Abstract
The presence of numerous bioactive compounds in fruit wastes indicates the potential to use them as low-cost resource for generating value-added ingredients. The integral use of residual biomass from wine and cider industries was proposed. Phenolic-rich liquid extracts were produced from grape marc (GM), grape stalk (GS), and apple pomace (AP) by applying hydrothermal (T), ultrasound-assisted (US), and enzymatic (EZ) extractions, and the remaining solid fraction (RSF) was then converted into fiber concentrated powders by air-drying. Additionally, the direct conversion of the whole initial wastes into integral powders rich in both fiber and phenolic compounds was proposed. An integral physicochemical study was performed: phenolic compounds, content and techno-functional properties of dietary fiber, and physical and stability properties. The T extraction conducted for 75 min at 75 °C demonstrated superior efficacy in terms of total polyphenol content (TPC) and antioxidant capacity (AC). Under these optimal conditions, the extracts exhibited a TPC content of 251 ± 2 mg GAE/100 g from GM, 1420 ± 5 mg GAE/100 g from GS, and 23 ± 2 mg GAE/100 g from AP. GS extract was mainly composed of procyanidins, catechin, and epicatechin, while in AP, flavonols (mainly rutin) represented the majority group. The GM extract showed a richer polyphenol profile including condensed tannins, flavonols (mainly rutin), flavan-3-ols (catechin), and a greater variety of phenolic acids. Within the fiber-rich powders produced from the RSF (optimal extraction conditions), those from grape wastes registered higher remaining TPC and total dietary fiber content (47–51%), mainly composed of insoluble fiber (IDF). AP powders (integral and from RSF) showed a higher soluble fiber content (SDF), a more balanced IDF/SDF ratio (1.9 and 3.3, respectively), and better hydration properties. Among the integral powders, GM and GS showed high level of TPC (997–1600 mg GAE/100 g), and suitable techno-functional properties. Physical properties are suggestive of free-flowing stable powdered ingredients in all cases. The findings offer a substantial advance in waste valorization, benefiting not only scientists, but also wine and cider producers, as well as the food industry. They pave the way for converting various fruit wastes into diverse food ingredients. This approach enables the economic value recovery while delivering substantial environmental benefits, achieving a zero waste state. Employing these ingredients in creating new functional foods could enhance public health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Consumption of natural products without chemical additives and with functional properties has been increasing in recent years, and as a consequence, the demand for new sources of bioactive compounds. In particular, the food industry has focused on the use of the waste generated for its nutritional, technological, and functional properties [1]. The fruit and vegetable industries produce higher volumes of waste compared to other food processing sectors, including 25–30% peels, followed by seeds, shells, pods, cores, pulp, and pomace. Its perishable nature, product traits, logistics issues, and disposal are some of the main concerns that make the valorization of this waste a challenging practice [2]. Hence, fruit wastes hold promising potential when transformed into valuable materials applicable in food, packaging, cosmetics, pharmaceuticals, wastewater treatment, and various other industrial sectors. This approach offers multiple advantages, such as waste reduction, resource conservation, and the development of environmentally friendly products [3]. Industries such as wineries, cider houses, and juicers generate large amounts of residual biomass, which, in addition to being an environmental threat, constitute an important source of biocompounds with industrial interest, such as dietary fiber and antioxidant compounds [4, 5]. These compounds have gained popularity since they appear to contribute significantly to lowering cholesterol level, preventing cardiovascular disease, and improving overall health [6]. In the wine industry, for every 100 kg of grapes processed, about 25 kg of waste is produced, comprising roughly 50% grape skins, 25% stalks, and the remaining 25% seeds. Grape marc, the main by-product of the winemaking process, is the solid waste generated after crushing and pressing the grapes in the juice separation step. Since it is composed of grape skin, seeds, and pulp, it is an important source of dietary fiber, anthocyanins, polyphenols, tannins, tartaric acids, and other antioxidant compounds [7, 8]. Grape stalks, obtained after the initial destemming process, are mainly composed of lignocellulosic materials containing cellulose, hemicelluloses, and lignin, also containing other compounds like polyphenols [9, 10]. According to recent studies, the discharge of grape stalks into soil originated inhibition of the soil germination properties, because of the biological oxygen demand, carbon, and phenolic compounds. Therefore, the valorization of grape stalks has become an important field of research to minimize the environmental impact [10]. Particularly in the juice and cider industry, apple pomace is the waste left after the fruit juice extraction during the pressing stage [11]. It is mainly composed of skin, seeds, and flesh tissues, containing nutrients and phytochemicals with antioxidant and biological activities [12]. It consists of cell wall polysaccharides (e.g., cellulose, hemicellulose, lignin, pectin, and gums) and skin-bound phenolic compounds.
The alternatives available for phenolic compound recovery are diverse. Numerous studies have demonstrated the effectiveness of traditional extraction techniques, including maceration processes and the Soxhlet extraction. However, using such techniques requires the use of a lot of time, energy, and solvent [13]. From the “Green Processes” concept, environment-friendly techniques are becoming more attractive; therefore, researchers are aiming at optimizing the most eco-friendly ways of extraction. Innovative methods, such as those assisted by microwaves, pulsed electric fields, ultrasound, enzymes, or supercritical fluid extraction, have shown to reduce the extraction time and therefore the consumed energy [14,15,16,17,18]. The use of safe solvents, moderate temperatures to minimize thermolabile chemical degradation, the ability to alter selectivity, and increased extraction efficiency are all features of these innovative extraction methods [16]. The ultrasound extraction technique takes advantage of the sonic waves that induce microbubble formation. The ultrasound frequencies collapse cavitation bubbles near the cell walls, leading to stronger agitation and allowing the solvent to penetrate the cells, thereby intensifying mass transfer [19]. Enzymatic pre-treatment is a biodegradation process that breaks the cellular wall in moderate circumstances. The enzymatic pre-treatment has also the benefit of not requiring complex equipment [16]. On the other hand, among the traditional methods, the hydrothermal extraction also appears as an eco-friendly option with several advantages, such as the absence of organic solvents and related corrosion problems, and the fact that it is easy to operate and cost-effective [20]. Moreover, some authors such as Kabir et al. [21] have shown a better performance for bioactive recovery compared to solvent extraction.
It is important to consider that the phytochemical composition of fruits, vegetables, and agro-industrial wastes is related to different proportions of simple and complex phenols, such as cinnamic acids, coumarins, tannins, lignins, lignans, and flavonoids [11, 22]. These diverse forms of phenolic compounds showed variable responses to different extraction conditions [23]. Several studies were reported on bioactive compound extraction from different fruits, such as maqui [24], elderberry [25], and blackcurrant [26, 27], as well as from by-products of vegetable origin such as orange pomace [28], apple pomace [29], grape pomace [30,31,32], mango peel [33], orange, mango, and tuna wastes [34], and olive leaves [35]. Most works have focused on the extraction process of bioactive compounds to later convert them into flours or powders, discarding the remaining solid fraction of the extraction process, without significantly reducing the waste volume. There is thus a need for processing alternatives to allow the condition of “zero waste” to be achieved. In this sense, the whole wastes from fruit industries can be used, turning them into different fractions, such as liquid extracts with antioxidant potential, and powders concentrated in fiber or in both, fiber and polyphenols. These fractions could be incorporated as ingredients into food products to add multifunctionality. For instance, powders can be used as cost-effective and low-calorie bulking agents to replace some of the fat, sugar, or flour in baked products. In addition, food functionality can be improved by enhancing water and oil retention and emulsion stability. Extracts can be used for phenolic enrichment in antioxidant product development [13].
This complete valorization strategy could allow healthier foods to be developed, whose consumption could increase the daily intake of fiber and/or polyphenols in addition to reducing the waste volume and contributing to the circular economy [1, 36].
The integral valorization of bio-wastes from wine and cider industries is proposed in this work by applying (1) the extraction of phenolic compounds using environment-friendly techniques, comparing traditional and emerging processes, and (2) the production of fiber-rich dry ingredients by direct processing of the initial wastes or by reconversion of the residual solids resulting from the extraction process. An integral evaluation of the physicochemical properties and bioactive potential was carried out on the obtained powders and liquid extracts in order to predict their potential use as nutraceutics or functional ingredients in foods.
2 Materials and methods
2.1 Materials
The fruit wastes were collected from industries located in the Río Negro province (Argentine Patagonia). Humberto Canale S.A. winery provided grape waste (var. Sauvignon Blanc), and La Flor S.A. cider industry provided green apple waste (var. Granny Smith). Three types of waste were studied: grape marc (GM), grape stalk (GS), and green apple pomace (AP). After collection, the waste samples were immediately frozen and stored at − 22 °C until use.
