustriales con vistas a su aprovechamiento. Centro Azúcar 43(4):27–35
Diaz Zavaleta PT (2021) Aprovechamiento de los residuos agroindustriales como prebióticos. Universidas Nacional de Trujillo. Tesis
Navas Miño GC, Ramírez Bayas SG (2012) Aprovechamiento de residuos agroindustriales, cascarilla de arroz (Oriza sativa) y residuos de papa (Solanum tuberosum) para la producción de Trichoderma spp. Universidad Técnica De Ambato. Tesis
Freitas LC, Barbosa JR, da Costa ALC, Bezerra FWF, Pinto RHH, de Carvalho Junior RN (2021) From waste to sustainable industry: how can agro-industrial wastes help in the development of new products? Resour Conserv Recycl 169(August 2020). https://doi.org/10.1016/j.resconrec.2021.105466
Duque-Acevedo M, Belmonte-Ureña LJ, Yakovleva N, Camacho-Ferre F (2020) Analysis of the circular economic production models and their approach in agriculture and agricultural waste biomass management. Int J Environ Res Public Health 17(24):1–34. https://doi.org/10.3390/ijerph17249549
Peñaranda Gonzalez LV, Montenegro Gómez SP, Giraldo Abad PA (2017) A provechamiento de residuos agroindustriales en Colombia. Revista de Investigación Agraria y Ambiental 8(2):141–150
Nazzaro F, Fratianni F, Ombra MN, d’Acierno A, Coppola R (2018) Recovery of biomolecules of high benefit from food waste. Curr Opin Food Sci 22:43–54. https://doi.org/10.1016/j.cofs.2018.01.012
Hilbig J, Alves VR, Müller CMO, Micke GA, Vitali L, Pedrosa RC, Block JM (2018) Ultrasonic-assisted extraction combined with sample preparation and analysis using LC-ESI-MS/MS allowed the identification of 24 new phenolic compounds in pecan nut shell [Carya illinoinensis (Wangenh) C. Koch] extracts. Food Res Int 106(October 2017):549–557. https://doi.org/10.1016/j.foodres.2018.01.010
Flores-Estrada RA, Gámez-Meza N, Medina-Juárez LA, Castillón-Campaña LG, Molina-Domínguez CC, Rascón-Valenzuela LA, García-Galaz A (2020) Chemical composition, antioxidant, antimicrobial and antiproliferative activities of wastes from pecan nut [Carya illinoinensis (Wagenh) K. Koch]. Waste Biomass Valorization 11(7):3419–3432. https://doi.org/10.1007/s12649-019-00681-2
Otles S, Kartal C (2018) Food waste valorization. In: Sustainable food systems from agriculture to industry (Issue November 1999). Elsevier Inc. https://doi.org/10.1016/b978-0-12-811935-8.00011-1
Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM (2013) Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng 117(4):426–436. https://doi.org/10.1016/j.jfoodeng.2013.01.014
Belwal T, Chemat F, Venskutonis PR, Cravotto G, Jaiswal DK, Bhatt ID, Devkota HP, Luo Z (2020) Recent advances in scaling-up of non-conventional extraction techniques: learning from successes and failures. TrAC - Trends Anal Chem 127:115895. https://doi.org/10.1016/j.trac.2020.115895
Rodríguez García SL, Raghavan V (2021) Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—a review. Crit Rev Food Sci Nutr 0(0):1–21. https://doi.org/10.1080/10408398.2021.1901651
Manousi N, Sarakatsianos I, Samanidou V (2019) Extraction techniques of phenolic compounds and other bioactive compounds from medicinal and aromatic plants. In: Eng Tools Beverage Ind. Elsevier Inc. https://doi.org/10.1016/b978-0-12-815258-4.00010-x
Bonifácio-Lopes T, Teixeira JA, Pintado M (2020) Current extraction techniques towards bioactive compounds from brewer’s spent grain–a review. Crit Rev Food Sci Nutr 60(16):2730–2741. https://doi.org/10.1080/10408398.2019.1655632
Zia S, Khan MR, Shabbir MA, Aslam Maan A, Khan MKI, Nadeem M, Khalil AA, Din A, Aadil RM (2020) An inclusive overview of advanced thermal and nonthermal extraction techniques for bioactive compounds in food and food-related matrices. Food Rev Int 00(00):1–31. https://doi.org/10.1080/87559129.2020.1772283
de la Rosa LA, Alvarez-Parrilla E, Shahidi F (2011) Phenolic compounds and antioxidant activity of kernels and shells of Mexican pecan (Carya illinoinensis). J Agric Food Chem 59(1):152–162. https://doi.org/10.1021/jf1034306
Olivas Tarango AL, Rodríguez Peña C, Cabrera Álvarez EN, Obregón Solís E, Longoria Garza GA, García Fajardo JA, Flores Montaño JL, Morales Landa JL, Reyes Vázquez N del C, Santos Moreno OO, Tarango Rivero SH (2019) Agronomía sustentable y aprovechamiento alternativo de la nuez
