Abstract
In this study, the use of fungal (Panaeolus papilionaceus) biomass as a biosorbent was investigated to effectively remove Pb2+ ions from aquatic medium. The removal of Pb2+ ions using a fungal biosorbent was examined in a batch system in terms of initial solution pH, temperature, time, and initial Pb2+ concentration. Optimal operating conditions for biosorption of Pb2+ ions; pH: 4.5, T: 25 °C, and t: 24 h. The max biosorption capacity for Pb2+ ions was found to be 31.2 mg g−1 from the Langmuir model. Thermodynamic studies showed that Pb2+ ions biosorption into fungal biomass was possible, spontaneous, and endothermic. Additionally, the antimicrobial activity and antibiofilm activity of the extract of fungus were also investigated. It was determined that the fungal extract did not have antimicrobial properties. On the other hand, the extract has been shown to have the potential to prevent biofilm formation. 1 mg of the extract prevented the biofilm formation of Staphylococcus aureus by 87.85%. It has been observed that the biosorption mechanism of Pb2+ ions into fungal biomass includes the steps of surface biosorption, film diffusion, and intra-particle diffusion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Today, the presence of heavy metals uncontrollably discharged into water bodies as a result of anthropogenic activities and rapid industrialization has become a global threat [1]. Heavy metals in dissolved or particle forms such as lead (Pb), mercury (Hg), copper (Cu), cadmium (Cd), zinc (Zn), and nickel (Ni) harm ecological systems because they are non-biodegradable and remain stable [2, 3]. Pb is one of the most common toxic heavy metals found in industrial wastewater, owing to its widespread use in various industrial processes such as mining, automotive manufacturing, battery production, and metal plating [4]. However, the World Health Organization (WHO) has reported that the maximum allowable concentration of Pb2+ ions in wastewater and drinking water should be 0.05 mg L−1 and 0.01 mg L−1, respectively [5]. Exceeding these limits in water consumption can lead to various health issues in living beings, including kidney, brain, and liver damage, hypertension, anemia, as well as neurological and hematological disorders [6, 7]. Therefore, effectively removing Pb2+ ions pollution from water bodies is critical for human health [8, 9]. Adsorption is a low-cost, simple, and efficient method, offering higher selectivity than alternatives such as coagulation, membrane removal, ion exchange, and chemical precipitation [10]. However, adsorption efficiency may vary depending on factors such as the surface area of the adsorbent, pore volume and size, and functional groups present on the surface [11, 12].
Biomass (biosorbents) has attracted the attention of researchers as an environmentally friendly, biodegradable, and cost-effective option for removing low concentrations of Pb2+ ions from wastewater [13]. These adsorbents are sustainable and do not cause secondary pollution, making them a promising solution for this issue [14]. Biosorbents such as bacteria [15], algae [16], fungi [17], plant residues [18], activated sludge [19], biopolymers [20], and yeast [21] have been investigated in Pb2+ ions removal studies.
Fungi are organisms with robust structures found worldwide, possessing physical properties that render them suitable for use as biosorbents [22]. They are increasingly employed in removal studies due to the diverse array of functional compounds present in their cell walls, as well as their low cost, ease of growth, simple fermentation techniques, and industrial abundance [23]. Fungal cell walls contain polysaccharides, lipids, and proteins with metal-binding functional groups, such as hydroxyl, carbonyl, carboxyl, amine, and amide [24]. These compounds enable fungi to efficiently remove heavy metals. Macrofungi such as Phanerochaete chrysosporium [25], Lentinula edodes, Agaricus bisporus [26], and Ganoderma lucidum [27] have been investigated for their ability to remove Pb2+ ions via biosorption. This study explores the potential of using inactivated and dried P. papilionaceus as a sustainable, inexpensive, readily available, and environmentally friendly biosorbent for removing Pb2+ ions from aqueous media. P. papilionaceus is a small, brown-blackish colored mushroom with conical or bell-shaped caps from the Bolbitiaceae family, inedible, non-poisonous, with remarkable health benefits and a common species [28].
In the literature review, no study was found investigating the biosorption capacity of P. papilionaceus. Therefore, the current study is significant as it represents the first exploration into this subject. The surface morphology and chemical structure of P. papilionaceus before and after biosorption were examined using FTIR, SEM, and EDX characterization techniques. Studies were conducted under various parameters including solution pH, contact time, amount of biosorbent, temperature, and initial Pb2+ ions concentration to assess their effects on the biosorption process. In addition, the determination of the antimicrobial and antibiofilm properties of P. papilionaceus was also investigated in the study.
2 Material and method
2.1 Chemicals and instrumentation
Pb(NO)3, KNO3, HCl, HNO3, NaOH, ethyl alcohol, and all other chemicals were of analytical purity (Sigma Aldrich).
The functional groups on the fungal biosorbent before/after Pb2+ ions biosorption were determined using the FT-IR (ATR, Bruker Model: Tensor II) technique. The changes in surface morphology of the fungal biosorbent before/after Pb2+ ions biosorption, along with the identification of different elements, were analyzed by SEM–EDX (TESCAN MIRA3 XMU). The Pb2+ ion concentrations were determined using the PAR method [29] with a UV–vis spectrophotometer (UV-DR-6000; Shimadzu, China) at λ = 519 nm. Fatty acid methyl esters in the fungi sample were analyzed by gas chromatography using flame ionization detection (GC-FID) equipment (Shimadzu, QP2010) and DB FastFame capillary column [30].
