Abstract
The utilization of renewable and cost-effective biomass for the production of activated carbon represents an innovative approach to environmental remediation. In this work, environmentally friendly carbon materials derived from cocopeat were employed to create a cocopeat-based magnetic activated carbon (CPAC-Fe3O4) nanocomposite for the removal of mercury from aqueous solutions. The CPAC-Fe3O4 nanocomposite underwent comprehensive characterization using SEM, FTIR, BET, XRD, and VSM analyses. The optimization process revealed a maximum adsorption capacity of 204.08 mg/g under specific conditions: initial Hg concentration of 20 mg/L, pH of 6, temperature of 25 °C, and adsorbent dose of 0.01 g within 60 min. Isotherm and kinetic modeling exhibited strong agreement with the Freundlich isotherm (0.9749) and pseudo-second-order (0.9997) kinetic models, indicating a favorable chemisorption process. Furthermore, thermodynamic analysis suggested that the adsorption process is endothermic and spontaneous. The adsorption mechanism was elucidated based on FTIR analysis. The results highlight the CPAC-Fe3O4 nanocomposite as a promising and sustainable candidate for effective water purification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The water crisis has tremendously increased due to the rapid development in agricultural activities, industrialization, and climate change, threatening the sustainability of ecological systems worldwide [1, 2]. According to the United Nations’ report, 2.3 billion people inhabit water-stressed countries [3], which is estimated to substantially upgrade by 2050 [4, 5]. Approximately 4 billion people experience water scarcity for at least 1 month per year, and 2 billion people will suffer from acute water scarcity by 2050 due to water shortage [6, 7]. Therefore, practical approaches are required to deal with water contamination and fulfill sustainable development goals [8,9,10].
The water quality has deteriorated as a result of heavy metals especially mercury (Hg), which is a priority pollutant due to hindering the enzyme binding sites and disturbing protein synthesis based on the WHO [11] and the US EPA reports [12, 13]. Hg is a neurotoxin and its low vapor pressure intensifies human poisoning [14, 15]. It is ranked second toxic waste by the US Agency for Toxic Substances and Disease Registry [16, 17], and a limit of 2 µg/L Hg2+ in drinking water has been established by the US Environmental Protection Agency (USEPA) [15, 18]. Therefore, Hg elimination from water resources and wastewater should receive more attention to avoid its adverse effects on ecosystems and human health.
Despite several accessible techniques for heavy metal removal including ion exchange, reverse osmosis, chemical precipitation, bioremediation, and coagulation, their implementation suffers from byproduct and sludge generation, required time, and cost of operation [19,20,21,22]. In the last few decades, researchers have focused on advanced porous materials regarding the exponential increase of nanomaterial demand [23, 24]. Although more than 99% heavy metal removal efficiency can be achieved through the adsorption process [15, 25], the adsorbent properties have fundamental roles in the removal potential. Magnetic nanoparticles have already exhibited special features including large surface area, high reactivity, selectivity, and catalytic potential. This can be associated with large oxygenated functional groups such as carboxylic, hydroxide, and amide on their surface that facilitate the metal ion bonding through surface complexation and ligand exchange [26]. However, some nanomaterials including CoFe2O4, MnFe2O4, and Fe3O4 have inherent poor adsorption capacity for heavy metals due to the lack of surface-active functional groups. Zhao et al. [27] believed that their characteristics can be improved using a coating layer of SiO2 under the outer layer of CoFe2O4 particles functionalized with polypyrrole. The CoFe2O4-based core–shell carriers with the grafted group of -NH2 is another proposed ideal adsorbent for Hg with 149.3 mg/g adsorption capacity [28]. Bao et al. [29] also synthesized mercaptoamine-functionalised silica-coated magnetic nanoparticles for the efficient removal of Hg and Pb from wastewater with maximum adsorption capacities of 355 and 292 mg/g, respectively. A novel magnetic diatomite-based material of DMT/CoFe2O4-p-ATP with an adsorption capability of 213.2 mg/g was also recommended for Hg removal [30]. Kaolin with a high specific surface area and negative charge is appropriate for heavy metal adsorption but easy agglomeration limits its further usage. Therefore, polypyrrole-functionalized magnetic Kaolin was proposed for Hg removal with substantial adsorption capacity of 317.1 mg/g [31].
Although numerous synthesized magnetic nanomaterials for Hg elimination have been proposed, a cost-effective and practical adsorbent with a simple design is indispensable for removing Hg from wastewater. Therefore, the synthesis, characterization, and adsorption efficiency of cocopeat-based magnetic activated carbon (CPAC-Fe3O4) nanocomposite were considered, and the following goals were determined: (1) preparation of magnetic nanocomposite using cocopeat; (2) characterization of CPAC-Fe3O4 nanocomposite structure and morphology; (3) optimization of Hg removal from aqueous solutions considering the impacts of pH, temperature, contact time, Hg concentration, and adsorbent dosage; and (4) interpretation of adsorption mechanism using isotherm, kinetics, and thermodynamic modeling. Summing up, the results of this study can provide a framework to elaborate cost-effective and promising nanocomposite for industrial wastewater treatment.
2 Materials and methods
2.1 Chemicals
Analytical-grade chemicals were utilized in experimental research. Hg chloride (HgCl2) was used for the preparation of Hg solutions. Iron (II) chloride (FeCl2. 4H2O) and iron (III) chloride (FeCl3. 6H2O) were applied for the magnetization of the adsorbent. Hydrochloric acid (HCl) and sodium hydroxide (NaOH) were also utilized to adjust the pH of the solutions. All chemicals were supplied by the Merck Company.
