Abstract
In this study, we utilize the agriculture waste of Portulaca quadrifida fiber-reinforced composite materials for lightweight structural and other engineering materials. A minimum amount of work was done on this Portulaca quadrifida fiber composite. Foremost is the attempt to extract Portulaca quadrifida fibers from the retting process. The extracted fibers are treated with 5%, 10%, and 15% alkali solutions. The physical, chemical, mechanical, thermal, and morphological properties of Portulaca quadrifida fiber composites are the focus of this investigation. For the 15% alkali-treated single fiber the maximum tensile strength attained is 5.45 MPa, whereas the untreated fiber is 2.70 MPa. From the chemical analysis, results showed that alkali treatment with 15% had a lower cell wall consistent with cellulose, hemicellulose, lignin, etc. On the surface of the treated fiber, the removal of contaminants, oil, particulates, and waxes caused the 5% modified fiber surfaces to be rough. Due to the acid carbonyl’s absorption, the band was visible at 1625 cm−1. The non-aromatic C–H stretching in all of the treated fibers is confirmed by the absorption bands at 3700–2950 cm−1 in the case of NaOH with 5%, 10%, and 15% treated composites. Thus, the CI values for untreated, alkali treated with 5%, 10%, and 15% of Portulaca quadrifida composite were 40.02% 56.25%, 84.31%, and 90.06%, respectively. Using Portulaca quadrifida fiber composites, water uptake ability can be decreased after alkali treatment. A lightweight material application is recommended for Portulaca quadrifida fiber composites based on the results of this study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The current scenario is moving towards the manufacturing of green products. The contribution of natural fibers to green products lies in their high strength-to-weight ratio, affordability, abundant availability, eco-friendliness, and biodegradability. It makes them a great choice for creating sustainable products that have a low impact on the environment [1]. The extraction of natural fiber from mature fruit stems and leaves has great potential for physical and chemical strength, which act as shock absorbers, acoustic insulation, and packing materials [2, 3]. Natural fiber serves socio-environmental purposes, such as the extraction of oil or bio-diesel from natural fiber, which reduces CO2 emissions [4]. Savio et al. [5] have reviewed a thermal insulation sheet made from natural fiber. Such a sheet maintains humidity control and decreases the evaporation rate while maintaining 17 °C in the inner chamber. The biodegradable nature of plant-based natural fiber composites has made them an essential substitute material for synthetic fiber-reinforced polymer matrix composites. Reducing the amount of synthetic fiber used and cutting greenhouse gas emissions can be achieved by using more natural fiber as reinforcement in composite products [6, 7].
The natural fiber is a hollow and lignocellulose in nature, that is collection of cellulose, hemicellulose, lignin, pectin, and waxy substances. Hence, increased moisture absorption and higher wettability increase the strength of natural fiber composites [8, 9]. The authors, Gopinath et al. [10] separated fiber from the Leucaena leucocephala tree and studied its mechanical and thermal properties. Results showed that the fiber exhibited the highest tensile strength and modulus and was thermally stable up to 188 °C. Overall, the results of this study revealed that L. leucocephala tree fiber has potential applications in the engineering industry. Similar studies have been conducted by Selvaraj et al. [11] on fibers extracted from Ageratina adenophora. Consequently, these fibers are applied in industrial applications due to their thermal stability at under polymerization temperatures. Furthermore, the strength of a natural fiber composite is also dependent on the fiber structure and the fiber-matrix interface. Therefore, proper surface modification of the fiber is necessary to improve the strength of the fiber composite [12]. Many researchers are concentrating on improving the strength of natural fiber through various surface treatments. Alkali treatment disturbed the hydrogen bonding of lignin and wax and caused crystallinity changes. During the treatment, cellulose groups swelled due to the adjacent binding of lignin and wax, which could be dissolved in solvents [13, 14]. The alkali solution affected the hydroxyl group (OH) of the fiber, which reacted with water (H–OH) and was removed from the fiber surface. The remaining reactive molecules form between the cellulose molecular chains [15]. This increased the cellulose crystallinity, resulting in higher stiffness and strength. The surface of the cellulose fiber was also modified, leading to improved reactivity and dyeability. The researchers used the following alkaline reactants and solutions: sodium hydroxide, sodium sulfite, and sodium carbonate [16,17,18,19]. The solutions were used to modify the surface of the cellulose fibers at different temperatures and concentrations. The results showed that the modified fibers had improved dyeability and reactivity [20]. Moreover, the modified fibers also displayed enhanced mechanical, thermal, and chemical properties when compared to untreated natural fibers [21]. Ramasamy et al. [22] found that the magnesium carbonate treatment enhanced the tensile strength of the Cissus quadrangularis. As a result, it increased its resistance to elongation and decreased its water absorption. All of these properties are desirable in fiber materials, making them better suited for applications that require strength and durability. Madhu et al.[23] studied a new study on the effect of alkali treatments on agave americana fiber (AAF) for composite reinforcement. The authors discovered that all of the modified AAFs had significantly enhanced tensile strength and elongation at break, but at the expanses of a decreased Young’s modulus, which is linked to a significant variance in fiber dimensions following the treatment.