2.2 Preparation of biosorbent
Fungal biosorbent was collected from Sivas, Turkey. The sample was photographed in the field and then transported to the laboratory by being carefully wrapped in individual paper parcels to prevent damage. The samples were diagnosed based on field data and microscopic features observed in laboratory examinations. Then, the biosorbent was washed several times with deionized water and then ıt was dried at 25 °C. It was then stored in a polystyrene container to be used in biosorption studies.
2.3 Biosorption procedure
Pb2+ ions biosorption was investigated using the batch biosorption method. The main factors examined are pH, contact time, adsorbent amount, temperature, and initial Pb2+ concentration. In each experimental set, a fixed dosage of 50 mg of biosorbent was added to 10 mL solutions consisting of 1000 mg L−1 Pb2+ with stirring for 24 h at room temperature. Then, the supernatant concentration and maximum absorbance of 519 nm for Pb2+ ions were measured. Pb2+ ion concentration was determined by the absorbance measurement. The batch adsorption conditions are given in Table 1. Q (mg g−1) and Biosorption% were calculated using (1) and (2) [31];
2.4 Preparation of the fungi extract for biological activity
The fungi extract, intended for use in antibacterial and antibiofilm activity studies, was prepared using the Soxhlet extraction method. Fungi sample was dried and pulverized into a fine powder by grinding them in a mortar. The extraction process was conducted for 12 h in 400 mL methanol. Following the extraction, the methanol was removed at 40 °C using an evaporator, resulting in the isolation of a dry, pure substance.
For the preparation of the main stock extract solution, 0.15 g of extract was dissolved in 3 mL of methanol. After sterilization with a 0.22 µm diameter syringe filter, the main stock solution was immediately used for bioactivity analysis. The rest of the pure substance was stored at + 4 °C.
2.5 Assessment of antibacterial activity
The antibacterial activity of fungi extract was assessed using the micro-broth dilution method, following the guidelines developed by the Clinical and Laboratory Standards Institute (CLSI) (Clinical and Laboratory Standards Institute, 2019). The study used a total of 6 different indicator bacteria, comprising 3 g-positive and 3 g-negative species. Indicator microorganisms include Listeria monocytogenes ATCC 7644, Bacillus subtilis (ATCC 6633) and Staphylococcus aureus (ATCC 43300), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 10031) and Pseudomonas aeruginosa (ATCC 27853). Ciprofloxacin was used as a control drugs. The sample and inoculum were prepared as follows.
For inoculum preparation, the indicator bacteria were cultured in Mueller Hinton Broth (MHB) at 37 °C for an overnight period. Subsequently, the density was adjusted to McFarland no 0.5 standard, and an additional dilution of 100 fold was performed in MHB to obtain an inoculum at a concentration of 106 colony-forming units per milliliter (CFU/mL). For sample preparation, the fungi extract was diluted in MHB within a concentration range of 2 mg/mL to 0.0312 mg/mL.
To analyze, 100 µl of the prepared dilutions of fungi sample and 100 µl inoculum were transferred to sterile ELISA plates. The plates were incubated at 37 °C for 24 h. Following the incubation period, the absorbance values of the plates were read at 600 nm by BMG LABTECH’s ultra-fast UV/vis spectrophotometer (SPECTROstar Nano, Germany). Thus, in the final analysis, antibacterial activity was evaluated in the concentration range of 1 mg to 0.0156 mg. The analysis was repeated twice. The well in which the minimum inhibitory concentration (MIC) was seen was identified, and the corresponding MIC value was computed.
To ascertain the absence of any impact of the solvent itself on bacterial growth, a control experiment was conducted. This control involved the use of inoculated broth supplemented solely with methanol, at the same dilutions employed in the experimental procedures.
2.6 Assessment of antibiofilm activity
The assessment of antibiofilm activity was conducted using a modified version of the Christensen method, as originally suggested by Christensen et al. [32]. In parallel with the antibacterial activity study, a total of 6 bacteria species (identical to the aforementioned in the antibacterial activity study) were used as indicator bacteria.
For inoculum preparation, the indicator bacteria were cultured in Tryptic Soy Broth (TSB) supplemented with 2% glucose at 37 °C for an overnight period and, the density was adjusted to McFarland no 0.5 standard to obtain an inoculum at a concentration of 108 CFU/mL. For sample preparation, the fungi extract was diluted in TSB supplemented with 2% glucose within a concentration range of 2 mg/mL to 0.0312 mg/mL.
To biofilm formation, 100 µl of the prepared dilutions of fungi sample and 100 µl inoculum were transferred to sterile ELISA plates. Then, the plates were incubated at 37 °C for 48 h. Thus, in the final analysis, antibiofilm activity was evaluated in the concentration range of 1 mg to 0.0156 mg. To assess the biofilm inhibition, after the incubation period, the medium present in the wells was discarded and, the plates were washed by using phosphate buffered saline solution. Following the washing step, plates were kept at 25 °C for 2 h. Then 1% crystal violet solution (200 µl) was added the each well, after waiting 15 min, and then washed by using phosphate-buffered saline solution. Finally, 200 µL absolute ethanol was added to the wells to dissolve the crystal violet and the absorbance values of the plates were read at 600 nm by BMG LABTECH’s ultra-fast UV/vis spectrophotometer (SPECTROstar Nano, Germany). Results were calculated as % biofilm inhibition concentration. In the analyses, Indicator bacterial suspensions (200 µl) were used as negative control and TSB containing 2% glucose was used as positive control.