2.2 Synthesis of CPAC-Fe 3 O 4 nanocomposite
Cocopeat samples were purchased from a local plant nursery and collected from the fiber separation of coconut husk. A total of 20 g of cocopeat samples was added to deionized water in a 1000-mL flask and heated to 65 °C for 20 min. Then, FeCl2. 4H2O and FeCl3. 6H2O with a molar ratio of 2 to 1 were added to the mixture and stirred for 30 min to ensure the diffusion of iron cations to the sample. Afterward, the sample was dehydrated and then transferred to 1 M NaOH solution and heated to 60 °C for 30 min to complete the synthesis of cocopeat-based magnetic nanoparticles. The color of the mixture was changed to black, indicating the successful synthesis of magnetic nanoparticles. The as-synthesized sample was collected and rinsed with deionized water two times to remove impurities and adjust the pH around neutral conditions. Afterward, the samples were dried under sunlight for 48 h, thus powdered and sieved to the size of 60 mesh no. (0.25 mm). The cocopeat samples were further subjected to heating at a rate of 5 °C per minute in a muffle furnace at 600 °C under nitrogen gas with a pressure of 100 mL/min for 2 h.
2.3 Characterization
The elemental analysis was applied by an elemental analyzer (Flash EA 1112, USA), which showed 42% C, 47% O, 6.5% H, 1.5% N, and 3% S contents of cocopeat. The morphology of the CPAC-Fe3O4 nanocomposite was analyzed with field emission scanning electron microscopy (FESEM, FEI NOVA NanoSEM 450, Japan). The crystal structure of the CPAC-Fe3O4 nanocomposite was determined by X-ray diffractometer (XRD, Ultima, Japan). Fourier-transform infrared spectroscopy (FTIR, Tensor 27, Bruker, Germany) was performed to assess the surface functional groups of the CPAC-Fe3O4 nanocomposite. The Brunauer–Emmett–Teller (BET, Belsorp mini II, Microtrac Bel Corp, Japan) method was employed to determine the specific surface area of the synthesized CPAC-Fe3O4 nanocomposite. The magnetic properties were measured using the vibrating sample magnetometer (VSM, LBKFB, Kashan Kavir Magnetic Co. Iran).
2.4 Batch adsorption experiment
The adsorption behavior of the CPAC-Fe3O4 nanocomposite was evaluated for Hg removal considering the impact of pH, adsorbent dosage, contact time, initial Hg concentration, and temperature. A 1000 mg/L Hg stock solution was prepared by dissolving the appropriate volume of HgCl2 in deionized water. To determine the optimum condition for maximum Hg removal, the pH of the solution was adjusted on 3, 4, 5, 6, 7, and 8 using 0.1 M HCl and NaOH, while other parameters were constant (0.01 g adsorbent dose, 20 mg/L Hg concentration, and contact time of 60 min at 25 °C). The mixture was shaken at 100 rpm and allowed to react with the magnetic nanocomposite. Then, the CPAC-Fe3O4 nanocomposite was removed from the solution by the magnetic field, and the mixture solution was centrifuged (HERMLE Z300, USA) at a rate of 4000 rpm for 5 min and then filtered. A total of 1 mL HNO3 was further added to the mixture to avoid Hg ions precipitation. The remained Hg concentration in the solution was measured by cold vapor technique with atomic absorption spectroscopy (AAS, Uniam 919). The limit of detection (LOD) for Hg was 0.01 μg/L. The potential of CPAC-Fe3O4 nanocomposite was also apprised at Hg concentrations of 5, 10, 20, 50, 70, and 100 mg/L at pH of 6 and 0.01 g adsorbent for 60 min at 25 °C. The same procedure was followed to optimize the contact time at 15, 30, 45, 60, 75, and 90 min at a fixed pH of 6, Hg concentration of 20 mg/L, and 0.01 g adsorbent at 25 °C. Adsorbent doses of 0.01, 0.02, 0.03, 0.04, 0.05, and 0.1 g were considered at a fixed pH of 6 and Hg concentration of 20 mg/L for 60 min at 25 °C. The impact of temperature (15, 20, 25, 30, 35, and 40 °C) was also evaluated at a fixed pH of 6, Hg concentration of 20 mg/L, and 0.01 g adsorbent for 60 min. All experiments were carried out in three replicates.
3 Results and discussion
3.1 Characterization of CPAC-Fe 3 O 4 nanocomposite
3.1.1 FESEM analysis
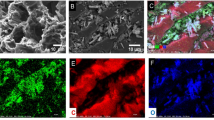
The morphology of CPAC-Fe3O4 nanocomposite was determined with SEM at 25 keV as depicted in Fig. 1. The activation with NaOH creates cavities with spherical structure, relatively smooth surface, and diameter size of < 100 nm. Several micropores and mesopores are formed, resulting in a high surface area as shown in Fig. 1b and c. Furthermore, the exterior pores facilitate the efficient transport of Hg ions from the aqueous solution to the inner micropores of the CPAC-Fe3O4 nanocomposite, increasing the adsorption performance [32]. As can be seen, the CPAC-Fe3O4 nanocomposite manifests various pore sizes with relatively uniform distribution. The large size of pores in the raw cocopeat sample and small pores in the cocopeat conversion to activated carbon were also reported by Varghese et al. [33]. The SEM image after Hg adsorption shows the vaccine sites on the surface of the CPAC-Fe3O4 nanocomposite, indicating a high potential of adsorbent to multiple adsorb Hg ions in a batch system (Fig. 1d).
3.1.2 FTIR analysis
The synthesized CPAC-Fe3O4 nanocomposite was analyzed by FTIR spectroscopy to determine the effective functional groups in the adsorption process. Figure 2 manifests the FTIR spectra for synthesized CPAC-Fe3O4 nanocomposite before and after Hg removal. A broad peak at 3434.21 cm−1 is attributed to the stretching vibrations of the O–H band [26, 34, 35]. A peak at 2922.56 cm−1 corresponds to C-H SP3 stretching, which verifies the presence of alkanes in the nanocomposite. Strong peaks at 1733.21 cm−1 and 1639.45 cm−1 can be ascribed to stretching of C = O [36]. Moreover, the absorption at 1200–1600 cm−1 is due to CH2 and CH3 stretching. A peak at 841.06 cm−1 can be associated with the stretching vibration of C-O. Absorption peaks at 618.62 cm−1 and 522.94 cm−1 correspond to the Fe–O band [36, 37], evidence of the formation of magnetic nanocomposite (Fig. 2a). Therefore, the presence of hydroxyl, carboxylic acid, and lactones functional groups on the surface of CPAC-Fe3O4 nanocomposite plays the main role in Hg ion adsorption. The changes in the vibrational spectra at 681.71, 747.42, 804.10, and 1077.28 cm−1 confirmed the adsorption of Hg ions by the functional groups (Fig. 2b).