2.2 Waste characterization
The waste characterization was done according to the Official Methods of Analysis of the Association of Official Agricultural Chemists [37]: soluble solids (932.12), acidity (945.26), pH (945.27), and moisture (925.09). Water activity (aw) was determined using an Aqualab Series 3 TE (Washington, USA). Acidity results were expressed as g tartaric acid per 100 g sample on dry basis (d.b.) for grape wastes (GM and GS) and as g malic acid per 100 g sample (d.b.) for apple pomace (AP) Total phenolic content (TPC and PCH) and antioxidant capacity (AC) methods are described below in Sect. 2.3.2 and in “Phenolic content and antioxidant capacity.”
2.3 Polyphenol-rich extract obtaining and characterization
Fruit wastes were processed as follows: thawing at room temperature for 10 min, waste weighing (40 g), milling with a mixer for 60 s, and subjected to three extraction processes (hydrothermal, enzyme-assisted, and ultrasound-assisted) using distilled water as solvent (solvent/waste ratio, 2/1) in all cases.
2.3.1 Operative conditions of the extraction processes
Hydrothermal extraction (T)
It was carried out in a Biosmartest thermostated bath (Buenos Aires, Argentina) with constant agitation, at different temperatures (30, 60, and 75 °C) and different extraction times (20, 75, and 120 min).
Ultrasound treatment (US)
A TB02TACA TestLab ultrasonic processor (Buenos Aires, Argentina) at a frequency of 40 kHz and 100% amplitude (80 W) was used. Two extraction temperatures (30 and 60 °C) and two extraction times (20 and 75 min) were evaluated.
Enzymatic treatment (EZ)
A pectinolytic enzyme (Novozymes Pectinex® Ultra Color, Bagsvaerd, Denmark) was used, following the methodology reported by Gomez Mattson et al. [38] at the optimal temperature–time condition defined by these authors (enzyme concentration, 160 ppm; extraction time, 75 min; temperature, 50 °C). The enzyme was added and the mixture was placed into a water bath set at the corresponding temperature (50 °C). After maceration (75 min), the mixture was immersed in boiling water to inactivate the enzyme (1 min at 80 °C); then, it was immediately cooled up to 30–35 °C.
After treatments, the processed wastes were cooled over an ice bath, and then centrifuged (6000 rpm, 15 min) and filtered. The supernatants (extracts) and the remaining solid fraction (RSF) were separated, and frozen for further processing.
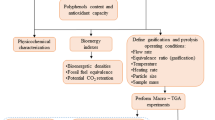
Figure 1 summarizes the sequence of treatments applied in order to achieve zero waste condition. Each treatment is described in detail below, along with the analytical methods used for the physicochemical study performed on all the obtained products (extracts and powders).
Flowchart of the processes proposed for the treatment of fruit wastes. Pathway 1: Phenolic extraction using different methods. Pathway 2: Reuse of the remaining waste for fiber-rich powder production by convective drying. Pathway 3: Direct processing of the initial waste obtaining a powder rich in both phenolic compounds and dietary fiber
2.3.2 Bioactive potential of the extracts
All chemical determinations were done on methanolic extracts obtained according to Sette et al. [39]. The spectrophotometric measurements were carried out using a spectrophotometer T60 UV–visible (PG instruments, Leicestershire, UK).
Polyphenol content
Spectrophotometric analysis: The total polyphenol content (TPC) was determined using the Folin–Ciocalteu reagent according to Sette et al. [39]. The calibration curve was made using gallic acid as standard. The results were expressed as mg gallic acid equivalents (mg GAE) per 100 g sample on dry basis (d.b.).
Qualitative-quantitative HPLC–DAD analysis: Individual phenolic compounds were determined according to the protocol described by Gomez Mattson et al. [38], using an Agilent 1260 HPLC system (Agilent Technologies, Waldbronn, Germany) with a diode array detector (DAD), controlled by Agilent’s OpenLAB Chem Station software.
Antioxidant capacity (AC)
AC was determined through three different methods: FRAP, ABTS, and DPPH. The ferric reducing-antioxidant power (FRAP) method and the bleaching method of the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) were both performed according to Sette et al. [39]. The ABTS bleaching method was carried out according to Franceschinis et al. [40]. A calibration curve was constructed using gallic acid as standard, and the results were expressed as mg GAE per 100 g sample on dry basis (d.b.).
2.4 Powdered waste obtaining and characterization
2.4.1 Drying and grinding processes
Different powders were obtained from (1) the initial fruit waste without the previous extraction processes (GM, GS, and AP), and (2) the remaining solid fraction obtained after the application of the selected processes to each fruit waste (RSFGM, RSFGS, RSFAP). The processing pathways can be observed in Fig. 1.
The samples were dried by convective drying at 60 °C (10% relative humidity, air rate of 1–1.5 m/s, and 12 h drying time) using an oven with forced circulation model Venticell 111 Standard (MMM Medcenter Einrichtungen GmbH, Munich, Germany). The drying time was set to achieve a final aw < 0.3.
Dried wastes were then ground at room temperature for 15 min using a hammer mill model FZ-102 (EKO, Arcano, China) at a speed of 1400 rpm. The ground samples were sifted using a sieve ASTM-USA, mesh 30 (particle size < 590 µm).
2.4.2 Bioactive potential of the powdered wastes
Phenolic content and antioxidant capacity
Powdered wastes were analyzed for total polyphenol content (TPC) and total polyphenols including those associated with cell wall components (PCH), which were quantified after alkaline hydrolysis to release the linked phenolic groups, according to Bunzel et al. [41]. The supernatants obtained from alkaline hydrolysis were separated by centrifugation. Total polyphenol content was determined using the Folin–Ciocalteu technique (previously described in the “Polyphenol content” section) on methanolic extracts obtained with and without alkaline hydrolysis to evaluate the PCH contribution to total content. This analysis was also performed on initial wastes.
The antioxidant capacity (AC) was determined through the methodologies described in Sect. 2.3.2.
Dietary fiber content
The contents of total dietary fiber (TDF) and insoluble fiber (IDF) were measured according to the AOAC method 991.43 [37]. The soluble fiber content (SDF) was calculated by subtracting the IDF proportion from the TDF value [42]. The results were expressed as g of dietary fiber per 100 g sample on dry basis (d.b.).
Analysis by Fourier transform infrared spectroscopy technique
The Fourier transform infrared spectroscopy (FTIR) spectra of the different waste powders were obtained according to Sette et al. [43], using an Infralum FT-08 FTIR spectrometer (Mission, Canada).
2.4.3 Techno-functional properties of the powdered wastes
The methodologies reported by Sette et al. [43] were employed to determine the swelling capacity (SC), the water holding capacity (WHC), the water retention capacity (WRC), the retention water (RW), the oil holding capacity (OHC), and the emulsifying activity (EA). The following expressions were used:
Swelling capacity (SC):
where Vh is the volume of the hydrated sample and wd is the weight of the dry sample.
Water holding capacity (WHC):
where Wd is the weight of the dehydrated sample and Wh is the weight of the hydrated sample.
Water retention capacity (WRC) and retention water (% RW).
where W represents the water mass (g) and R represents the dry residue (g). The percentage of RW was calculated with the following equation:
where RW is the percentage of retained water, W is the water mass (g), and Wt is the total water added (g).
Emulsifying activity (EA):
where Vel refers to the volume of the emulsified layer (mL) and V is the total volume of fluid (mL).
2.5 Physical properties of the powdered wastes
2.5.1 Superficial color
The superficial color was measured according to Sette et al. [43]. A photocolorimeter model CR400 (Minolta Co, Tokyo, Japan) was used. Color functions were obtained in the CIELAB uniform color space for illuminant C at 2° standard observer. Values of L* (luminosity/darkness), a* (redness/greenness), and b* (yellowness/blueness) were obtained. These numerical values were converted into “global color difference” (ΔE*ab) using the following equation:
where
\({\mathrm{L}}_{0}^{*}\mathrm{, }{ \, {\mathrm{a}}}_{0}^{*}\mathrm{, and }{\mathrm{b}}_{0}^{*}\) correspond to the values of the powders without the previous extraction processes.
\({\mathrm{L}}^{*}\mathrm{, }{ \, {\mathrm{a}}}^{*}\mathrm{, and }{\mathrm{b}}^{*}\) correspond to the values of the powders obtained from the remaining solid fraction (obtained after the application of the extraction process).
2.5.2 Bulk, compacted density, and cohesiveness (Hausner ratio)
Bulk and compacted density (g/mL) and Hausner ratio (H) were determined according to Sette et al. [43]. The Hausner ratio (H) is related to the powder cohesiveness: levels below 1.2 are considered low, between 1.2 and 1.4 are intermediate, and above 1.5 are high.