3 Results and discussion
3.1 Effect of pH on adsorption and pHpzc for fungal biosorbent
One of the vital parameters affecting biosorption efficiency in the biosorption process is the initial pH value of the medium. The solution for fungal biosorbent Pb2+ ion biosorption was investigated in the pH range of 1.0–5.0 and the results obtained are shown in Fig. 1. When Fig. 1 is examined, it was seen that there was an increase in biosorption efficiency from 26 to 56% as the pH value increased from pH:1.0 to pH:4.0. After pH: 4.0, biosorption efficiency decreased relatively.
At low pH values, biosorption efficiency decreases due to partial protonation of surface functional groups of the fungal biosorbent and strong competition between H+ ions and Pb2+ ions. Since there is competition between H+ ions and Pb2+ ions for binding sites at low pH values, biosorption efficiency is less at low pH values. With increasing pH values, the surface functional groups became deprotonated, causing strong electrostatic interactions with Pb2+ ions, and biosorption increased. It was observed that Pb2+ ion biosorption reached its maximum at pH: 4.0. After pH: 4.0, biosorption was observed to decrease relatively.
At high pH values, metal hydroxides of Pb2+ ions begin to precipitate. At pH > 5.5, Pb2+ ions undergo a series of hydrolysis reactions and as a result, hydroxide species are formed [33]. In this case, the repulsion forces between the functional groups on the adsorbent surface and the Pb2+ ions in the solution decrease, and the number of Pb2+ ions removed from the solution decreases. For this reason, Pb2+ ions were not studied since they tend to precipitate at pH > 5.5.
The zero charge point (pHpzc) represents the pH value at which the biosorbent surface is electrically neutral. The surface charge solid addition technique of fungal biosorbent was found by adding the same amount of solid to a series of solutions with the same ionic strength at different pH values [34]. The pHpzc value for the fungal biosorbent was found to be 4.49 (Fig. 2).
3.2 Effect of biosorbent dose
The effect of fungal biomass amount on Pb2+ ion biosorption was (Fig. 3). Figure 3 shows that the adsorption rate increases from 28 to 85% with the increase of the fungal dosage from 1 to 20 g L−1, while the quantity adsorbed decreases from 75 to 40 mg g−1. The amount of Pb2+ removed increases with the biosorbent mass because more active sites can be accessed to take part in the biosorption process [35]. Fungal cell walls contain various functional groups such as carboxyl, hydroxyl, amino, sulfhydryl, and phosphate [36]. These functional groups are also of interest for Pb2+ ions. This reveals the excellent biosorption properties of fungi.
3.3 Biosorption isotherms
Biosorption equilibrium isotherms, whether graphical or mathematical, play a crucial role in understanding and describing biosorption processes. They provide valuable information about how a biosorption system reaches equilibrium and the characteristics of the biosorbent involved in the process. These isotherms are used to elucidate the fundamental nature of biosorption, the mechanisms behind it, the optimum biosorption capacity, and the affinity of the biosorbent for the target substances in a solution [37].
Langmuir [38], Freundlich [39], and D-R [40] isotherm models were used to explain the concentration effect on biosorption. Nonlinear biosorption isotherms for Pb2+ ions into the fungal biosorbent are presented in Fig. 4, and the equations and determine parameters of these isotherms are presented in Table 2. The optimum biosorption capacity (QL) determined for the fungal biosorbent from the Langmuir biosorption isotherm model was calculated as 31.2 mg g−1. The KL was determined as 0.745 L mg−1. When the Freundlich and Langmuir isotherm models are compared, it is understood that the R2 value of the Freundlich model is higher and it is the model that describes the biosorption process better. This shows that biosorption follows multilayer biosorption behavior [41]. Freundlich capacity (XF) was determined as 3.89 and heterogeneity factor (β) was determined as 0.332. Since the EDR value was found as 11.9 kJ mol−1, it was concluded that the biosorption process proceeded chemically. The maximum biosorption capacity of the fungal biosorbent determined for the removal of Pb2+ ions and the isotherm model they are compatible with are compared with various sorbents in Table 3. As a result of this comparison, it was determined that the maximum biosorption capacity of fungal biomass was high enough to be an alternative to other sorbents. This indicates that the fungal biosorbent is a competitive and potentially cost-effective alternative to other sorbents for the removal of Pb2+ ions from solutions.
3.4 Biosorption kinetics
The mechanism of the biosorption process depends on the chemical and physical properties of the biosorbent as well as the system conditions. The biosorption process must reach equilibrium in a short time. Because increasing contact time may cause higher treatment costs in wastewater treatment plants. When Fig. 5 was examined, it was observed that Pb2+ ion biosorption increased with contact time. It was observed that biosorption was rapid until the first 240 min and reached equilibrium after that. The high biosorption rate in the first 240 min is initially due to the number of empty active centers present in the fungal biosorbent. Therefore, increasing concentration gradients exist between Pb2+ ions in solution and the surface of the fungal biomass. This increase in the concentration gradient causes an increase in the removal rate of Pb2+ ions in the initial stages. Over time, this concentration decreases due to Pb2+ ions adhering to empty active centers, which causes the adsorption rate to decrease.