3.1.3 XRD analysis
X-ray diffraction is a practical technique for investigating the characteristics of the crystal structure, such as the qualitative features of unknown materials, lattice geometry, crystal phase, crystal size, single crystal orientation, and lattice defects [38]. To determine the crystalline phase of the CPAC-Fe3O4 nanocomposite, XRD analysis was performed in the range of angle 2θ = 0–80° and temperature 25 °C. Regarding Fig. 3, the peaks at 2θ = 30.12° and 35.36° reveal the crystalline phase, with 100% purity and maximum intensity of 141 at 36.36°. Based on the obtained spectrum, 6 peaks at 2θ = 35.4°, 39.36°, 43.16°, 53.6°, 56.84°, 64.48° in inverse cubic spinel confirm the presence of Fe3O4 nanoparticles on the CPAC-Fe3O4 nanocomposite [39]. In addition, the obtained peaks are compatible with the standard peak (No JCPDS. 19-0629) and prove the existence of magnetic activated carbon and Fe3O4 particles.
3.1.4 BET analysis
The BET theory is based on a simplified model of physisorption and determines a specific surface area of adsorbent by nitrogen sorption–desorption measurement at a constant temperature of liquid nitrogen (77 K) [40]. The pore size distribution was achieved by the Barrett-Joyner-Halenda (BJH) equation during the desorption phase. The BJH method attributes the relative pressure of nitrogen in equilibrium with the porous adsorbent to the size of the pores, considering the Kelvin equation [41]. The pore size radii which are covered by the BJH calculations ranged from 1.7 to 300 nm [42]. The results of the BET analysis of the CPAC-Fe3O4 nanocomposite revealed that the size of the nanopores was around 2.27 nm, and the total volume was 0.067 cm3/g of the CPAC-Fe3O4 nanocomposite (Table 1). The specific surface area of the prepared nanocomposite was also equal to 118.25 m2/g. The volume of required gas to create a single layer on the CPAC-Fe3O4 nanocomposite was estimated as 27.168 cm3/g. Notably, the high surface area of the nanocomposite indicates a suitable substrate for bonding with Fe3O4 nanoparticles. The shape of the adsorption/desorption isotherms might be included in type IV based on the International Union of Pure and Applied Chemistry (IUPAC), with a hysteresis loop, which showed the porosity of activated carbon (Fig. 4). A lower p/p0 ratio depicted narrow and micropores, raising the agglomeration of Hg ions and adsorption efficiency [43, 44].
3.1.5 VSM analysis
Figure 5 illustrates the magnetization curves of Fe3O4 and the CPAC-Fe3O4 nanocomposite at room temperature and − 10,000 to 10,000 oersted (Oe) field. The saturation values for Fe3O4 particles and the CPAC-Fe3O4 nanocomposite are 63.77 (Fig. 5a) and 2.5 (Fig. 5b) emu/g, respectively, and have an S-like form. The decrease of magnetization properties in the CPAC-Fe3O4 nanocomposite was attributed to the existence of nonmagnetic particles. Moreover, the CPAC-Fe3O4 nanocomposite follows the behavior pattern of superparamagnetic materials and has no hysteresis loop [45]. Therefore, the magnetization curve passes through the origin, implying no coercive field and remanent magnetization. The curve primarily moves away from the center with an almost constant slope. It indicates that the relationship between magnetization and the magnetic field is linear. Then, the slope of the curve gradually begins to reduce and the acceleration of magnetization decreases to reach saturation magnetization. After that, increasing the applied external magnetic field density does not affect enhancing the magnetization, and the magnetic material is completely saturated.
3.2 Effect of pH
Figure 6a illustrates the adsorption efficiency of Hg ions on the CPAC-Fe3O4 nanocomposite. As the pH of the solution was increased from 3 to 6, the Hg adsorption enhanced from 101.8 to 182.4 mg/g with a maximum removal rate of 91.2%. Whereas, a further increase in pH to 8 led to a 14.97% reduction in Hg removal. The functional groups such as carboxyl and amino groups are protonated at low pH causing the electrostatic repulsion between the functional groups and positively charged Hg ions and subsequently hindering the adsorption [35, 46]. However, the increase in pH deprotonates the functional groups and the Hg species (Hg (II), Hg(OH)+, and Hg(OH)2) can chelate with the neutral functional groups, leading to high adsorption [35, 46]. Einollahipeer and Okati [47] also referred to a strict competition between Hg2+ and H3O+ at low pH accompanied by HgOH formation at pH higher than 7 with a lower affinity towards amine grafted magnetic graphene oxide (m-GO-NH2), which could reduce the Hg adsorption [48]. The formation of a colloidal precipitate at higher pH was also attributed to lower adsorption efficiency [35]. Ge et al. [49] highlighted the increasing trend of Hg removal with a pH increase to 6, using poly(itaconic acid)-grafted crosslinked chitosan nanoadsorbent. Similar results were reported in the literature [50, 51].