2.5.3 Angle of repose and Carr’s index (CI)
The angle of repose (AR) and Carr’s index (CI) were determined according to Garrido Makinistian et al. [44]. Based on Carr’s compressibility index, the following flow conditions are considered to evaluate the powders: CI < 10% excellent, 11% < CI < 15% good, 16% < CI < 20% fair, 21% < CI < 25% acceptable, and 26% < CI < 31% poor flow [45]. Repose angles between 25° and 30° indicate excellent flowability, within 31°–35° the flow is good, and within 36°–40° the flow is fair. Values higher than 41° represent poor flow properties.
2.6 Powder stability
2.6.1 Water activity and water content
Water activity and water content were determined according to Sect. 2.2.
2.6.2 Glass transition temperature (T g)
The glass transition temperatures were determined by differential scanning calorimetry (DSC; onset values) using a calorimeter model 822 (Mettler Toledo, Schwerzenbach, Switzerland) according to Franceschinis et al. [46]. Thermograms were evaluated using Stare software v. 3.1 (Mettler Thermal Analysis).
2.6.3 Hygroscopicity (Hi)
The hygroscopicity of powders was determined according to the methodology described by Garrido Makinistian et al. [44]. Powders (approximately 0.3 g) were stored in containers at 20 °C and 75% RH (given by a saturated solution of NaCl). Hygroscopicity was expressed as the average of grams of adsorbed water per 100 g of dry solids after 7 days.
3 Results and discussion
3.1 Fruit waste characterization
Physicochemical properties of fruit wastes are variable and highly dependent on many factors, such as type of fruit, growing conditions, industrial operation, and storage conditions [47]. Additionally, it is also necessary to consider other variables such as the kind of biomass for the same process (i.e., grape stalk or grape marc) and previous treatments of the waste. Table 1 shows some physicochemical and potentially functional properties of the different fruit wastes. The apple pomace (AP) registered significantly higher water content compared to grape wastes (GS and GM), due to the higher proportion of flesh presented in AP compared to GS and GM wastes that have a higher proportion of seeds and stems. AP water content value was similar to that reported by Gal [48], for apple pomace collected under the same conditions as those used in this work, after the pressing process in a cider industry (water content = 80.9% w.b.). The three wastes (grape marc, grape stalks, and apple pomace) exhibited similar acidity and pH values, in accordance with results previously reported for these fruit wastes (acidity values between 3.3 and 3.5 g tartaric acid/100 g d.b. for grape wastes and 4.2 g malic acid/100 g d.b. for apple pomace, and pH values between 3.9 and 4.5) [8, 49]. Regarding waste color, all samples presented yellow-brownish tones.
Concerning the bioactive compounds (Table 1), the grape stalk (GS) exhibited significantly higher levels of total polyphenols (TPC) and antioxidant capacity (AC) in comparison with grape marc (GM) and apple pomace (AP). Apple pomace showed the lowest TPC values (≈73% lower compared to GS, and ≈59% lower compared to GM). When comparing GM and GS, the main differences were observed in AC, regardless of the analytical method employed. These results coincided with those obtained by other authors when comparing the phenolic content of grape stalk, grape pomace, and different fruit wastes (apple, pear, and quince) [21, 50, 51]. The TPC value of apple pomace from cider industries reported by Diñeiro Garcia et al. [52] ranged between 550 and 720 mg gallic acid/100 g (d.b.), a range consistent with the findings of our study. At subcellular level, phenolic compounds accumulate at two major sites in plant cells: in the vacuole, containing various soluble phenolic compounds and derivatives, but also in the cell wall matrix, a porous polysaccharide-based material where lignin is deposited and also simpler molecules, such as flavonoids and esterified ferulic acid, can be found. At tissue level, in most cases, soluble phenolic compound concentration is higher in the external tissues of fleshy fruits (epidermal and subepidermal layers) than in internal tissue (mesocarp, pulp). It has been shown that in apples and grapes, polyphenols are mostly linked to the cell wall matrix, which makes it difficult to recover these compounds by traditional solvent extraction methods [53]. The polyphenols’ binding capacity of plant cell walls, which has been extensively studied for these fruits, occurs due to a combination of hydrogen bonding, hydrophobic interactions, and ionic interactions [54]. According to the results of Budak et al. [55], since apple polyphenols are concentrated in the peel, after the pressing process for the juice extraction, the remaining waste having a high content of peel consequently presents high polyphenol content. Polyphenols in grapes are located mainly in the seeds and skin cell walls; therefore, a considerable proportion of polyphenol compounds would not be extracted during the winemaking process, remaining in the residual biomass [8, 56, 57]. Additionally, differences between industries regarding the equipment used for juice extraction or processing methods modify the extractability of the cell wall compounds. In this context, it was worthy to further analyze the proportion of polyphenols within the wastes, considering not only soluble compounds (upon methanolic extraction), but also cell-bound compounds, both represented by the PCH variable. It is noteworthy that bound phenolic compounds account for more than 80% of the total polyphenol content in all the analyzed wastes (83% in GM, 87% in GS, and 85% in AP).
3.2 Selection of extraction conditions
Table 2 shows TPC and AC results, as well as the soluble solid content (SS) of the extracts obtained from all the fruit wastes subjected to the different extraction processes (ultrasound (US); hydrothermal (T); enzymatic (EZ)). As expected from the waste characterization results, substantially higher TPC and AC values were observed with grape wastes (GP and GM) when compared with apple pomace, especially in the extracts produced from stalks. Fuchs et al. [58] obtained polyphenol-rich extracts from grape waste using methanol as solvent, with a solvent/solid ratio of 25/1 and stirring at 25 °C for 90 min. They obtained grape stalk extracts with the highest TPC content (6650 mg GAE/100 g d.b.), followed by the grape pomace extract (5890 mg GAE/100 g d.b.). As for apple pomace extracts, similar values of TPC (684 ± 0.38 mg GAE/100 g d.b.) were reported by Pollini et al. [59] when using 4 h maceration at 25 °C and ethanol as solvent, and a solvent/pomace ratio of 2/1.
On the other hand, except for some conditions with AP, richer extracts were achieved (Table 2), if a comparison with the corresponding initial waste (Table 1) is performed. Even for GS, the amount of extracted phenolic compounds in most of operating conditions exceeds that obtained when alkaline hydrolysis is carried out for the analysis (PCH values). The applied processes affected the integrity of cell walls and membranes differently depending on the type of extraction, making the phenolic compounds in fruit waste more accessible and resulting in extracts substantially enriched in antioxidant compounds, despite the use of water as solvent.
When comparing the different extraction processes studied, the highest content of TPC and AC was obtained when the hydrothermal method (T) was applied at 75 °C. At low temperatures (30 °C and 60 °C), no major differences were observed when the extraction time was increased from 20 to 75 min, while at 75 °C considerable higher extraction of bioactive compounds occurred at 75 min. However, no differences were observed between 75 and 120 min for GM and AP extracts, while in GS extract, both TPC and AC decreased with increasing extraction time from 75 to 120 min. Long extraction times could have accelerated the phenolic compound degradation in this case while producing a greater consumption of time and energy. It can be concluded that, when using the traditional method (T), the temperature variable has major effect on the extraction of phenolic compounds, achieving the best condition at 75 °C for a period of 75 min.
Regarding US treatment, the best condition was observed at 60 °C for 20 min. When comparing the alternative extraction methods concerning T, in the case of GM, a lower TPC content was observed for US and EZ (23% and 29% respectively). When analyzing GS and AP, a TPC decrease of 11% and 18% was observed for US and EZ, respectively. In the case of antioxidant capacity, GM and AP showed 40% and 45% decrease for US and EZ, respectively, while GS showed a decrease of around 22–25%. According to these results, the T method showed greater efficiency (Fig. 2).
Total polyphenol content (TPC) and antioxidant capacity (AC) corresponding to the best condition for each extraction method. US, ultrasound treatment (60 °C, 20 min); T, hydrothermal extraction process (75 °C, 75 min); EZ, enzyme-assisted extraction process (50 °C, 75 min, 160 ppm). Results are expressed in mg GAE/100 g dry basis (d.b.)
The optimal operative conditions in terms of total polyphenol content (TPC) and antioxidant capacity (AC) for the T method were found to be an extraction time of 75 min and a temperature of 75 °C. Under these extraction conditions, the TPC content (wet basis) was 251 ± 2 mg GAE/100 g for GM, 1420 ± 5 mg GAE/100 g for GS, and 23 ± 2 mg GAE/100 g for AP, which could be considered valuable considering that the consumption of commercial apple juice provides between 15 and 60 mg TPC/100 g of juice [59] while commercial grape juice provides between 111.2 and 343 mg TPC/100 g juice, indicating the high potential of the extracts as source of natural phenolic compounds.