Experimental data were applied to pseudo first order (PFO) [48], pseudo second order (PSO) [49], and intra particle diffusion (IPD) [50] models to predict the mechanism involved in the biosorption process [51, 52]. The applicability of PFO and PSO kinetic models in the removal of Pb2+ ions using fungal biomass was evaluated by comparing their correlation coefficients and the most appropriate model was selected. According to Table 4, it can be seen that the R2: 0.777 value of the PFO model is smaller than the R2: 0.834 value of the PSO model. In addition, it has been determined that the theoretical Qt value and the experimental Qe values of the PSO model are in agreement with each other in explaining the experimental data. It was seen that the PSO model described the biosorption process better. The multicollinearity of the Q-t0.5 graph drawn using experimental data indicates that the biosorption mechanism follows two or more steps (Fig. 6). When Fig. 6 is examined, it is seen that the IPD model graph is divided into two linear parts. The first linear part of the IPD model plot shows a boundary layer effect, while the second linear part represents intra-particle diffusion. When all this information was evaluated together, it was concluded that the biosorption process followed the PFO and IPD models.
3.5 Biosorption thermodynamics
To explain the thermodynamic behavior of the biosorption process, the studies were carried out at 5 °C, 25 °C, and 40 °C. Thermodynamic parameters were derived from data obtained from temperature effect studies on biosorption (Eq. 3–6). The following equations were used to determine the ΔHo, the ΔSo, and the ΔGo parameters [53].
As seen in Fig. 6, a 1/T graph was drawn against ln Kd for Pb2+ removal on the fungal biosorbent. Figure 6 represents a graph where 1/T is plotted against ln Kd (the natural logarithm of the distribution coefficient) for the removal of Pb2+ ions on the fungi. ΔSo was calculated from the intersection point of this obtained graph, and ΔHo was determined from the slope of the ln Kd vs. 1/T graph. The obtained parameters are given in Table 5. The positive ΔHo value of 92.9 kJ mol−1 determined for Pb2+ biosorption indeed indicates an endothermic nature of the biosorption process. The positive ΔSo value of 380 kJ mol−1 determined for the biosorption process indicates that randomness increases at the biosorbate-biosorbent interface during the process and ion exchange reactions occur [54]. Additionally, high negative ΔGo values indicate that biosorption occurs chemically [55].
3.6 FT-IR and SEM–EDX analyses
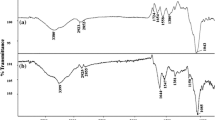
FT-IR analysis was performed to identify surface functional groups of the fungus as well as groups that would assist in Pb2+ biosorption. FT-IR spectra of the fungus before and after Pb2+ biosorption are given in Fig. 7 (a, b). The broad and strong band in the range of approximately 3000–3600 cm−1 observed in the FT-IR spectrum of fungus without Pb2+ loading can be explained by the stretching vibration of –OH and N–H groups [56]. The observed peak at 2926 cm−1 can be attributed to the symmetric and asymmetric vibration of the CH3 and CH2 groups [57], and the peak at 1638 cm−1 can be attributed to the stretching vibration of the carboxyl group (-COOH) [22]. In addition, the peaks at 1539 cm−1, 1410 cm−1 and 1040 cm−1 can be associated with the amide (II) vibration, bending vibration of the C-H group, and stretching vibrations of the C-O-C and C-O groups, respectively. The peak observed at 609 cm−1 can be explained as the vibration of phosphate groups (P = O) [58]. In line with this information obtained from the FTIR spectrum, it was determined that the fungus has functional groups that help metal biosorption such as carboxyl, hydroxyl, amino, and amide. When looking at the FT-IR spectrum of the fungus after Pb2+ biosorption, a shift in some characteristic absorption peaks and an increase or decrease in their intensity were detected. It is observed that the peaks where the vibrations of –CH, -COOH, C-O, and phosphate groups detected in the structure of the fungus are determined to have shifted to 2916 cm−1, 1623 cm−1, 1310 cm−1, 732 cm−1, respectively. The changes observed before and after biosorption can be attributed to surface complexation and electrostatic interaction reactions between these functional groups and Pb2+ ions [58].
SEM images of the fungal biosorbent before and after Pb2+ ion biosorption are given in Fig. 8. In the SEM image of the fungal biomass, the structure consisting of sporangiospore mass is seen (Fig. 8 (a)). The SEM image of the fungal biomass (Fig. 8 (a)) was observed to be obviously different from that of the Pb2+ ions biosorbed (Fig. 8 (b)). It was seen that the sporangium surface of the fungus became irregular and rough after the biosorption of Pb2+ ions. As shown in Fig. 8 (c-d), EDX analysis results showed that there were changes in percentage by weight in the inorganic components after Pb2+ ions biosorption, and there were increases and decreases indicating ion exchange. Based on this information, it was thought that more than one mechanism was involved in Pb2+ ion biosorption onto fungal biomass, such as surface complexation and electrostatic interactions.
3.7 Biological activity of the fungal extract
Certain species belonging to the Panaeolus genus have garnered attention due to their notable psilocin production, as well as their diverse range of biological activities, including antioxidative, anti-inflammatory, and antibacterial capabilities. However, there is limited data available regarding the antibacterial properties of P. papilionaceus, a species known for its capability to produce psychoactive compounds. Although Coprophilous mushroom species are generally considered to have a high potential for producing antimicrobial compounds, the limited number of studies conducted on this subject indicate the absence of antimicrobial activity by this species [59]. The current investigation aimed to evaluate the antibacterial efficacy of the methanol extract derived from P. papilionaceus against a selected set of six distinct bacterial strains. However, it was shown that there was no notable impact on any of the bacteria examined when exposed to concentrations ranging from 1 to 0.0156 mg.