3.3 Effect of Hg concentration
The effect of Hg concentration in the range of 5 to 100 mg/L at pH 6 is exhibited in Fig. 6b. The maximum removal efficiency (98.3%) was observed at 5 mg/L, which was decreased to 91.2% at a concentration of 20 mg/L. A further increase to 94.8% was detected at a concentration of 50 mg/L, while reduced to 90.21% at 100 mg/L concentration. A reduction in Hg adsorption at high Hg concentration can be related to the repulsive force between adsorbed Hg ions and the remaining ones in the solution due to limited available sites on the adsorbent [52, 53]. In contrast, at low Hg concentrations, the adsorption occurs on high-energy sites on the adsorbent [15]. The adsorption capacity was also increased from 49.15 to 902.15 mg/g in parallel with the increase in initial Hg concentration. This can be ascribed to the interaction between adsorption sites and Hg ions, which can diffuse the adsorption sites rapidly until all are fully occupied and equilibrium occurs [54]. The obtained adsorption capacity in this study was higher than that for Pistachio-nut/licorice residues (147.1 mg/g) [55], curcumin-based biocomposite (144.9 mg/g) [56], and carboxymethyl CS-sewage sludge (594 mg/g) [57].
3.4 Effect of adsorbent dose
The adsorbent dose as a critical and effective parameter in the adsorption process was investigated. As shown in Fig. 6c, the common pattern is that the increase in adsorbent dose resulted in more Hg ion removal and then decreased when the adsorbent dose exceeded a threshold. Notably, even in the lowest adsorbent dose (0.01 g), substantial removal efficiency was achieved (91.2%). The increase in nanocomposite dose from 0.01 to 0.03 g revealed a 4.3% enhancement in adsorption efficiency. This can be justified by more available active binding sites on the surface of the adsorbent [58]. A further reduction to 88.3% was attained at 0.1 g adsorbent dose. The adsorption capacity was decreased from 182.4 (0.01 g dosage) to 17.66 mg/g (0.1 g dosage), which was because of more vacant sites at higher doses of adsorbent [59].
3.5 Effect of contact time
Adsorption is a time-dependent process and prediction of the removal rate of Hg ions is indispensable to optimize the process in the full-scale operation. Figure 6d illustrates the increase in adsorption efficiency and capacity when the contact time was upgraded from 15 (71.7%) to 90 min (96.07%). It is interesting to mention that a rapid increase in Hg removal at the initial time is relevant to a greater number of available active sites, which gradually are occupied with time increasing [60, 61]. Moreover, the adsorption is controlled through the diffusion process from the solution to the adsorbent surface, which is slowed down due to fewer available adsorption sites [62]. Regarding literature [47], the capability of m-GO-NH2 to adsorb Hg ions displayed a rise from 79.02 to 95.54%, while the contact time was increased from 15 to 90 min. Another study revealed 60% Hg removal by Fe3O4@SiO-NH-COOH in 5 min and reached 75% after 1 h [63]. The highest adsorption capacity in the present study was 192.15 mg/g at 90 min, higher than reported values as 157.9 mg/g in 60 min [64] and 118.55 mg/g in 180 min [65], highlighting the potential of the CPAC-Fe3O4 nanocomposite in Hg ion adsorption.
3.6 Effect of temperature
The increase in the temperature of a solution from 15 to 40 °C enhanced the removal efficiency in the range of 82.07 to 92.67% alongside the adsorption capacity from 164.15 to 185.35 mg/g (Fig. 6e). The temperature enhancement decreases the solution viscosity and accelerates the diffusion of Hg ions into the adsorbent, rapidly occupying the surface of the adsorbent in a shortened time [66, 67]. It should be noticed that the excessive mobility of the metal ions at high temperatures could lessen their accessibility and lower removal efficiency [15]. Although the most common optimal temperature for the adsorption process is an ambient temperature (25 °C), however, various temperature values were also reported. The adsorption capacity of the FeCu-based biochar was as high as 3901 ng/g at an optimal adsorption temperature of 200 °C [68]. The highest adsorption capacity of coconut pith-based char [69] was also achieved at 50 °C (2395.98 µg/g) for an initial Hg concentration of 200 µg/m3. Another study referred to a temperature of 150 °C for maximum Hg removal (120 µg/g) using sunflower husk-based char [70].
3.7 Adsorption isotherms and kinetics
The degree of pollutant adsorption onto the surface of the adsorbent commonly depends on the equilibrium concentration in the aqueous solution and temperature, while at a constant temperature, the adsorption capacity (qe) mainly relates to the final pollutant concentration (Ce) [2, 71]. Hence, Langmuir and Freundlich isotherm models were assessed to determine the Hg adsorption mechanism of the CPAC-Fe3O4 nanocomposite. Table 2 demonstrates the isotherm equations accompanied by the constant values and maximum adsorption capacity. The fitting results derived from the isotherm modeling are depicted in Fig. 7. As can be seen, the Freundlich isotherm model gave the highest R2 (0.9749) with a KF value of 434.85 mg/g (l/mg)1/n, fitting well with the empirical data. The isotherm results confirmed the multilayer adsorption of Hg ions and the heterogeneous surface of the CPAC-Fe3O4 nanocomposite [34, 52]. The value of 1/n in the Freundlich isotherm model was less than 1, indicating the desirable adsorption of Hg ions [47]. Bhatnagar et al. [72] modified pectin and cellulose composites with cocopeat biochar and SDS and highlighted the best fit with Freundlich isotherm, which showed multilayer chemisorption via complexation. The pseudo-first-order and pseudo-second-order kinetic models were employed to further analyze the adsorption kinetic (Table 2). The determination coefficient values demonstrated a better fit of empirical data with the pseudo-second-order kinetic model (R2 = 9997), manifesting that the adsorption of Hg ions through the CPAC-Fe3O4 nanocomposite followed a chemisorption process [73] as depicted in Fig. 7d. The qe value was 204.08 mg/g, higher than that reported in the literature [35, 56]. Sireesha and Sreedhar [32] noted that the chromium adsorption mechanism of H3PO4 surface-modified cocopeat biochar (PSMCB) was chemisorption and followed a pseudo-second-order kinetic model.