Unfortunately, emerging technologies did not always result in positive outcomes, even when using organic solvents. For instance, Ntourtoglou et al. [60] studied the combination of pulsed electric field and ultrasound-assisted on polyphenol extraction in grape stalk, using methanol and water as extraction solvent, and concluded that the US method needs the combination with another method to optimize the polyphenol extraction in this waste. Haas et al. [61] studied grape wastes and three extraction methods (ultrasound, orbital shaker, and plate shaker) using a hydroalcoholic solution (80% v/v methanol), obtaining higher polyphenol concentrations when using orbital and plate shaking.
The ability of US to enhance recovery of specific metabolites has been attributed to its ability to disrupt cell membranes through the transmission of ultrasound pressure waves and the subsequent phenomenon of cavitation [19, 62]. On the other hand, the pectic substances located in the middle lamella and the primary cell of plant tissues are attacked by the pectolytic enzyme used in EZ extraction. The enzyme-assisted extraction dissolves cell wall elements and increases the release of intracellular content [4, 13]. Heating treatments under the conditions used in this work often result in tonoplast and plasmalemma disruption, as well as disorganization of cell wall structure, representing alteration of individual cell wall polysaccharides (pectic polysaccharides, cellulose, hemicellulose) [63]. In addition, water immersion for long times generally leads to solubilization and depolymerization of hemicellulose and pectin contributing to wall loosening and disintegration. Besides, during heat treatments, in addition to compound degradation, new phenolic compounds or interactions between them can occur, increasing polyphenol concentration, as observed for instance by Marsiglia et al. [64], when studying polyphenol extraction from jaboticaba peel. Therefore, the T extraction process, especially the one performed at higher temperatures, affected to a greater extent the binding capacity of cell walls, favoring the polyphenol extractability. The time–temperature combination must be then carefully analyzed to determine the specific range that improves the extraction performance and prevents the specific compound degradation according to the waste type to be addressed.
The worst performance of alternative methods could probably be ascribed to the damage already produced on tissue structure during the industrial processing; therefore, the US ability to disrupt the cell membranes through the cavitation phenomenon or the enzymes to destroy cell wall structure did not have a significant effect for linked phenolic release.
Quantification of individual phenolic compounds by HPLC (Table 3) was carried out to analyze the composition achieved using the best extraction process for each fruit waste. In agreement with spectrophotometric measurements (Fig. 2), higher content of individual compounds was observed by HPLC in grape stalk extract. In GS extract, procyanidins represented the main phenolic group (≈ 57%), followed by catechin and epicatechin (≈ 27%). In apple pomace extract (AP), flavonols represented 84% (mainly rutin) of the total quantified phenolic compounds, accounting for the rest of the compounds as condensed tannins. Grape marc extract showed a richer polyphenol profile compared to the other wastes (Table 3), identifying in these samples a greater variety of polyphenols types (40% of condensed tannins, 23% of flavonols and flavan-3-ols, and 14% of phenolic acids). Similar results were obtained by Teixeira et al. [65] who also quantified procyanidins as the major polyphenol group in grape stalk extracts. According to Tylewicz et al. [66], apple pomace mainly contains flavan-3-ols (catechin and epicatechin), proanthocyanidins, and chlorogenic acid; and grape pomace contains condensed tannins, phenolic acids (gallic in the seeds), quercetin and its glycosides, and flavan-3-ols such as catechin, epicatechin, and proanthocyanidins. These authors, when studying grape stalk, also reported the presence of flavan-3-ols (catechin and epicatechin), flavonols (quercetin glycosides, including rutin), and phenolic acids. Nevertheless, fruit by-products like apple pomace are complex matrices presenting variation in their composition as they have undergone different industrial processes that impact their final state [67]. Finally for grape marc extracts, similar results were found by Perra et al. [68], who found the highest level of flavan 3-ols, accounting for approximately 50% of the total phenolic content after solid–liquid extraction from grape pomace using a hydroethanolic mixture.
3.3 Physicochemical and functional properties of fruit waste powders
Table 4 and Fig. 3 show relevant properties of the powders obtained from the residual solid fractions (RSF) of the selected phenolic extraction, to be evaluated together with those produced from the integral use of the initial wastes (GS, GM, and AP). Both valorization options would allow zero waste dumping to be achieved at the industrial sites. It is worth pointing out that RSF accounted for ≈ 87% of the total waste weight in GM, 64% in GS, and 57% in AP wastes.
Antioxidant capacity (AC) of waste powders through three analytical methods: FRAP (
 ), DPPH (
), DPPH (
 ), ABTS (
), ABTS (
 ) (d.b. = dry basis). Powders produced from initial wastes: apple pomace (AP), grape marc (GM), and grape stalk (GS). Produced from the RSF of selected extraction processes: apple pomace (RSFAP), grape marc (RSFGM), and grape stalk (RSFGS)
) (d.b. = dry basis). Powders produced from initial wastes: apple pomace (AP), grape marc (GM), and grape stalk (GS). Produced from the RSF of selected extraction processes: apple pomace (RSFAP), grape marc (RSFGM), and grape stalk (RSFGS)
Powder water content was less than 5% (wet basis) in all cases being the values consistent with those obtained by other authors when using similar dehydration processes in fruit wastes [69]. In general, a significant effect of the extraction process on chemical properties was observed, because many compounds initially present in the fruit wastes migrated to the extracts (Fig. 2; Table 2), directly impacting on several properties such as acidity and bioactive potential.
Regarding potentially bioactive compounds, wine wastes registered higher TDF content, mainly composed of IDF (62–70%), while powders from apple waste presented a more balanced ratio between types of fiber and the highest proportion of SDF (24.6% and 45%, for RSFAP and AP, respectively) that is related to the higher proportion of soluble hemicellulose and pectin of apple matrices [70]. On the other hand, the extraction process produced SDF decrease, which led to an increase in the IDF/SDF ratio in all cases. As expected, the RSFGS and RSFGM samples showed a substantially higher IDF percent (87–93%) and a greater IDF/IDS ratio. Taking into account that an IDF/SDF ratio ranging between 1 and 3 is considered desirable to yield maximum health benefits [71], the RSFAP value would indicate a better quality for use as a food ingredient. The data reported, for example, on apple pomace powder obtained by drying waste provided by a juicer at 70 °C [72] fall within the range of TDF values obtained in this study for AP and RSFAP samples (37.7 and 47.9 g/100 g, d.b.).
In terms of polyphenol content (TPC) (Table 4) and antioxidant capacity (AC) (Fig. 3), in line with the extraction results, GS powder was the one showing the highest bioactive potential. The extraction process led to a significant decrease in powder TPC and AC, being the RSFGS sample the one that registered the greatest decreases in both variables: TPC (52%) and AC through most of the tested methods (64–78%). It can also be observed from PCH data that most of the phenolic compounds seem to be bound to the solid phase in powders, mainly composed of dietary fiber.
Recent reports show that dietary fiber exerts a variety of biological benefits on the host’s health [73]. Fiber provides a source of nutrition for the gut microbiota that is able to decompose it and produce short-chain fatty acids and other valuable metabolites that affect directly the host’s health [74]. Additionally, polyphenols have shown a protective effect on the gastrointestinal tract, which was related to the maintenance of the eubiosis of the gut microbiota [75]. Other studies show that the gastrointestinal system is continuously exposed to reactive oxygen species [76]; then, the antioxidant compounds like polyphenols may help maintain the redox balance in these organs [77].
FTIR spectra of the waste powders are presented in Fig. 4. All spectra exhibited the same shape and only variations in the absorption intensity were detected.
Samples showed two characteristic absorption peaks at 3350 and 1040 cm−1, which were attributed to the stretching vibration of the hydroxyl group mainly due to cellulose and hemicelluloses or phenols in which hydrogen atoms participate in hydrogen bonds [78,79,80]. These two peaks were more intense in grape stalk samples (GS and RSFGS), which could be related to the higher dietary fiber and polyphenol content (Table 4). The characteristic peaks at 1635 cm−1 corresponded to the aromatic benzene ring of lignin [81] or C = C vibrations of polyphenols [82] which were observed in all samples. This peak is more intense in the grape waste samples (Fig. 4b), which could be related to the higher content of insoluble fiber and polyphenols in these samples. The strong bands about 2900 cm−1 were representative of C-H vibrations from some methyl groups of polysaccharides and carbonyl groups of lignin [82, 83].