In the current study, were examined not only the antimicrobial effects of the fungal extract but also its ability to inhibit biofilm formation. Biofilms are complex structures that result from the attachment of microbes to one other and a surface, accompanied by the release of extracellular polymeric substrates (EPS) into the surrounding environment. Microorganisms engage in the formation of biofilms as a means to enhance their resilience against antimicrobial substances and various environmental stressors present in natural settings. The production of biofilms is considered unfavorable in terms of hygiene and safety, especially within the food industry, due to its propensity to facilitate the attachment of pathogenic microbes or those responsible for food spoiling onto food and related surfaces. Therefore, understanding the formation of biofilms on surfaces and their removal is of great importance [60]. There is a lack of available evidence related to the antibiofilm capabilities exhibited by Panaeolus species, including P. papilionaceus. To the best of our current understanding, this study represents the initial documentation of the anti-biofilm properties of P. papilionaceus. The findings of our investigation revealed that P. papilionaceus could prevent biofilm formation at varying rates depending on the type of biofilm-forming bacteria (Table 6). It was determined that 1 mg of fungal extract noteworthy inhibitory effect on the formation of biofilms by indicator bacteria, particularly S. aureus and Klebsiella, with reductions of 87.85% and 72.73% respectively. However, no discernible impact was observed on the biofilm formation of P. aeruginosa and B. subtilis. Upon analysis of the fungal sample, it was determined that the predominant fatty acid component was linoleic acid (%80.6) (Fig. 9). In addition to linoleic acid, the presence of palmitic acid, stearic acid, and oleic acid in small amounts has also been observed.
3.8 Biosorption mechanism
The biggest challenge in a biosorption study is to elucidate the biosorption mechanism. To elucidate the biosorption mechanism, the surface properties and structure of the biosorbent need to be examined. In this context, it should be noted that; Fungi are biological materials that can remove pollutants such as heavy metals and dyes [2]. The binding mechanism of heavy metals to fungi can be explained by the strong attraction forces of cell wall components. Fungi have great advantages over other biosorbent materials in terms of biosorption and bioaccumulation activities due to their cell wall materials showing metal binding properties [61]. The fungal cell wall consists of glycoproteins, chitin, and glucans, as well as chitosan and other components [62]. The complex structure of fungal cell walls containing various functional groups such as hydroxyl (OH−), carboxyl (COOH−), amino (NH2), phosphate (PO43−), and sulfhydryl (SH−) reveals the excellent biosorption properties of fungi (confirmed by the FT-IR spectrum, SEM images, and fatty acid profile). The biosorption mechanism is assumed to include the following steps [63]; i) bulk solution transport to the surface of the biosorbent, ii) diffusion of Pb2+ ions across the boundary layer to the surface of the fungal biosorbent (film diffusion), iii) accumulation of increasing amount of Pb2+ ions to the surface of the fungal biosorbent (biosorption). Electrostatic interactions occur between biological groups such as OH−, COOH−, NH2, PO43−, SH−, and Pb2+ ions in the fungal biosorbent cell wall, which is one of the active centers for biosorption. Additionally, during the process of biosorption to the fungal surface, chelation occurs between one or more Pb2+ ions in the solution and the active functional groups on the fungal cell wall surface. In addition, solution pH supports ion exchange because at the natural pH of Pb2+ ions: 4.5, the competitive effect of H+ ions decreases, and negative ion gaps are formed on the fungal cell wall surface that can biosorb more Pb2+ ions. iv) Pb2+ ions into the internal pores and capillary spaces of the fungal biosorbent (intra-particle diffusion). Each of these four mechanisms are independent process (Fig. 10). When all this information is evaluated together, it is thought that the biosorption process of Pb2+ ions into fungal biomass is accompanied by the steps of surface biosorption, intra-particle diffusion, and film diffusion on the fungal cell walls. In addition, during the biosorption process, protons or light metal cations such as Na+, K+, Mg2+, and Ca2+ attached to the functional groups on the biosorbent surface are exchanged with metal cations in the aqueous solution (ion exchange) [64].
4 Conclusion
In this study, to effectively remove Pb2+ ions from aqueous solutions fungal (P. papilionaceus) biomass was used. The optimum biosorption of Pb2+ ions was achieved in 240 min at 25 °C at a natural pH of 4.5. It was observed that biosorption isotherms fit the Freundlich model better. The optimum biosorption capacity calculated from the Langmuir model was found to be 31.2 mg g−1. The biosorption energy found from the D-R model showed that the biosorption process was chemical. Negative values of the Gibbs free energy change, one of the thermodynamic parameters, showed that the biosorption process was spontaneous, and positive enthalpy values showed that the biosorption process was endothermic. Biosorption kinetics showed that the biosorption process followed PSO and IPD patterns. Biosorption of Pb2+ ions into fungal biomass was observed to involve surface biosorption, film diffusion, and intra-particle diffusion mechanisms. In light of all this information, it was seen that the fungal biosorbent is a low-cost, natural, effective, and potentially promising biosorbent in the removal of Pb2+ ions from aqueous solution.
Data availability
Not applicable.