Rovirosa JEC (2017) Planeación Agrícola Nacional 2017–2030: Nuez Pecanera Mexicana
Retes López R, Moreno Medina S, Ibarra Flores FA, Martín Rivera MH, Suárez Suárez NE (2021) Cultivo de nogal pecanero en la costa de hermosillo asociado a su rentabilidad, ciclo 2020. Rev Mex Agroneg 48:714–723
Cason C, Yemmireddy VK, Moreira J, Adhikari A (2021) Antioxidant properties of pecan shell bioactive components of different cultivars and extraction methods. Foods 10(4). https://doi.org/10.3390/foods10040713
Ribas LE, Baravalle ME, Gasser FB, Renna MS, Addona S, Ortega HH, Savino GH, Van de Velde F, Hein GJ (2021) Extraction of phenolic compounds from the shells of pecan nuts with cytotoxic activity through apoptosis against the colon cancer cell line HT-29. J Food Sci 86(12):5409–5423. https://doi.org/10.1111/1750-3841.15950
Martínez-Casillas DC, Mascorro-Gutiérrez I, Arreola-Ramos CE, Villafán-Vidales HI, Arancibia-Bulnes CA, Ramos-Sánchez VH, Cuentas-Gallegos AK (2019) A sustainable approach to produce activated carbons from pecan nutshell waste for environmentally friendly supercapacitors. Carbon 148:403–412. https://doi.org/10.1016/j.carbon.2019.04.017
Eldeen IMS, Seow EM, Abdullah R, Sulaiman SF (2011) In vitro antibacterial, antioxidant, total phenolic contents and anti-HIV-1 reverse transcriptase activities of extracts of seven Phyllanthus sp. S Afr J Bot 77(1):75–79. https://doi.org/10.1016/j.sajb.2010.05.009
Perla V, Holm DG, Jayanty SS (2012) Effects of cooking methods on polyphenols, pigments and antioxidant activity in potato tubers. LWT Food Sci Technol 45(2):161–171. https://doi.org/10.1016/j.lwt.2011.08.005
Pérez-Perez LM, Del Toro Sánchez CL, Sánchez Chavez E, González Vega RI, Reyes Díaz A, Borboa Flores J, Soto Parra JM, Flores-Cordova MA (2019). Bioaccesibilidad de compuestos antioxidantes de diferentes variedades de frijol (Phaseolus vulgaris L.) en México, mediante un sistema gastrointestinal in vitro//bioaccessibility of antioxidant compounds from different bean varieties (Phaseolus vulgaris L). Biotecnia 22(1):117–125. https://doi.org/10.18633/biotecnia.v22i1.1159
Re R, Pellergrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an impoved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Del-Toro-Sánchez, C. L., Rodríguez-Félix, F., Cinco-Moroyoqui, F. J., Juárez, J., Ruiz-Cruz, S., Wong-Corral, F. J., Borboa-Flores, J., Castro-Enríquez, D. D., Barreras-Urbina, C. G., & Tapia-Hernández, J. A. (2021). Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J Food Process Preserv 45(9):1–16. https://doi.org/10.1111/jfpp.15765
do Prado ACP, da Silva HS, da Silveira SM, Barreto PLM, Vieira CRW, Maraschin M, Ferreira SRS, Block JM (2014) Effect of the extraction process on the phenolic compounds profile and the antioxidant and antimicrobial activity of extracts of pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell. Ind Crops Prod 52:552–561.https://doi.org/10.1016/j.indcrop.2013.11.031
Prado ACP do, Manion BA, Seetharaman K, Deschamps FC, Barrera Arellano D, Block JM (2013) Relationship between antioxidant properties and chemical composition of the oil and the shell of pecan nuts [Caryaillinoinensis (Wangenh) C. Koch]. Ind Crops Prod 45:64–73.https://doi.org/10.1016/j.indcrop.2012.11.042
Rodríguez-Roque MJ, Del-Toro-Sánchez CL, Chávez-Ayala JM, González-Vega RI, Pérez-Pérez LM, Sánchez-Chávez E, Flores-Córdova MA (2022) Digestibility, antioxidant and anti-inflammatory activities of pecan nutshell (Carya illioinensis) extracts. J Renew Mater 10(10):2569–2580. https://doi.org/10.32604/jrm.2022.021163
Girardi Müller L, Simonetti Pase C, Reckziegel P, Barcelos RCS, Boufleur N, Prado ACP, Fett R, Block JM, Pavanato MA, Bauermann LF, Teixeira da Rocha JB, Escobar Burger M (2013) Experimental and toxicologic pathology hepatoprotective effects of pecan nut shells on ethanol-induced liver damage. Exp Toxicol Pathol 65(1–2):165–171. https://doi.org/10.1016/j.etp.2011.08.002
Dunford NT, Gumus ZP, Gur CS (2022) Chemical composition and antioxidant properties of pecan shell water extracts. Antioxidants 11(1127):1–13. https://doi.org/10.3390/antiox11061127
Engler Ribeiro PC, de Britto Policarpi P, Dal Bo A, Barbettac PA, Blocka JM (2017) Impact of pecan nut shell aqueous extract on the oxidative properties of margarines during storage. J Sci Food Agric 97:3005–3012. https://doi.org/10.1002/jsfa.8141