Physical, chemical, and morphological changes were observed after the various surface treatments of natural fibers [6, 7]. The removal of cell walls due to treatment results in the mass loss (16–40%) of the fiber, which changes the density of the fiber. This affects the mechanical and thermal properties of the fiber, which leads to an increase in the stiffness and strength of the fiber [24]. Based on FTIR analysis conducted among the cell groups in natural fibers a peak variation of hemicellulose (1700 cm−1), ester, phenol, and carboxyl groups (1238 cm−1), and deformation of cellulose and hemicellulose groups (1370–1390 cm−1) are present in untreated fiber. Cellulose hydroxyl groups (3300 cm−1) are observed in untreated fiber, but they are lower in treated fiber. The decrease in these groups implies that the treated fiber has a lower degree of crystallinity. Furthermore, the treated fiber shows an increase in amide groups (1650 cm−1), which is due to the addition of amide groups via the chemical treatment process [25, 26]. Change in crystallinity index between untreated and treated fiber was examined through XRD analysis. In treated fiber, the crystalline nature of the cellulose and the amorphous nature of the lignin and hemicellulose differ from raw fiber [27]. Treated fiber topography was wavier, rougher, and had some scratches on the surface after the removal of waxy, hemicellulose, and non-cellulosic substances [28, 29]. As part of the development of lightweight, degradable composite fabrication for lightweight materials, Rao et al. [30] characterized fiber extracted from Careya arborea. The authors found cell wall constituents are cellulose, lignin (71%), 14% with a crystal size of 7.4 nm and a crystallinity of 85%. But Bauhinia vahlii fiber has cellulose 75% and lignin 13% with crystallinity 56% [31]. Similarly, extracted fiber from Glycyrrhiza glabra has cellulose and lignin, which are 40% and 12% [32]. But the average density of these Careya arborea, Bauhinia vahlii, and Glycyrrhiza glabra fiber is 1.1–1.47 g/cm3.
Portulaca quadrifida is a relatively common agricultural waste. It is not as widely used by humans as other plants like spinach, bananas, sisal, etc. Stem fibers extracted from Portulaca quadrifida were subjected under alkali treatment, and their effects on the physical and chemical properties such as single fiber tensile strength, morphological analysis, crystallinity index, Fourier transform infrared spectroscopy analysis, mechanical testing, thermogravimetric analysis, water absorption were explored.
2 Experimental details
2.1 Material
The reinforcement is a Portulaca quadrifida that is sourced from a farm in Sivakasi, Tamil Nadu, India. Then, the plants are divided into their parts once they have been gathered. Among the matrix materials, we purchased two-pack epoxy (Araldite, LY556) and hardener (Aradur, HY917) from Araldite, Tamil Nadu, India. Sodium hydroxide (NaOH), a chemical of analytical grade, was supplied by Nice in Tamil Nadu, India.
2.1.1 Portulaca quadrifida fiber extraction
Initially, the Portulaca quadrifida agricultural waste was found in Tamil Nadu, India’s southern region. The majority of agricultural grounds are covered with this shrub. This Portulaca quadrifida plant stem often has yellow blooms and a tiny leaf, about 2 to 3 cm in length. Then, the Portulaca quadrifida plant is harvested from agricultural grounds. After being gathered, the Portulaca quadrifida plants were dried. The plant is then divided into its constituent parts, such as its leaves, petioles, and roots. Fiber from Portulaca quadrifida plants is harvested by using their stems. The retting process is the only method used to work with Portulaca quadrifida plants. In our opinion, no fiber extraction work had to be done on the Portulaca quadrifida plant before. The stems and fibers extracted from Portulaca quadrifida are shown in Fig. 1.