References
Alamrani NA, Almutairi FM, Alotaibi FA, Alenazi DAK, Monier M, Abdel-Latif DA, Elsayed NH (2023) Developing thiosemicarbazide-modified/ion-imprinted chitosan for selective cadmium ion biosorption. Mater Today Chem 30. https://doi.org/10.1016/j.mtchem.2023.101547
Vieira RHSF, Volesky B (2000) Biosorption: a solution to pollution? Int Microbiol 3:17–24
Samimi M, Nouri J (2023) Optimized zinc uptake from the aquatic environment using biomass derived from Lantana camara L. stem. Pollution. https://doi.org/10.22059/POLL.2023.363363.2014
Batool F, Mohyuddin A, Amjad A, ul Hassan A, Nadeem S, Javed M, Hafiz Dzarfan Othman M, Wayne Chew K, Rauf A, Kurniawan TA (2023) Removal of Cd(II) and Pb(II) from synthetic wastewater using Rosa damascena waste as a biosorbent: an insight into adsorption mechanisms, kinetics, and thermodynamic studies. Chem Eng Sci 280. https://doi.org/10.1016/j.ces.2023.119072
Wu S, Jiang H, Lu J (2023) Adsorptive performance and mechanism exploration of L-lysine functionalized celluloses for enhanced removal of Pb(II) from aqueous medium. Int J Biol Macromol 242. https://doi.org/10.1016/j.ijbiomac.2023.124997
Negi A, Joshi SK, Bhandari NS (2023) Estimation of sorption–desorption characteristics of biosorbent of Lantana camara leaves for removal of Pb (II) ions from wastewater. Environ Monit Assess 195. https://doi.org/10.1007/s10661-022-10629-0
Kumar K, Singh D (2023) Toxicity and bioremediation of the lead: a critical review. Int J Environ Health Res. https://doi.org/10.1080/09603123.2023.2165047
Samimi M, Shahriari-Moghadam M (2021) Isolation and identification of Delftia lacustris Strain-MS3 as a novel and efficient adsorbent for lead biosorption: Kinetics and thermodynamic studies, optimization of operating variables. Biochem Eng J 173. https://doi.org/10.1016/j.bej.2021.108091
Aljohani MS, Alnoman RB, Alharbi HY, Al-Anazia M, Monier M (2024) Designing of a cellulose-based ion-imprinted biosorbent for selective removal of lead (II) from aqueous solutions. Int J Biol Macromol 259:129145. https://doi.org/10.1016/j.ijbiomac.2023.129145
Mosoarca G, Vancea C, Popa S, Dan M, Boran S (2022) The use of bilberry leaves (Vaccinium myrtillus L.) as an efficient adsorbent for cationic dye removal from aqueous solutions. Polymers (Basel) 14. https://doi.org/10.3390/polym14050978
Rehman MU, Taj MB, Carabineiro SAC (2023) Biogenic adsorbents for removal of drugs and dyes: a comprehensive review on properties, modification, and applications. Chemosphere 338. https://doi.org/10.1016/j.chemosphere.2023.139477
Samimi M, Zakeri M, Alobaid F, Aghel B (2023) A brief review of recent results in arsenic adsorption process from aquatic environments by metal-organic frameworks: classification based on kinetics, isotherms and thermodynamics behaviors. Nanomaterials 13. https://doi.org/10.3390/nano13010060
Eliescu A, Georgescu AA, Nicolescu CM, Bumbac M, Cioateră N, Mureșeanu M, Buruleanu LC (2020) Biosorption of Pb(II) from aqueous solution using mushroom (Pleurotus ostreatus) biomass and spent mushroom substrate. Anal Lett 53. https://doi.org/10.1080/00032719.2020.1740722
Samimi M (2024) Efficient biosorption of cadmium by Eucalyptus globulus fruit biomass using process parameters optimization. Glob J Environ Sci Manag 10:27–38. https://doi.org/10.22034/gjesm.2024.01.03
Liu G, Geng W, Wu Y, Zhang Y, Chen H, Li M, Cao Y (2024) Biosorption of lead ion by lactic acid bacteria and the application in wastewater. Arch Microbiol 206:18. https://doi.org/10.1007/s00203-023-03755-x
Gu S, Lan CQ (2023) Effects of culture pH on cell surface properties and biosorption of Pb(II), Cd(II), Zn(II) of green alga Neochloris oleoabundans. Chem Eng J 468. https://doi.org/10.1016/j.cej.2023.143579
Senol ZM, Gül ÜD, Gurbanov R, Simsek S (2021) Optimization the removal of lead ions by fungi: Explanation of the mycosorption mechanism. J Environ Chem Eng 9. https://doi.org/10.1016/j.jece.2020.104760
Al-Amrani WA, Fathi NSSM, Hanafiah MAKM, Zakaria H (2023) Comparative biosorption of Cu (II) and Pb (II) Ions from aqueous solutions using soursop (Annona muricata L) leaf powder: characterisation, isotherm and kinetic studies. Bioresour Environ 1(1):66–79
Hua Liu G, Tang X, Yuan J, Li Q, Qi L, Wang H, Ye Z, Zhao Q (2023) Activated sludge process enabling highly efficient removal of heavy metal in wastewater. Environ Sci Pollut Res 30. https://doi.org/10.1007/s11356-022-23693-3
Berdous D, Abdellaoui N, Arous O, Akretche DE (2023) Low cost biopolymer adsorbents for the removal of heavy metal contaminant from wastewater. Macromol Symp 408. https://doi.org/10.1002/masy.202200066
Sagar Jena P, Pradhan A, Prakash Nanda S, Kishore Dash A, Naik B (2022) Biosorption of heavy metals from wastewater using Saccharomyces cerevisiae as a biosorbent: a mini review. Mater Today Proc 67. https://doi.org/10.1016/j.matpr.2022.07.306