Do Prado ACP, Aragão AM, Fett R, Block JM (2009) Antioxidant properties of pecan nut [Carya illinoinensis (Wangenh.) C. Koch] shell infusion. Grasas Aceites 60(4):330–335. https://doi.org/10.3989/gya.107708
Md Yusof AH, Abd Gani SS, Zaidan UH, Halmi MIE, Zainudin BH (2019) Optimization of an ultrasound-assisted extraction condition for flavonoid compounds from cocoa shells (Theobroma cacao) using response surface methodology. Molecules (Basel, Switzerland) 24(4):1–16. https://doi.org/10.3390/molecules24040711
Wang S, Lin AH, Han Q, Xu Q (2020) Evaluation of direct ultrasound-assisted extraction of phenolic compounds from potato peels. Processes 8(1665):2–14
Lo Fiego MJ, Lorenzetti AS, Silbestri GF, Domini CE (2021) Ultrasonics sonochemistry The use of ultrasound in the South Cone region. Advances in organic and inorganic synthesis and in analytical methods. Ultrason Sonochem 80(2021):105834. https://doi.org/10.1016/j.ultsonch.2021.105834
Oroian M, Ursachi F, Dranca F (2020) Influence of ultrasonic amplitude, temperature, time and solvent concentration on bioactive compounds extraction from propolis. Ultrason Sonochem 64(January):105021. https://doi.org/10.1016/j.ultsonch.2020.105021
Capelo-Martínez JL (2009) Ultrasound in chemistry: analytical and applications. John Wiley & Sons
Flores-Córdova MA, Sánchez E, Muñoz-Márquez E, Ojeda-Barrios DL, Soto-Parra JM, Preciado-Rangel P (2017) Phytochemical composition and antioxidant capacity in Mexican pecan nut. Emirates J Food Agric 29(5):346–350. https://doi.org/10.9755/ejfa.EJFA-2016-08-1075
Agustin-Salazar S, Cerruti P, Medina-Juárez LÁ, Scarinzi G, Malinconico M, Soto-Valdez H, Gamez-Meza N (2018) Lignin and holocellulose from pecan nutshell as reinforcing fillers in poly (lactic acid) biocomposites. Int J Biol Macromol 115:727–736. https://doi.org/10.1016/j.ijbiomac.2018.04.120
Robbins KS, Ma Y, Wells ML, Greenspan P, Pegg RB (2014) Separation and characterization of phenolic compounds from dry-blanched peanut skins by liquid chromatography-electrospray ionization mass spectrometry. J Agric Food Chem 69(19):4332–4341. https://doi.org/10.1021/jf500909h
Sarkis JR, Côrrea APF, Michel I, Brandeli A, Tessaro IC, Marczak LDF (2014) Evaluation of the phenolic content and antioxidant activity of different seed and nut cakes from the edible oil industry. JAOCS, J Am Oil Chem Soc 91(10):1773–1782. https://doi.org/10.1007/s11746-014-2514-2
Kureck I, Policarpi P de B, Toaldo IM, Maciel MV de OB, Bordignon-Luiz MT, Barreto PLM, Block JM (2018) Chemical characterization and release of polyphenols from pecan nut shell [Carya illinoinensis (Wangenh) C. Koch] in zein microparticles for bioactive applications. Plant Foods Hum Nutr 73(2):137–145. https://doi.org/10.1007/s11130-018-0667-0
Gur CS, Dunford NT, Gumus ZP (2023). Cytotoxicity of subcritical water extracts obtained from byproducts generated at commercial pecan shelling operations on cancer cells. Bioresour Bioprocess 10(1). https://doi.org/10.1186/s40643-023-00666-z
Acknowledgements
Frida Lourdes Garcia Larez acknowledges the National Council of Humanities, Sciences and Technologies (CONAHCYT, Mexico) for providing a scholarship for this project.
Author information
Authors and Affiliations
Contributions
Frida Lourdes Garcia-Larez: conceptualization, methodology, investigation, original draft writing, formal analysis. Javier Esquer and Héctor Guzmán: formal analysis, supervision, review and editing. María Jesús Moreno-Vásquez, Francisco Rodríguez-Félix and Carmen Lizette Del-Toro-Sánchez: supervision, investigation, review and editing. Betzabe Ebenhezer López-Corona: review and editing. David Slim Zepeda Quintana and José Agustín Tapia-Hernández: conceptualization, methodology, software, validation, supervision, investigation, original draft writing, formal analysis, resources, review and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garcia-Larez, F.L., Esquer, J., Guzmán, H. et al. Effect of Ultrasound-Assisted Extraction (UAE) parameters on the recovery of polyphenols from pecan nutshell waste biomass and its antioxidant activity. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05901-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05901-x