Figure 5 shows the contributions of the main phenolic groups (flavan-3-ols, flavonols, phenolic acids, and condensed tannins) to the phenolic profile of the waste powders. The grape pomace and grape stalk powders presented a richer phenolic profile. The flavan-3-ols were predominant in the three types of wastes, except for the RSFGM sample, which showed a greater proportion of condensed tannins. In the case of apple waste powders (AP and RSFAP), phenolic acids were not detected, but condensed tannins (procyanidins), flavan-3-ols (catechin), and flavonols (rutin and quercetin) were quantified. Catechin accounted for 59% of the total phenolic compounds in AP and 83% in RSFAP powders. In the grape stalk powders, the main quantified compounds were gallic acid (17–19%), catechin (43%), and rutin/quercetin (38–40%). Mohammadi et al. [84] also investigated the chemical profile of apple pomace powder generated from industrial apple juice production. Their findings revealed that flavanols and flavonols were abundant, comprising 53% of the total phenolic compounds.
Main phenolic groups (%) of powdered samples determined by HPLC–UV/DAD. Fruit waste powders without previous extraction: apple pomace (AP), grape marc (GM), and grape stalk (GS). The powders generated by the remaining solid fraction of the selected extraction process: apple pomace (RSFAP), grape marc (RSFGM), and grape stalk (RSFGS)
Techno-functional properties of fiber depend on plant source, degree of processing, and IDF/SDF ratio, as well as on powder particle size, porosity, and density. Regarding the hydration properties (Table 5), the apple waste powders (AP and RSFAP) absorbed greater amounts of water (> SC, > WHC, and > WRC) when compared to grape ones in accordance with the higher content of SDF (24.6–45%). The loss of water holding capacity of powders previously subjected to phenolic extraction (RSF) could be ascribed to alterations in dietary fiber content and composition, as well as to changes in waste structural features such as breakdown of the cell wall polysaccharide network but heat and solubilization of pectins during water immersion. The greater capacity to retain water of these powders may be more related to the IDF proportion increase (% IDF = 75–93%), since insoluble fiber forms a matrix in which water is entrapped. All the waste powders exhibited higher WRC values compared with those of other reported agricultural by-product powders, such as from grape pomace (2.26 g/g), lemon juice by-product (1.85 g/g), and orange juice by-product (1.65 g/g) reported by Santos et al. [36]. OHC is another important fiber property; dietary fibers with high OHC can be used to stabilize food emulsions and decrease fat digestion and absorption in the gastrointestinal tract [85]. RSF powders also showed a greater oil holding capacity, because of the higher proportion of IDF (75–93%) when compared with powders without extraction (55–70%). The RSFGM and RSFGS samples presented the highest OHC values, in accordance with those reported by Lopez-Marcos et al. [86] in air-dried grape pomace (OHC = 2.3 g oil/g). On the other hand, the EA of the six powders was comparable to that of commercial fibers [87] without appreciable differences between samples. According to these results, the low OHC values of the powders (< 1.9 g/g) suggest they would provide a non-fatty sensation in food, with great potential as ingredient in for instance fried products [88]. In general, all powders presented good hydration capacity; therefore, they could be used to increase the viscosity of those foods to which they are added [89]. In particular, the best hydration properties were observed for RSFAP and AP samples, which indicate that these powders can be used as functional food ingredients to reduce calories, to avoid syneresis, and to modify the viscosity and texture of the final product [86]. Manthei et al. [90] also obtained good hydration properties (WHC = 13.2 g H2O/g d.b.) in powders obtained from apple wastes by convective drying at 55 °C.
Figure 6 shows images of the different powders together with the chromatic parameters (L*, a*, and b*). All the powders presented similar brownish tones. Regarding the global color difference (ΔE*ab), no significant differences were observed between powders with and without extraction (3.15, 4.1, and 5.3, for GM, GS, and AP, respectively).
The Hausner ratio and Carr’s index are used for assessing the tendency of powders to flow and determine the interaction among the powder particles that affect the flow behavior [91]. The extraction process had no significant effect on powder flowability (Table 6). All the powders presented low cohesiveness (i.e., Hausner ratio values < 1.12) and excellent or good flowability (Carr’s index values between 6.44 and 12.3%). The apple waste powders (AP and RSFAP) presented the poorest flowability (> density, > angle of repose, > Carr index). These results were similar to those obtained by Kaur et al. [92] who observed good flowability on air-dried carrot pomace powder, having an angle of repose = 33.69 and a Hausner ratio of 1.46. In addition, Alam et al. [93] obtained air-dried banana flour of good quality with Carr’s index values ranging from 9.38 to 13.65 and good cohesiveness (Hausner ratio between 1.13 and 1.16).
In this work, the waste powders showed Tg values higher than 39 °C (Table 7), suggesting physical stability for storage at temperatures lower than ~ 30 °C. Ferrari et al. [94] obtained similar results in mango peel powders, with Tg values between 31.37 and 42.40 °C.
Additionally, the samples presented aw values lower than 0.3, indicating that the microorganism growth would be hindered, while chemical and physical changes would be limited [95]. Regarding hygroscopicity (Table 7), the studied powders showed values between 9.3 and 18 g/100 g d.b., which, according to Tontul et al. [95], can be considered not very hygroscopic materials. Overall, the physical properties of the waste powders show adequate properties for handling and storage.
4 Conclusions
The combination of a phenolic extraction step using environment-friendly techniques, followed by convective dehydration of the residual solid fraction, was successfully applied for the total valorization of apple pomace and grape wastes from regional industries. It was found that all the extracts obtained using the different extraction methods, especially those produced from the grape stalks, presented high polyphenolic content and antioxidant capacity, even overcoming the content of commercial fruit juices, suggesting their potential as functional ingredients in foods or for nutraceutical development. On the other hand, a great variety of powders rich in dietary fiber and antioxidant compounds could be produced, with physical and techno-functional properties adequate for different applications. Both the liquid extracts and the powders obtained from grape and apple wastes could be used as potentially functional ingredients.
Finally, considering that fruit waste represents approximately 50% of the raw materials processed by fruit industries, this entails economic losses due to the underutilization of raw materials and costs associated with waste collection and final disposal. In this regard, industries need to take advantage of the generated wastes or by-products to mitigate production costs. Even in some cases, large investments would not be necessary; for instance, juice producers could utilize existing juice extraction lines, and powdered products could be obtained in simple convective dryers with subsequent grinding stage. Valorizing wastes will enable their reintroduction into the production system, thereby reducing environmental impact.
The main limitations of this work are related to the high variability of the raw material, depending on the biological variability of the fruit available for industrial processing each season. However, the broad spectrum of technologies presented in this work has the potential to overcome the variability in raw wastes, thereby enabling the generation of multiple valuable ingredients.