3.8 Thermodynamic studies
The CPAC-Fe3O4 nanocomposite performance in Hg removal was investigated at different temperatures (288, 293, 298, 303, 308, and 313 K). The enthalpy (∆H) and entropy (∆S) were estimated from the slope and intercept of 1/T versus lnKc. Gibbs free energy (∆G) was also calculated using the equation in Table 2. The negative values of ∆G presented the spontaneous nature of Hg adsorption [35]. Moreover, the increase in temperature from 288 to 313 K decreased the ∆G value from − 3.643 kJ/mol to − 6.604 kJ/mol, implying the endothermic adsorption of Hg ions. The positive and high value of ∆H verified the endothermic adsorption process and strong interaction between Hg ions and adsorbent [65]. The positive value of ∆S also presented the randomness adsorption of Hg ions due to the interference of water molecules in the solution [52, 74].
3.9 Comparison with other adsorbents
Table 3 compares the CPAC-Fe3O4 nanocomposite efficiency with other synthesized adsorbents for Hg removal. As can be seen in Table 3, more than 94% Hg removal was achieved for the various applied adsorbents, whereas a significant distinction was observed in adsorption capacity. Arshadi et al. [76] manifested the maximum adsorption efficiency of 3232 mg/L for Hg removal by a novel heterogeneous nanodendrimer. They increased the removal capacity through several pretreatment processes and heterogenized l-cysteine methyl ester dendrimer on the surface. A lower adsorption capacity was also reported for Hg removal due to differences in adsorbent characteristics and operational conditions [71, 77]. However, it is worth mentioning that the CPAC-Fe3O4 nanocomposite could remove 71.7% of Hg ions within 15 min, indicating the significant potential of the synthesized adsorbent. On the other hand, the maximum adsorption capacity was substantially higher than that of other adsorbents. Furthermore, the CPAC-Fe3O4 nanocomposite underlined high stability after 13 cycles with more than 80% Hg removal (Fig. 8). Therefore, the CPAC-Fe3O4 nanocomposite is recommended to be applied for Hg removal due to its simplicity, high potential, and low cost.
3.10 Adsorption mechanism
Various forms of Hg such as Hg2+, HgOH+, HgCl+, and Hg(OH)2 are present in an aqueous solution under pH < 3. The formation of Hg2+ is observed with pH enhancement and further dissolved Hg(OH)2 at pH higher than 6 [27]. When the pH of the solution increases more than 4, H3O+ ions decrease and more ionized functional groups are formed [79]. Oxygen functional groups such as carboxyl and carbonyl groups significantly contribute to mercury adsorption [80]. The reaction of Hg ions and these functional groups leads to the formation of surface complexes on the adsorbent surface. The chemical reactions are as follows [55]:
The role of the surface chemistry of CPAC-Fe3O4 nanocomposite on the adsorption mechanism was assessed using FTIR. The peaks at 1639.45 and 1733.21 cm−1 ascribed to the C = O group changed after the Hg adsorption and a new peak at 1629 cm−1 appeared, which was associated with O-Hg. A sharp peak at 841.62 cm−1 also decreased after adsorption, indicating chemisorbed C-O group contribution [81].
4 Conclusion
The application of low-cost and eco-friendly CPAC-Fe3O4 nanocomposite derived from cocopeat, a by-product of coconut husk processing, was apprised for Hg removal from aqueous solution. The CPAC-Fe3O4 nanocomposite illustrated spherical morphology with an average pore diameter of 2.27 nm. The saturation magnetization value was 2.5 emu/g, confirming the appropriate magnetic properties of the synthesized nanocomposite. The equilibrium adsorption capacity of the CPAC-Fe3O4 nanocomposite was 204.08 mg/g with a substantial Hg removal efficiency of 98%. The empirical data was well fitted with pseudo-second-order kinetic and the Freundlich isotherm models. In addition, Hg adsorption was endothermic and spontaneous based on thermodynamic study. It can be concluded that the CPAC-Fe3O4 nanocomposite can be an eco-friendly candidate with a noticeable capability for Hg removal.
Data availability
Not applicable.
References
Abyar H, Nowrouzi M, Rostami A (2022) A comprehensive study of biological phosphorus removal systems from economic and environmental perspectives based on the optimization approach. Environ Technol Innov 28:102811
Nowrouzi M, Abyar H, Rohani S (2023) A comparison of nitrogen removal systems through cost-coupled life cycle assessment and energy efficiency analysis. Sci Total Environ 858:159787
J Rajapakse M Otoo G Danso 2023 Progress in delivering SDG6: safe water and sanitation Cambridge Prisms: Water 1 e6
Barati AA, Pour MD, Sardooei MA (2023) Water crisis in Iran: a system dynamics approach on water, energy, food, land and climate (WEFLC) nexus. Sci Total Environ 882:163549
AMMA Caretta, RBM Arfanuzzaman, SMR Morgan, M Kumar Water. In: Climate change 2022: impacts, adaptation, and vulnerability. Contribution of Working Group II to the in: I.P.o.C. Change (Ed.) 2022
He C, Liu Z, Wu J, Pan X, Fang Z, Li J, Bryan BA (2021) Future global urban water scarcity and potential solutions. Nat Commun 12(1):4667
Mekonnen MM, Hoekstra AY (2016) Four billion people facing severe water scarcity. Sci Adv 2(2):e1500323