Naeemullah, Tuzen M, Sarı A, Turkekul I (2020) Influential bio-removal of mercury using Lactarius acerrimus macrofungus as novel low-cost biosorbent from aqueous solution: Isotherm modeling, kinetic and thermodynamic investigations. Mater Chem Phys 249. https://doi.org/10.1016/j.matchemphys.2020.123168
Sharma R, Talukdar D, Bhardwaj S, Jaglan S, Kumar R, Kumar R, Akhtar MS, Beniwal V, Umar A (2020) Bioremediation potential of novel fungal species isolated from wastewater for the removal of lead from liquid medium. Environ Technol Innov 18:100757. https://doi.org/10.1016/j.eti.2020.100757
Ayele A, Haile S, Alemu D, Kamaraj M (2021) Comparative utilization of dead and live fungal biomass for the removal of heavy metal: a concise review. Sci World J 2021. https://doi.org/10.1155/2021/5588111
Huang C, Lai C, Xu P, Zeng G, Huang D, Zhang J, Zhang C, Cheng M, Wan J, Wang R (2017) Lead-induced oxidative stress and antioxidant response provide insight into the tolerance of Phanerochaete chrysosporium to lead exposure. Chemosphere 187. https://doi.org/10.1016/j.chemosphere.2017.08.104
Castanho NRCM, De Oliveira RA, Batista BL, Freire BM, Lange C, Lopes AM, Jozala AF, Grotto D (2021) Comparative study on lead and copper biosorption using three bioproducts from edible mushrooms residues. J Fungi 7. https://doi.org/10.3390/jof7060441
Rozman U, Kalčíková G, Marolt G, Skalar T, Žgajnar Gotvajn A (2020) Potential of waste fungal biomass for lead and cadmium removal: characterization, biosorption kinetic and isotherm studies. Environ Technol Innov 18. https://doi.org/10.1016/j.eti.2020.100742
Strauss D, Ghosh S, Murray Z, Gryzenhout M (2023) Global species diversity and distribution of the psychedelic fungal genus Panaeolus. Heliyon 9 https://doi.org/10.1016/j.heliyon.2023.e16338
Şenol ZM (2021) A chitosan-based composite for adsorption of uranyl ions; mechanism, isothems, kinetics and thermodynamics. Int J Biol Macromol 183:1640–1648. https://doi.org/10.1016/j.ijbiomac.2021.05.130
Zou Y, Yu H (2018) Improving the analysis of 37 fatty acid methyl esters using three types of capillary GC columns. Agilent Technologies Application Note
Samimi M, Mansouri E (2023) Efficiency evaluation of Falcaria vulgaris biomass in Co(II) uptake from aquatic environments: characteristics, kinetics and optimization of operational variables. Int J Phytoremediation. https://doi.org/10.1080/15226514.2023.2250462
Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH (1985) Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22. https://doi.org/10.1128/jcm.22.6.996-1006.1985
Wang X, Wang L, Wang Y, Tan R, Ke X, Zhou X, Geng J, Hou H, Zhou M (2017) Calcium sulfate hemihydrate whiskers obtained from flue gas desulfurization gypsum and used for the adsorption removal of lead. Crystals 7. https://doi.org/10.3390/cryst7090270
Şenol ZM, Elma E, El Messaoudi N, Mehmeti V (2023) Performance of cross-linked chitosan-zeolite composite adsorbent for removal of Pb2+ ions from aqueous solutions: experimental and Monte Carlo simulations studies. J Mol Liq 391. https://doi.org/10.1016/j.molliq.2023.123310
Elsayed NH, Monier M, Alatawi RAS, Al-Anazi M, Albalawi M, Alatawi MJ (2023) Selective removal of uranyl ions using ion-imprinted amino-phenolic functionalized chitosan. Int J Biol Macromol 237. https://doi.org/10.1016/j.ijbiomac.2023.124073
Elgarahy AM, Elwakeel KZ, Mohammad SH, Elshoubaky GA (2021) A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean Eng Technol 4:100209. https://doi.org/10.1016/j.clet.2021.100209
Şen NE, Şenol ZM (2023) Effective removal of Allura red food dye from water using cross-linked chitosan-diatomite composite beads. Int J Biol Macromol 253. https://doi.org/10.1016/j.ijbiomac.2023.126632
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40. https://doi.org/10.1021/ja02242a004
Freundlich H (1907) Über die Adsorption in Lösungen. Zeitschrift Für Phys Chemie 57U. https://doi.org/10.1515/zpch-1907-5723
Rand B (1976) On the empirical nature of the Dubinin-Radushkevich equation of adsorption. J Colloid Interface Sci 56. https://doi.org/10.1016/0021-9797(76)90259-9
Hou L, Xing B, Guo H, Zeng H, Cheng S, Meng M, Qu X, Valdivieso López A, Zhang C, Cao Y (2023) Effect of mineralogical characteristics evolution of vermiculite upon thermal and chemical expansions on its adsorption behavior for aqueous Pb(II) removal. Powder Technol 430. https://doi.org/10.1016/j.powtec.2023.119040
Wang Q, Wang B, Lee X, Lehmann J, Gao B (2018) Sorption and desorption of Pb(II) to biochar as affected by oxidation and pH. Sci Total Environ 634. https://doi.org/10.1016/j.scitotenv.2018.03.189
Patel PK, Pandey LM, Uppaluri RVS (2023) Adsorptive removal of Zn, Fe, and Pb from Zn dominant simulated industrial wastewater solution using polyvinyl alcohol grafted chitosan variant resins. Chem Eng J 459. https://doi.org/10.1016/j.cej.2023.141563