The findings of this study provide insights into the development of liquid extracts and powdered ingredients derived from fruit wastes, varying in technological and functional quality.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Leong Y, Chang J (2022) Valorization of fruit wastes for circular bioeconomy: current advances, challenges, and opportunities. Biores Technol 359:127459. https://doi.org/10.1016/j.biortech.2022.127459
Nirmal N, Khanashyam A, Mundanat A, Shah K, Babu K, Thorakkattu P, Al-Asmari F, Pandiselvam R (2023) Valorization of fruit waste for bioactive compounds and their applications in the food industry. Foods 12:556. https://doi.org/10.3390/foods12030556
Tripathi A, Srivastava S, Kumar Pandey V, Singh R, Panesar P, Dar A, Rustagi S, Shams R, Pandiselvam R (2023) Substantial utilization of food wastes for existence of nanocomposite polymers in sustainable development: a review. Environ Dev Sustain. https://doi.org/10.1007/s10668-023-03756-2
Da Silva Martins L, Komesu A, Rocha de Oliveira J, Bichara C, Moreira D, Rai M (2022) Enzymatic extraction of polyphenols from wastes of Amazon fruits industry. In: Pintado M, Saraiva J, Da Cruz Alexandre E (eds) Technologies to recover polyphenols from agrofood by-products and wastes, 3rd edn. Academic Press, Cambridge, pp 225–246. https://doi.org/10.1016/C2020-0-02128-6
Portilla O, Saavedra Leos M, Solis V, Domínguez J (2021) Recent trends on the valorization of winemaking industry wastes. Curr Opin Green Sustain Chem 27:100415. https://doi.org/10.1016/j.cogsc.2020.100415
Banwo K, Olojede A, Adesulu-Dahunsi A, Verma D, Thakur M, Tripathy S, Singh S, Patel A, Gupta A, Aguilarj C, Utama G (2021) Functional importance of bioactive compounds of foods with potential health benefits: a review on recent trends. Food Biosci 43:101320. https://doi.org/10.1016/j.fbio.2021.101320
Peixoto C, Dias M, Alves M, Calhelha R, Barros L, Pinho S, Ferreira I (2018) Grape pomace as a source of phenolic compounds and diverse bioactive Properties. Food Chem 253:132–138. https://doi.org/10.1016/j.foodchem.2018.01.163
Rani J, Rautela A, Kumar S (2020) Biovalorization of winery industry waste to produce value-added products. In: Rathinam NK, Sani RK (Eds) Biovalorisation of wastes to renewable chemicals and biofuels. Elsevier, Netherlands, 4:63–85. https://doi.org/10.1016/B978-0-12-817951-2.00004-3
Ahmad B, Yadav V, Yadav A, Rahman M, Yuan W, Li Z, Wang X (2020) Integrated biorefinery approach to valorize winery waste: a review from waste to energy perspectives. Sci Total Env 719:137315. https://doi.org/10.1016/j.scitotenv.2020.137315
Atatoprak T, Amorim M, Ribeiro T, Pintado M, Madureira A (2022) Grape stalk valorization for fermentation purposes. Food Chem Mol Sci 4:100067. https://doi.org/10.1016/j.fochms.2021.100067
Rana S, Guptad S, Rana A, Bhushan S (2015) Functional properties, phenolic constituents and antioxidant potential of industrial apple pomace for utilization as active food ingredient. Food Sci Hum Wellness 4:180–187. https://doi.org/10.1016/j.fshw.2015.10.001
Kumar M, Barbhai M, Esatbeyoglu T, Sheri B, Dhumal S, Rais N, Al R, Masry EM, Chandran D, Pandiselvam R, Senapathy M, ADey A, Deshmukh S, Negm M, Vishvanathan M, Sathyaseelan S, Viswanathan S, Mohankumar P, Lorenzo J (2022) Apple (Malus domestica Borkh.) seed: a review on health promoting bioactivities and its application as functional food ingredient. Food Bioscie. 50:102155. https://doi.org/10.1016/j.fbio.2022.102155
Nirmal N, Khanashyam A, Mundanat A, Shah K, Babu K, Thorakkattu P, Al-Asmari F, Pandiselvam R (2022) Valorization of fruit waste for bioactive compounds and their applications in the food industry. Foods 12:556. https://doi.org/10.3390/foods12030556
Airouyuwa J, Mostafa H, Riaz A, Maqsood S (2022) Utilization of natural deep eutectic solvents and ultrasound-assisted extraction as green extraction technique for the recovery of bioactive compounds from date palm (Phoenix dactylifera L.) seeds: an investigation into optimization of process parameters. Ultrasonics Sonochem 91:106233. https://doi.org/10.1016/j.ultsonch.2022.106233
More P, Jambrak A, Arya S (2022) Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci Technol 128:296–315. https://doi.org/10.1016/j.tifs.2022.08.016
Jha A, Sit N (2023) Effect of ultrasound, microwave, and enzymatically pre-treated Terminalia chebula pulp on extraction of bioactive compounds using supercritical CO2. Sustain Chem Pharm 33:101098. https://doi.org/10.1016/j.scp.2023.101098
Teixeira-Guedes C, Gomes-Diasa J, Cunha S, Pintado M, Pereira R, Teixeira J, Rocha C (2023) Enzymatic approach for the extraction of bioactive fractions from red, green and brown seaweeds. Food Bioprod Process 138:25–39. https://doi.org/10.1016/j.fbp.2022.12.005
Nandhu Lal A, Prince M, Kothakota A, Pandiselvam R, Thirumdas R, Kumar Mahanti N, Sreeja R (2021) Pulsed electric field combined with microwave-assisted extraction of pectin polysaccharide from jackfruit waste. Innovative Food Sci Em Technol 74:102844. https://doi.org/10.1016/j.ifset.2021.102844
Gavahian M, Mathad G, Pandiselvam R, Lin J, Da-Wen Sun D (2021) Emerging technologies to obtain pectin from food processing by-products: a strategy for enhancing resource efficiency. Trends Food Sci Technol 115:42–54. https://doi.org/10.1016/j.tifs.2021.06.018
Sepúlveda L, Romaní A, Aguilar C, Teixeira J (2018) Valorization of pineapple waste for the extraction of bioactive compounds and glycosides using autohydrolysis. Innov Food Sci Emerg Technol 47:38–45. https://doi.org/10.1016/j.ifset.2018.01.012
Kabir F, Tow WW, Hamauzu Y, Katayama S, Tanaka S, Nakamura S (2015) Antioxidant and cytoprotective activities of extracts prepared from fruit and vegetable wastes and by products. Food Chem 167:358–362. https://doi.org/10.1016/j.foodchem.2014.06.099
Naeem U, Umair M, Saeed F, Imran A (2022) Extraction and characterization of polyphenols from fruits and vegetable waste through green extraction technologies with special reference to antioxidant profile. J Food Process Preserv 00:e16807. https://doi.org/10.1111/jfpp.16807
Bakić M, Pedisić S, Zorić Z, Dragović-Uzela V, Grassino A (2019) Effect of microwave-assisted extraction on polyphenols recovery from tomato peel waste. Acta Chim Slov 66:367–377. https://doi.org/10.17344/acsi.2018.4866
Garrido Makinistian F, Sette P, Gallo L, Bucalá V, Salvatori D (2019) Optimized aqueous extracts of maqui (Aristotelia chilensis) suitable for powder production. J Food Sci Technol 56(7):3553–3560. https://doi.org/10.1007/s13197-019-03840-4
Gomez Mattson M, Sozzi A, Corfield R et al (2022) Colorant and antioxidant properties of freeze-dried extracts from wild berries: use of ultrasound-assisted extraction method and drivers of liking of colored yogurts. J Food Sci Technol 59:944–955. https://doi.org/10.1007/s13197-021-05096-3
Gagneten M, Leiva G, Salvatori D, Schebor C, Olaiz N (2019) Optimization of pulsed electric field treatment for the extraction of bioactive compounds from blackcurrant. Food Bioprocess Technol Int J 12(7):1102–1109. https://doi.org/10.1007/s11947-019-02283-1
Toscano Martinez H, Gagneten M, Diaz-Calderón P, Enrione J, Salvatori D, Schebor C, Leiva G (2020) Natural food colorant from blackcurrant spray-dried powder obtained by enzymatic treatment: characterization and acceptability. J Food Process Preserv 45(1):e15011. https://doi.org/10.1111/jfpp.15011
Perez-Pirotto C, Moraga G, Quiles A, Hernando I, Cozzano S, Arcia P (2022) Techno functional characterization of green-extracted soluble fibre from orange by-product. LWT 166:113765. https://doi.org/10.1016/j.lwt.2022.113765
Li W, Yang R, Ying D, Yu J, Sanguansri L, Augustin M (2020) Analysis of polyphenols in apple pomace: a comparative study of different extraction and hydrolysis procedures. Ind Crops Products 147:112250. https://doi.org/10.1016/j.indcrop.2020.112250
Beutinger B, Speroni C, Bolson M, Dal Pont M, Rheinheimer D, Picolli L, Garcia P (2020) Effects of micronization on dietary fiber composition, physicochemical properties, phenolic compounds, and antioxidant capacity of grape pomace and its dietary fiber concentrate. LWT 117:108652. https://doi.org/10.1016/j.lwt.2019.108652
Moro B, Beutinger B, Da Silva L (2021) Green extraction methods and microencapsulation technologies of phenolic compounds from grape pomace: a review. Food Bioprocess Technol 14(2):1407–1431. https://doi.org/10.1007/s11947-021-02665-4