Dixit A, Madhav S, Mishra R, Srivastav AL, Garg P (2022) Impact of climate change on water resources, challenges and mitigation strategies to achieve sustainable development goals. Arab J Geosci 15(14):1296
Mikulčić H, Baleta J, Wang X, Duić N, Dewil R (2022) Sustainable development in period of climate crisis. J Environ Manage 303:114271
Stringer LC, Mirzabaev A, Benjaminsen TA, Harris RM, Jafari M, Lissner TK, Stevens N, Tirado-von Der Pahlen C (2021) Climate change impacts on water security in global drylands. One Earth 4(6):851–864
Attari M, Bukhari SS, Kazemian H, Rohani S (2017) A low-cost adsorbent from coal fly ash for mercury removal from industrial wastewater. J Environ Chem Eng 5(1):391–399
Monjane-Mabuie A, Mondlane-Milisse A, Pedro O, Leão-Buchir J, Correia D (2022) Mercury pollution assessment and metallothionein gene expression in tilapia (Oreochromis mossambicus): a case study of Revuè River in Manica, Mozambique. Rendiconti Lincei Scienze Fisiche e Naturali 33(3):513–526
Zarei S, Raanaei H, Niad M (2023) Investigation of mercury removal by Fe3O4@ SiO2-NH2-GO-NC as magnetic nanocomposite. Inorg Chem Commun 152:110665
Adibkia M, Tajjarod S, Talavari A, Noviery E, Azimi Maleki S (2017) The effect of amino acid coating on the performance of magnetic nanoparticles for the elimination of mercury from waste. Chem Metall Eng J 4(1):1–11
EC Emenike, AG Adeniyi, KO Iwuozor, CJ Okorie, AU Egbemhenghe, PE Omuku, KC Okwu, OD Saliu 2023 A critical review on the removal of mercury (Hg2+) from aqueous solution using nanoadsorbents Environ Nanotechnol Monit Manag 100816
Behjati M, Baghdadi M, Karbassi A (2018) Removal of mercury from contaminated saline wasters using dithiocarbamate functionalized-magnetic nanocomposite. J Environ Manage 213:66–78
EC Emenike, KO Iwuozor, SU Anidiobi Heavy metal pollution in aquaculture: sources, impacts and mitigation techniques Biol Trace Elem Res (2021) 1–17
Awual MR (2017) Novel nanocomposite materials for efficient and selective mercury ions capturing from wastewater. Chem Eng J 307:456–465
Chen J, Wang Y, Wei X, Xu P, Xu W, Ni R, Meng J (2018) Magnetic solid-phase extraction for the removal of mercury from water with ternary hydrosulphonyl-based deep eutectic solvent modified magnetic graphene oxide. Talanta 188:454–462
Igwegbe CA, Obiora-Okafo IA, Iwuozor KO, Ghosh S, Kurniawan SB, Rangabhashiyam S, Kanaoujiya R, Ighalo JO (2022) Treatment technologies for bakers’ yeast production wastewater. Environ Sci Pollut Res 29(8):11004–11026
Iwuozor KO (2019) Prospects and challenges of using coagulation-flocculation method in the treatment of effluents. Adv J Chem A 2(2):105–127
Saleh TA, Mustaqeem M, Khaled M (2022) Water treatment technologies in removing heavy metal ions from wastewater: a review. Environ Nanotechnol Monit Manag 17:100617
Talebi J, Halladj R, Askari S (2010) Sonochemical synthesis of silver nanoparticles in Y-zeolite substrate. J Mater Sci 45:3318–3324
Yaqoob AA, Ahmad H, Parveen T, Ahmad A, Oves M, Ismail IM, Qari HA, Umar K, Mohamad Ibrahim MN (2020) Recent advances in metal decorated nanomaterials and their various biological applications: a review. Front Chem 8:341
Nejati B, Adami P, Bozorg A, Tavasoli A, Mirzahosseini AH (2020) Catalytic pyrolysis and bio-products upgrading derived from Chlorella vulgaris over its biochar and activated biochar-supported Fe catalysts. J Anal Appl Pyrol 152:104799
Nowrouzi M, Younesi H, Bahramifar N (2017) High efficient carbon dioxide capture onto as-synthesized activated carbon by chemical activation of Persian Ironwood biomass and the economic pre-feasibility study for scale-up. J Clean Prod 168:499–509
Zhao Y, Xia K, Zhang Z, Zhu Z, Guo Y, Qu Z (2019) Facile synthesis of polypyrrole-functionalized CoFe2O4@ SiO2 for removal for Hg (II). Nanomaterials 9(3):455
Wang X, Zhang Z, Zhao Y, Xia K, Guo Y, Qu Z, Bai R (2018) A mild and facile synthesis of amino functionalized CoFe2O4@ SiO2 for Hg (II) removal. Nanomaterials 8(9):673
Bao S, Li K, Ning P, Peng J, Jin X, Tang L (2017) Highly effective removal of mercury and lead ions from wastewater by mercaptoamine-functionalised silica-coated magnetic nano-adsorbents: behaviours and mechanisms. Appl Surf Sci 393:457–466
Zhang S, Qian L, Zhou Y, Guo Y (2023) High selective removal towards Hg (II) from aqueous solution with magnetic diatomite-based adsorbent functionalized by poly (3-aminothiophenol): conditional optimization, application, and mechanism. Environ Sci Pollut Res 30(19):56121–56136
Lin Z, Pan Z, Zhao Y, Qian L, Shen J, Xia K, Guo Y, Qu Z (2020) Removal of Hg2+ with polypyrrole-functionalized Fe3O4/kaolin: synthesis, performance and optimization with response surface methodology. Nanomaterials 10(7):1370
S. Sireesha, I. Sreedhar 2023 Holistic and parametric optimization study on Cr (VI) removal using acid-treated coco peat biochar adsorbent, Bioresource Technology Reports 101486
Varghese SM, Chowdhury AR, Arnepalli DN, Rao GR (2023) Delineating the effects of pore structure and N-doping on CO2 adsorption using coco peat derived carbon. Carbon Trends 10:100250
Peer FE, Bahramifar N, Younesi H (2018) Removal of Cd (II), Pb (II) and Cu (II) ions from aqueous solution by polyamidoamine dendrimer grafted magnetic graphene oxide nanosheets. J Taiwan Inst Chem Eng 87:225–240