Xu W, Liu X, Tang K (2022) Adsorption of hydroquinone and Pb(II) from water by β-cyclodextrin/polyethyleneimine bi-functional polymer. Carbohydr Polym 294. https://doi.org/10.1016/j.carbpol.2022.119806
Wang J, Zhang W (2021) Evaluating the adsorption of Shanghai silty clay to Cd(II), Pb(II), As(V), and Cr(VI): kinetic, equilibrium, and thermodynamic studies. Environ Monit Assess 193. https://doi.org/10.1007/s10661-021-08904-7
Lai KC, Lee LY, Hiew BYZ, Thangalazhy-Gopakumar S, Gan S (2020) Facile synthesis of xanthan biopolymer integrated 3D hierarchical graphene oxide/titanium dioxide composite for adsorptive lead removal in wastewater. Bioresour Technol 309. https://doi.org/10.1016/j.biortech.2020.123296
Şenol ZM, Şimşek S (2022) Insights into effective adsorption of lead ions from aqueous solutions by using chitosan-bentonite composite beads. J Polym Environ 30. https://doi.org/10.1007/s10924-022-02464-8
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf Environ Prot 76. https://doi.org/10.1205/095758298529326
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34. https://doi.org/10.1016/S0032-9592(98)00112-5
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89. https://doi.org/10.1061/jsedai.0000430
Elsayed NH, Monier M, Alatawi RAS, Al-Anazi M (2023) Design of ion-imprinted cellulose-based microspheres for selective recovery of uranyl ions. Carbohydr Polym 313. https://doi.org/10.1016/j.carbpol.2023.120873
Aljohani MS, Alnoman RB, Alharbi HY, Bukhari AAH, Monier M (2024) Development and evaluation of thiosalicylic-modified/ion-imprinted chitosan for selective removal of cerium (III) ion. Carbohydr Polym 326:121620. https://doi.org/10.1016/j.carbpol.2023.121620
Lima EC, Hosseini-Bandegharaei A, Moreno-Piraján JC, Anastopoulos I (2019) A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J Mol Liq 273. https://doi.org/10.1016/j.molliq.2018.10.048
Ebelegi AN, Ayawei N, Wankasi D (2020) Interpretation of adsorption thermodynamics and kinetics. Open J Phys Chem 10. https://doi.org/10.4236/ojpc.2020.103010
Borandegi M, Nezamzadeh-Ejhieh A (2015) Enhanced removal efficiency of clinoptilolite nano-particles toward Co(II) from aqueous solution by modification with glutamic acid. Colloids Surf A Physicochem Eng Asp 479. https://doi.org/10.1016/j.colsurfa.2015.03.040
Amin F, Talpur FN, Balouch A, Afridi HI (2017) Eco-efficient fungal biomass for the removal of Pb(II) ions from water system: a sorption process and mechanism. Int J Environ Res 11. https://doi.org/10.1007/s41742-017-0029-z
Yang S, Wang Y, Liu Y (2021) Biosorption of Cu(II) from aqueous solutions by a macrofungus (ganoderma lobatum) biomass and its biochar. Nat Environ Pollut Technol 20. https://doi.org/10.46488/NEPT.2021.v20i02.014
Isik B, Ugraskan V, Cankurtaran O (2022) Effective biosorption of methylene blue dye from aqueous solution using wild macrofungus (Lactarius piperatus). Sep Sci Technol 57. https://doi.org/10.1080/01496395.2021.1956540
Janeš D, Kreft S, Jurc M, Seme K, Štrukelj B (2007) Antibacterial activity in higher fungi (mushrooms) and endophytic fungi from Slovenia. Pharm Biol 45. https://doi.org/10.1080/13880200701575189
Szczepanski S, Lipski A (2013) Essential oils show specific inhibiting effects on bacterial biofilm formation. Food Control 36. https://doi.org/10.1016/j.foodcont.2013.08.023
Kapoor A, Viraraghavan T (1995) Fungal biosorption - an alternative treatment option for heavy metal bearing wastewaters: a review. Bioresour Technol 53. https://doi.org/10.1016/0960-8524(95)00072-M
Dhankhar R, Hooda A (2011) Fungal biosorption-an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ Technol 32. https://doi.org/10.1080/09593330.2011.572922
Aragaw TA, Bogale FM (2021) Biomass-based adsorbents for removal of dyes from wastewater: a review. Front Environ Sci 9. https://doi.org/10.3389/fenvs.2021.764958
Michalak I, Chojnacka K, Witek-Krowiak A (2013) State of the art for the biosorption process - a review. Appl Biochem Biotechnol 170. https://doi.org/10.1007/s12010-013-0269-0
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The present study was partly supported by the Sivas Cumhuriyet University Projects Commission.
Author information
Authors and Affiliations
Contributions
Zeynep Mine Şenol: experimental work, visualization, methodology, supervision, writing, guidance, editing, and review. Zehra Saba Keskin: experimental work, visualization, methodology writing, editing, and review. Emine Dinçer: experimental work, biological analysis, writing, editing, and review. Amina Ben Ayed: writing, editing, and review.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Şenol, Z.M., Keskin, Z.S., Dinçer, E. et al. Influential lead uptake using dried and inactivated-fungal biomass obtained from Panaeolus papilionaceus: biological activity, equilibrium, and mechanism. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05584-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05584-4