Krstonosic M, Sazdanic D, Cirin D, Maravic N, Mikulic M, Cvejic J, Krstonosic V (2023) Aqueous solutions of non-ionic surfactant mixtures as mediums for green extraction of polyphenols from red grape pomace. Sust Chem Pharm 33:101069. https://doi.org/10.1016/j.scp.2023.101069
Tariq A, Sahar A, Usman M, Sameen A, Azhar M, Tahir R, Younas R, Khan M (2023) Extraction of dietary fiber and polyphenols from mango peel and its therapeutic potential to improve gut health. Food Biosci 53:102669. https://doi.org/10.1016/j.fbio.2023.102669
Garcia-Amezquita L, Tejada-Ortigoza V, Campanella O, Welti-Chanes J (2018) Influence of drying method on the composition, physicochemical properties, and prebiotic potential of dietary fibre concentrates from fruit peels. J Food Quality 2018:9105237. https://doi.org/10.1155/2018/9105237
Dios-Perez I, Nieto C, Vega M, Del Valle E (2023) Polyphenol extraction from olive leaves to show chemical engineering students the importance of revaluating residues while improving their hands-on experience. Education Chem Eng 43:10–22. https://doi.org/10.1016/j.ece.2023.01.002
Santos D, da Silva J, Pintado M (2022) Fruit and vegetable by-products’ flours as ingredients: a review on production process, health benefits and technological functionalities. LWT 154:112707. https://doi.org/10.1016/j.lwt.2021.112707
Association Official Analitycal Chemistry (2000) Official Methods of Analysis of AOAC International. AOAC International, Maryland
Gomez Mattson M, Corfield R, Bajda L, Perez O, Schebor C, Salvatori D (2021) Potential bioactive ingredient from elderberry fruit: process optimization for a maximum phenolic recovery, physicochemical characterization, and bioaccesibility. J Berry Res 11:51–68. https://doi.org/10.3233/JBR-200629
Sette P, Franceschinis L, Schebor C, Salvatori D (2017) Fruit snacks from raspberries: influence of drying parameters on colour degradation and bioactive potential. Int J Food Sci Technol 52:313–328. https://doi.org/10.1111/ijfs.13283
Franceschinis L, Salvatori D, Sosa N, Schebor C (2014) Physical and functional properties of blackberry freeze- and spray-dried powders. Dry Technol 32:197–207. https://doi.org/10.1080/07373937.2013.814664
Bunzel M, Ralph J, Marita J, Steinhart H (2000) Identification of 4-O-5’-coupled diferulic acid from insoluble cereal fiber. J Agric Food Chemistry 48:3166–3169. https://doi.org/10.1021/jf000125n
López-Vargas J, Fernandez-Lopez J, Perez-Alvarez J, Viuda-Martos M (2013) Chemical, physico-chemical, technological, antibacterial and antioxidant properties of dietary fiber powder obtained from yellow passion fruit (Passiflora edulis var. flavicarpa) co-products. Food Res Int 51:756–763. https://doi.org/10.1016/j.foodres.2013.01.055
Sette P, Garrido Makinistian F, Maturano C, Salvatori D (2022) Particulate systems from maqui (Aristotelia chilensis) wastes to be used as nutraceutics or high value-added ingredients. Dry Tech 40(13):2669–2684. https://doi.org/10.1080/07373937.2021.1953521
GarridoMakinistian F, Gallo L, Sette P, Salvatori D, Bucala V (2020) Nutraceutical tablets from maqui berry (Aristotelia chilensis) spray-dried powders with high antioxidant levels. Dry Technol 38:1231–1242. https://doi.org/10.1080/07373937.2019.1629589
The United States Pharmacopeial Convention (2007) United States Pharmacopeia and National Formulary, USP 30-NF 25. Mack Printing, Rockville
Franceschinis L, Sette P, Salvatori D, Schebor C (2018) Valorization of postharvest sweet cherry discard for the development of dehydrated fruit ingredients: compositional, physical, and mechanical properties. J Sci Food Agri 98(14):5450–5458. https://doi.org/10.1002/jsfa.9089
Beres C, Simas-Tosin F, Cabezudo I, Freitas S, Iacomini M, Mellinger-Silva C, Cabral L (2016) Antioxidant dietary fibre recovery from Brazilian Pinot noir grape pomace. Food Chem 201:145–152. https://doi.org/10.1016/j.foodchem.2016.01.039
Gal T (2020) Cascade valorization of apple pomace into polyphenols and pectins by green extraction processes. KTH Royal Institute of Technology School of Engineering Sciences in Chemistry, Biotechnology and Health, Stockholm
Dhillon G, Kaur S, Brar S (2013) Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: a review. Renew Sustain Energy Rev 27:789–805. https://doi.org/10.1016/j.rser.2013.06.046
Alonso A, Guillén D, Barroso C, Puertas B, García A (2002) Determination of antioxidant activity of wine by-products and it correlation with polyphenolic content. J Agric Food Chem 50:5832–5836. https://doi.org/10.1021/jf025683b
Llobera A, Cañellas J (2007) Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): pomace and stem. Food Chem 101:659–666. https://doi.org/10.1016/j.foodchem.2006.02.025
Diñeiro GY, Suárez VB, Lobo A (2009) Phenolic and antioxidant composition of by-products from the cider industry: apple pomace. Food Chem 117:731–738. https://doi.org/10.1016/j.foodchem.2009.04.049
Macheix J, Fleuriet A, Billot J (1990) Fruit phenolics. CRC Press, Boca Raton
Liu D, Lopez-Sanchez P, Martinez-Sanz M, Gilbert E, Gidley M (2019) Adsorption isotherm studies on the interaction between polyphenols and apple cell walls: effects of variety, heating and drying. Food Chem 282:58–66
Budak N, Ozcelik F, Güzel-Seydim Z (2015) Antioxidant activity and phenolic content of apple cider. Turk J Agric-Food Sci Technol 3(6):356–360. https://doi.org/10.24925/turjaf.v3i6.356-360.265
Amrani-Joutei K, Glories Y, Mercier M (1994) Localisation des tanins dans la pellicule de baie de raisin. Vitis 33:133–138. https://doi.org/10.5073/vitis.1994.33.133-138
Boulet J, Abi-Habib E, Carrillo S et al (2023) Focus on the relationships between the cell wall composition in the extraction of anthocyanins and tannins from grape berries. Food Chem 406:135023. https://doi.org/10.1016/j.foodchem.2022.135023
Fuchs C, Bakuradze T, Steinke R, Grewal R, Eckert G, Richling E (2020) Polyphenolic composition of extracts from winery by-products and effects on cellular cytotoxicity and mitochondrial functions in HepG2 cells. J Functional Foods 70:103988. https://doi.org/10.1016/j.jff.2020.103988
Pollini L, Blasi F, Ianni F, Grispoldi L, Moretti S, Di Veroli A, Cossignani L, Cenci-Goga B (2022) Ultrasound-assisted extraction and characterization of polyphenols from apple pomace, functional ingredients for beef burger fortification. Molecules 27:1933. https://doi.org/10.3390/molecules27061933
Ntourtoglou G, Drosou F, Chatzimitakos T et al (2022) Combination of pulsed electric field and ultrasound in the extraction of polyphenols and volatile compounds from grape stems. Appl Sci 12:6219. https://doi.org/10.3390/app12126219
Da Silva HI, Toaldo I, Burin V, Bordignon-Luiz M (2018) Extraction optimization for polyphenolic profiling and bioactive enrichment of extractives of non-pomace residue from grape processing. Ind Crops Prod 112:593–601. https://doi.org/10.1016/j.indcrop.2017.12.058
Tzima K, Brunton N, Lyng J, Frontuto D, Rai D (2021) The effect of pulsed electric field as a pre-treatment step in ultrasound assisted extraction of phenolic compounds from fresh rosemary and thyme by-products. Innovative Food Sci Emerging Technol 69:102644. https://doi.org/10.1016/j.ifset.2021.102644
Alzamora SM, Castro MA, Nieto AB, Vidales SL, Salvatori D (2000) The role of tissue microstructure in the textural characteristics of minimally processed fruits. In: Alzamora SM, Tapia MS, López-Malo A (eds) Minimally processed fruits and vegetables, 2nd edn. Aspen Publishers Inc., Maryland
Marsiglia W, Oliveira L, Almeida R, Santos N, da Silva NJ, Santiago A, Amorim de Melo B, Honorato da Silva F (2023) Thermal stability of total phenolic compounds and antioxidant activities of jaboticaba peel: effect of solvents and extraction methods. J Indian Chem Soc 100:100995. https://doi.org/10.1016/j.jics.2023.100995
Teixeira N, Mateus N, de Freitas V, Oliveira J (2018) Wine industry by-product: full polyphenolic characterization of grape stalks. Food Chem 268:110–117. https://doi.org/10.1016/j.foodchem.2018.06.070
Tylewicz U, Nowacka M, Martín-García B, Wiktor A, Gómez Caravaca A (2018) Target sources of polyphenols in different food products and their processing by-products. In: Galanakis CM (ed) Polyphenols: properties, recovery, and applications, 3rd edn. Woodhead Publishing, Cambridge