Venkateswarlu S, Yoon M, Kim MJ (2022) An environmentally benign synthesis of Fe3O4 nanoparticles to Fe3O4 nanoclusters: rapid separation and removal of Hg (II) from an aqueous medium. Chemosphere 286:131673
Yang L, Wang Z, Yang L, Li X, Zhang Y, Lu C (2017) Coco peat powder as a source of magnetic sorbent for selective oil–water separation. Ind Crops Prod 101:1–10
Okoli CP, Naidoo EB, Ofomaja AE (2018) Role of synthesis process variables on magnetic functionality, thermal stability, and tetracycline adsorption by magnetic starch nanocomposite. Environ Nanotechnol Monit Manag 9:141–153
Bunaciu AA, UdriŞTioiu EG, Aboul-Enein HY (2015) X-ray diffraction: instrumentation and applications. Crit Rev Anal Chem 45(4):289–299
Verma S, Kujur S, Sharma R, Pathak DD (2022) Cucurbit [6] uril-supported Fe3O4 magnetic nanoparticles catalyzed green and sustainable synthesis of 2-substituted benzimidazoles via acceptorless dehydrogenative coupling. ACS Omega 7(11):9754–9764
Tan YH, Davis JA, Fujikawa K, Ganesh NV, Demchenko AV, Stine KJ (2012) Surface area and pore size characteristics of nanoporous gold subjected to thermal, mechanical, or surface modification studied using gas adsorption isotherms, cyclic voltammetry, thermogravimetric analysis, and scanning electron microscopy. J Mater Chem 22(14):6733–6745
Ncibi M, Jeanne-Rose V, Mahjoub B, Jean-Marius C, Lambert J, Ehrhardt J, Bercion Y, Seffen M, Gaspard S (2009) Preparation and characterisation of raw chars and physically activated carbons derived from marine Posidonia oceanica (L.) fibres. J Hazard Mater 165(1–3):240–249
Fu H, Yan D, Yao C, Su X, Wang X, Wang H, Li Y (2022) Pore structure and multi-scale fractal characteristics of adsorbed pores in marine shale: a case study of the Lower Silurian Longmaxi shale in the Sichuan Basin China. J Earth Sci 33(5):1278–1290
Kianfar E (2018) Synthesis and characterization of AlPO4/ZSM-5 catalyst for methanol conversion to dimethyl ether. Russ J Appl Chem 91(10):1711–1720
Mangi HN, Chi R, DeTian Y, Sindhu L, He D, Ashraf U, Fu H, Zixuan L, Zhou W, Anees A (2022) The ungrind and grinded effects on the pore geometry and adsorption mechanism of the coal particles. J Nat Gas Sci Eng 100:104463
Ebadi M, Rifqi Md Zain A, Tengku Abdul Aziz TH, Mohammadi H, Tee CA, Rahimi Yusop M (2023) Formulation and characterization of Fe3O4@ PEG nanoparticles loaded sorafenib; molecular studies and evaluation of cytotoxicity in liver cancer cell lines. Polymers 15(4):971
Ma Z, Liu F, Liu N, Liu W, Tong M (2021) Facile synthesis of sulfhydryl modified covalent organic frameworks for high efficient Hg (II) removal from water. J Hazard Mater 405:124190
Einollahipeer F, Okati N (2022) High efficient Hg (II) and TNP removal by NH2 grafted magnetic graphene oxide synthesized from Typha latifolia. Environ Technol 43(25):3956–3972
Ahmad M, Wang J, Xu J, Yang Z, Zhang Q, Zhang B (2020) Novel synthetic method for magnetic sulphonated tubular trap for efficient mercury removal from wastewater. J Colloid Interface Sci 565:523–535
Ge H, Hua T, Wang J (2017) Preparation and characterization of poly (itaconic acid)-grafted crosslinked chitosan nanoadsorbent for high uptake of Hg2+ and Pb2+. Int J Biol Macromol 95:954–961
Das S, Samanta A, Kole K, Gangopadhyay G, Jana S (2020) MnO2 flowery nanocomposites for efficient and fast removal of mercury (II) from aqueous solution: a facile strategy and mechanistic interpretation. Dalton Trans 49(20):6790–6800
Zhang Z, Xia K, Pan Z, Yang C, Wang X, Zhang G, Guo Y, Bai R (2020) Removal of mercury by magnetic nanomaterial with bifunctional groups and core-shell structure: synthesis, characterization and optimization of adsorption parameters. Appl Surf Sci 500:143970
Awad FS, AbouZied KM, Abou El-Maaty WM, El-Wakil AM, El-Shall MS (2020) Effective removal of mercury (II) from aqueous solutions by chemically modified graphene oxide nanosheets. Arab J Chem 13(1):2659–2670
Khorshidi P, Shirazi RHSM, Miralinaghi M, Moniri E, Saadi S (2020) Adsorptive removal of mercury (II), copper (II), and lead (II) ions from aqueous solutions using glutathione-functionalized NiFe2O4/graphene oxide composite. Res Chem Intermed 46:3607–3627
Falahian Z, Torki F, Faghihian H (2018) Synthesis and application of polypyrrole/Fe3O4 nanosize magnetic adsorbent for efficient separation of Hg2+ from aqueous solution. Global Chall 2(1):1700078
Asasian N, Kaghazchi T, Soleimani M (2012) Elimination of mercury by adsorption onto activated carbon prepared from the biomass material. J Ind Eng Chem 18(1):283–289
Naushad M, Ahamad T, AlOthman ZA, Ala’a H (2019) Green and eco-friendly nanocomposite for the removal of toxic Hg (II) metal ion from aqueous environment: adsorption kinetics & isotherm modelling. J Mol Liq 279:1–8
Ifthikar J, Jiao X, Ngambia A, Wang T, Khan A, Jawad A, Xue Q, Liu L, Chen Z (2018) Facile one-pot synthesis of sustainable carboxymethyl chitosan–sewage sludge biochar for effective heavy metal chelation and regeneration. Biores Technol 262:22–31
Xia K, Guo Y, Shao Q, Zan Q, Bai R (2019) Removal of mercury (II) by EDTA-functionalized magnetic CoFe2O4@ SiO2 nanomaterial with core-shell structure. Nanomaterials 9(11):1532