Da Silva L, Vigano J, de Souza ML et al (2021) Recent advances and trends in extraction techniques to recover polyphenols compounds from apple by-products. Food Chem: X 12:100133. https://doi.org/10.1016/j.fochx.2021.100133
Perra M, Cuena-Lombraña A, Bacchetta G et al (2021) Combining different approaches for grape pomace valorization: polyphenols extraction and composting of the exhausted biomass. Sustainability 14:10690. https://doi.org/10.3390/su141710690
Gouw VP, Jung J, Zhao Y (2017) Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT 80:136–144. https://doi.org/10.1016/j.lwt.2017.02.015
Sette P, Fernandez A, Soria J, Rodriguez R, Salvatori D, Mazza G (2020) Integral valorization of fruit waste from wine and cider industries. J Clean Prod 242:118486. https://doi.org/10.1016/j.jclepro.2019.118486
Alba K, Campbell GM, Kontogiorgos V (2019) Dietary fibre from berry-processing waste and its impact on bread structure: a review. J Sci Food Agr 99:4189–4199. https://doi.org/10.1002/jsfa.9633
Salari S, Ferreira J, Lima A, Sousa I (2024) Effects of particle size on physicochemical and nutritional properties and antioxidant activity of apple and carrot pomaces. Foods 13:710. https://doi.org/10.3390/foods13050710
Li L, Yan S, Liu S, Wang P, Li W, Yi Y, Qin S (2023) In-depth insight into correlations between gut microbiota and dietary fiber elucidates a dietary causal relationship with host health. Food Res Int 172:113133. https://doi.org/10.1016/j.foodres.2023.113133
Coker J, Moyne O, Rodionov D, Zengler K (2021) Carbohydrates great and small, from dietary fiber to sialic acids: how glycans influence the gut microbiome and affect human health. Gut Microbes 13(1):1–18. https://doi.org/10.1080/19490976.2020.1869502
Lippolis T, Cofano M, Caponio GR, De Nunzio V, Notarnicola M (2023) Bioaccessibility and bioavailability of diet polyphenols and their modulation of gut microbiota. Int J Mol Sci 24(4):3813. https://doi.org/10.3390/ijms24043813
Halliwell B, Rafter J, Jenner A (2005) Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? Am J Clin Nutr 81(1):268S-276S. https://doi.org/10.1093/ajcn/81.1.268S
Leopoldini M, Chiodo SG, Russo N, Toscano M (2011) Detailed investigation of the OH radical quenching by natural antioxidant caffeic acid studied by quantum mechanical models. J Chem Theory Comput 7(12):4218–4233. https://doi.org/10.1021/ct200572p
Alzate-Arbelaez A, Dorta E, Lopez-Alarcon C, Cortes F, Rojano B (2019) Immobilization of Andean berry (Vaccinium meridionale) polyphenols on nanocellulose isolated from banana residues: a natural food additive with antioxidant properties. Food Chem 294:503–517. https://doi.org/10.1016/j.foodchem.2019.05.085
Grassino A, Ostojic J, Miletic V et al (2020) Application of high hydrostatic pressure and ultrasound-assisted extractions as a novel approach for pectin and polyphenols recovery from tomato peel waste. Innov Food Sci Emerg Technol 64:102424. https://doi.org/10.1016/j.ifset.2020.102424
Umdale S, Ahire M, Aiwale V, Jadhav A, Mundada P (2020) Phytochemical investigation and antioxidant efficacy of wild, underutilized berries of economically important Indian sandalwood (Santalum album L.). Biocatalysis Agric Biotechnol 27:101705. https://doi.org/10.1016/j.bcab.2020.101705
Rouhou M, Abdelmoumen S, Thomas S, Attia H, Ghorbel D (2018) Use of green chemistry methods in the extraction of dietary fibers from cactus rackets (Opuntia ficus indica): structural and microstructural studies. Int J Biol Macromol 116:901–910. https://doi.org/10.1016/j.ijbiomac.2018.05.090
Nogales-Bueno J, Baca-Bocanegra B, Rooney A, Hernandez-Hierro J, Byrne H, Heredia F (2017) Study of phenolic extractability in grape seeds by means of ATR-FTIR and Raman Spectroscopy. Food Chem 232:602–609. https://doi.org/10.1016/j.foodchem.2017.04.049
Pathania S, Kaur N (2022) Utilization of fruits and vegetable by-products for isolation of dietary fibres and its potential application as functional ingredients. Bioact Carbohydrates Diet Fibre 27:100295. https://doi.org/10.1016/j.bcdf.2021.100295
Mohammadi N, Guo Y, Wang K, Granato D (2024) Macroporous resin purification of phenolics from Irish apple pomace: chemical characterization, and cellular antioxidant and anti-inflammatory activities. Food Chem 437:137815. https://doi.org/10.1016/j.foodchem.2023.137815
López-Marcos M, Bailina C, Viuda-Martos M, Pérez-Alvarez J, Fernández-López J (2015) Properties of dietary fibers from agroindustrial coproducts as source for fiber-enriched foods. Food Bioprocess Technol 8:2400–2408. https://doi.org/10.1007/s11947-015-1591-z
Martinez-Las Heras R, Landines E, Heredia A, Castello M, Andres A (2017) Influence of drying process and particle size of persimmon fibre on its physicochemical, antioxidant, hydration and emulsifying properties. J Food Sci Technol 54:2902–2912. https://doi.org/10.1007/s13197-017-2728-z
Vázquez-Ovando A, Rosado-Rubio G, Chel-Guerrero L, Betancur-Ancona D (2009) Physicochemical properties of a fibrous fraction from chia (Salvia hispanica L.). LWT 42:168–173. https://doi.org/10.1016/j.lwt.2008.05.012
Moraes Crizel T, Jablonski A, de Oliveira RA, Rech R, Hickmann Flôres S (2013) Dietary fiber from orange byproducts as a potential fat replacer. LWT 53:9–14. https://doi.org/10.1016/j.lwt.2013.02.002
Khoozani A, Birch J, Bekhit A (2020) Textural properties and characteristics of whole green banana flour produced by air-oven and freeze-drying processing. J Food Meas Charact 14:1533–1542. https://doi.org/10.1007/s11694-020-00402-7
Manthei A, Elez-Martínez P, Soliva-Fortuny R, Murciano-Martínez P (2024) Ultrasonication and enzymatic treatment of apple and orange bagasses: molecular characterization of released oligosaccharides and modification of techno-functional and health-related properties. LWT 194:115816. https://doi.org/10.1016/j.lwt.2024.115816
Kaur N, Aggarwal P, Kaur S (2023) Phytochemical profile and techno-functional properties of black carrot (Daucus carota) pomace powder for the formulation of nutraceutical tablets: an impact of drying methods. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-023-04511-3
Alam M, Biswas M, Hasan M, Hossain M, Zahid M, Al-Reza S, Islam T (2023) Quality attributes of the developed banana flour: effects of drying methods. Heliyon 9:e18312. https://doi.org/10.1016/j.heliyon.2023.e18312
Ferrari C, Morgano M, Germer S (2021) Evaluation of water sorption isotherm, glass transistion temperature, vitamin C and color stability of mango peel powder during storage. SN Appl Sci 3:210. https://doi.org/10.1007/s42452-021-04251-x
Daza LD, Fujita A, Favaro-Trindade CS, Rodrigues-Ract J, Granato D, Genovese MI (2016) Effect of spray drying conditions on the physical properties of cagaita (Eugenia dysenterica DC.) Fruit Extracts. Food Bioprod Process 97:20–29. https://doi.org/10.1016/j.fbp.2015.10.001
Tontul I, Topuz A (2017) Spray-drying of fruit and vegetable juices: effect of drying conditions on the product yield and physical properties. Trends Food Sci Technol 63:91–102. https://doi.org/10.1016/j.tifs.2017.03.009
Acknowledgements
The authors thank L. Bajda, M. Amaro, and R. C. Maturano (PROBIEN, CONICET-UNCo) for their technical assistance in the HPLC analysis and waste physicochemical characterization.
Funding
This work was supported by the National University of Comahue (PIN 04/L007), and the National Agency for the Promotion of Scientific and Technical Research of Argentina (PICT-2019–02658).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by PS and MGM. The first draft of the manuscript was written by PS and DS, and all authors commented on previous versions of the manuscript. Funding acquisition and reviewing of the manuscript were performed by CS and DS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The authors confirm that there were no ethical requirements in preparing this manuscript.
Consent to participate
All authors consent to participating in this work.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sette, P., Mattson, M.G., Schebor, C. et al. Strategies for bioactive compound recovery from grape and apple wastes: traditional and emerging technologies to reach zero waste discharge. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05630-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05630-1








 ) (AP and RSF ap). b Grape marc powders (
) (AP and RSF ap). b Grape marc powders (
 ) (GM and RSF gm) and grape stalk powders (
) (GM and RSF gm) and grape stalk powders (
 ) (GS and RSF gs)
) (GS and RSF gs)