Huang S, Ma C, Liao Y, Min C, Du P, Jiang Y (2016) Removal of mercury (II) from aqueous solutions by adsorption on poly (1-amino-5-chloroanthraquinone) nanofibrils: equilibrium, kinetics, and mechanism studies. J Nanomater 2016:7245829
Mensah MB, Lewis DJ, Boadi NO, Awudza JA (2021) Heavy metal pollution and the role of inorganic nanomaterials in environmental remediation. R Soc Open Sci 8(10):201485
Rahmanzadeh L, Ghorbani M, Jahanshahi M (2016) Effective removal of hexavalent mercury from aqueous solution by modified polymeric nanoadsorbent. J Water Environ Nanotechnol 1(1):1–8
AlOmar MK, Alsaadi MA, Jassam TM, Akib S, Hashim MA (2017) Novel deep eutectic solvent-functionalized carbon nanotubes adsorbent for mercury removal from water. J Colloid Interface Sci 497:413–421
Tabatabaiee Bafrooee AA, Ahmad Panahi H, Moniri E, Miralinaghi M, Hasani AH (2020) Removal of Hg2+ by carboxyl-terminated hyperbranched poly (amidoamine) dendrimers grafted superparamagnetic nanoparticles as an efficient adsorbent. Environ Sci Pollut Res 27:9547–9567
Zhang Y, Yan L, Xu W, Guo X, Cui L, Gao L, Wei Q, Du B (2014) Adsorption of Pb (II) and Hg (II) from aqueous solution using magnetic CoFe2O4-reduced graphene oxide. J Mol Liq 191:177–182
Cui L, Guo X, Wei Q, Wang Y, Gao L, Yan L, Yan T, Du B (2015) Removal of mercury and methylene blue from aqueous solution by xanthate functionalized magnetic graphene oxide: sorption kinetic and uptake mechanism. J Colloid Interface Sci 439:112–120
Goci MC, Leudjo Taka A, Martin L, Klink MJ (2023) Chitosan-based polymer nanocomposites for environmental remediation of mercury pollution. Polymers 15(3):482
Liu F, Liu Y, Xu Y, Ni L, Meng X, Hu Z, Zhong G, Meng M, Wang Y, Han J (2015) Efficient static and dynamic removal of Sr (II) from aqueous solution using chitosan ion-imprinted polymer functionalized with dithiocarbamate. J Environ Chem Eng 3(2):1061–1071
Zhao R, Jia L, Yao Y-X, Huo R-P, Qiao X-L, Fan B-G (2019) Study of the effect of adsorption temperature on elemental mercury removal performance of iron-based modified biochar. Energy Fuels 33(11):11408–11419
Johari K, Saman N, Song ST, Cheu SC, Kong H, Mat H (2016) Development of coconut pith chars towards high elemental mercury adsorption performance–effect of pyrolysis temperatures. Chemosphere 156:56–68
Fuente-Cuesta A, Diaz-Somoano M, Lopez-Anton M, Cieplik M, Fierro J, Martínez-Tarazona M (2012) Biomass gasification chars for mercury capture from a simulated flue gas of coal combustion. J Environ Manage 98:23–28
Safari N, Ghanemi K, Buazar F (2020) Selenium functionalized magnetic nanocomposite as an effective mercury (II) ion scavenger from environmental water and industrial wastewater samples. J Environ Manage 276:111263
Bhatnagar P, Sireesha S, Siddiqui S, Sreedhar I (2023) Novel pectin-cellulose-biochar composite with SDS modification for copper removal: optimization, characterization, and regeneration. Bioresour Technol Rep 21:101382
Samaniego JO, Tanchuling MAN (2019) Removal of heavy metals from an actual small scale gold mining wastewater by sorption onto Cocopeat. ASEAN J Sci Technol Dev 36(1):1–7
Hosseini-Bandegharaei A, Hosseini MS, Jalalabadi Y, Sarwghadi M, Nedaie M, Taherian A, Ghaznavi A, Eftekhari A (2011) Removal of Hg (II) from aqueous solutions using a novel impregnated resin containing 1-(2-thiazolylazo)-2-naphthol (TAN). Chem Eng J 168(3):1163–1173
Jena KK, Reddy KSK, Karanikolos GN, Choi DS (2023) l-Cysteine and silver nitrate based metal sulfide and Zeolite-Y nano adsorbent for efficient removal of mercury (II) ion from wastewater. Appl Surf Sci 611:155777
Arshadi M, Mousavinia F, Khalafi-Nezhad A, Firouzabadi H, Abbaspourrad A (2017) Adsorption of mercury ions from wastewater by a hyperbranched and multi-functionalized dendrimer modified mixed-oxides nanoparticles. J Colloid Interface Sci 505:293–306
Chen F, Ma N, Peng G, Xu W, Zhang Y, Meng F, Huang Q, Hu B, Wang Q, Guo X (2023) Camellia oleifera shell biochar as a robust adsorbent for aqueous mercury removal. Fermentation 9(3):295
Esrafili A, Ghambarian M, Tajik M, Baharfar M (2020) Adsorptive removal of Hg2+ from environmental water samples using thioglycerol-intercalated magnetic layered double hydroxides. Anal Methods 12(17):2279–2286
Hadi P, To M-H, Hui C-W, Lin CSK, McKay G (2015) Aqueous mercury adsorption by activated carbons. Water Res 73:37–55
Sun X, Hwang J-Y, Xie S (2011) Density functional study of elemental mercury adsorption on surfactants. Fuel 90(3):1061–1068
Duan L, Hu X, Sun D, Liu Y, Guo Q, Zhang T, Zhang B (2020) Rapid removal of low concentrations of mercury from wastewater using coal gasification slag. Korean J Chem Eng 37:1166–1173
Funding
This work was supported by Gorgan University of Agricultural Sciences and Natural Resources, Iran [grant number 01–474-96].
Author information
Authors and Affiliations
Contributions
Hassan Rezaei: conceptualization, investigation, methodology, and supervision; Negar Movazzaf Rostami: investigation, methodology, original draft; Hajar Abyar: data analysis, original draft, and draft review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rezaei, H., Rostami, N.M. & Abyar, H. Magnetic nanocomposite synthesized from cocopeat for highly efficient mercury removal from aqueous solutions. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05425-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05425-4