2.1.2 Sodium hydroxide treatment (NaOH)
Prior to alkalization (NaOH) treatment, the Portulaca quadrifida was washed with ordinary distilled water to eliminate any impurities on the fibers. The dry fiber was immersed in a concentration of 5%, 10%, and 15% alkali solution for 1 h at room temperature (30 °C). After that, the fiber was then rinsed with distilled water [33]. To eliminate any remaining moisture, the fibers are then dried in an oven set at 100 °C. Table 1 illustrates the notation used in this experimental work.
2.1.3 Composite preparation
Retting extraction procedures are utilized to separate the fibers from Portulaca quadrifida plant stem. Subsequently, a 10:1 mixture of epoxy and hardener is added to the matrix materials. Composites are made using an epoxy matrix and reinforcement Portulaca quadrifida fiber that has been alkali-treated and filled into a molding plate with dimensions of 300 × 300 × 3 mm. Then matrix materials are poured into the fiber filled mold cavity. In order to avoid any voids forming when applying epoxy to the Portulaca quadrifida fiber, a pressure of 1.8 MPa is then applied. During the 12-h curing period, the mold was maintained at this pressure at room temperature. As per ASTM standards, the specimens were cut into pieces after de-molding.
2.2 Characterization
2.2.1 Single fiber tensile strength
The tensile properties of Portulaca quadrifida natural fiber are measured using INSTRON—5500R universal testing machine. In this experiment, five fiber samples of the same 30-mm length are investigated, and their values are noted. In accordance with ASTM D3822-7, this single fiber tensile strength test was performed at 65% relative humidity, 21 °C ambient temperature, and 0.1 mm/min test speed [34].
2.2.2 Physical and chemical aspects of Portulaca quadrifida fibers
The Klason method was used to determine the amount of lignin in each fraction. A solvent extraction method based on hydrocarbons was used to determine the wax content of each component [30]. By using a pycnometer technique and distilled water as an immersion liquid, density was evaluated. The samples were exposed to mineral acid at a high temperature for 30 min to identify hemicellulose. An alkali solution containing the residual sample was heated to a high temperature and agitated, after which the extract was dried and weighed. The hemicelluloses are taken out by an alkali treatment. After subjecting the samples to a high-temperature alkali solution and mineral acid treatment, the presence of cellulose was confirmed. The sample was then bleached at a high temperature at the next stage. Cellulose was the precipitate that formed as a result, and it was repeatedly cleaned with hot deionized water to bring the filtrate’s pH to a neutral level [21]. The final result was calculated using the average of five samples and a standard deviation.
2.2.3 Morphological analysis
Using scanning electron microscope (Carl Zeiss V18, Germany), a morphological examination of treated and untreated fibers was carried out with acceleration at 20 kV, and the working distance was 15.31 mm.
2.2.4 Crystallinity index
By using an X-ray diffractometer (D8 advance ECO XRCD—Bruker) with monochromatic Cu Kα radiation and operating parameters of λ = 1.544°A, 43 kV, and 32 mA, the structure of Portulaca quadrifida fiber-reinforced composite was examined [3]. It was possible to get the X-ray spectra of both treated and untreated Portulaca quadrifida fiber composites. The employed scanning parameters were 2θ = 5 – 90˚ and 0.05 deg/min speed. In the Portulaca quadrifida fiber composite spectrum, the integrated intensities of the Bragg peaks were identified. Based on the procedure used, the following Eq. (1) was used for assessing their crystallinity indices:
2.2.5 Fourier transform infrared spectroscopy analysis
Prior to being milled into a fine powder using a mortar and pestle, the Portulaca quadrifida fiber composite samples had to be chopped up into little pieces. In order to record using IR Tracer—100 Shimadzu, Japan under standard conditions, this powder was combined with kBr and pelletized by pressurization [31]. A KBr matrix was scanned at a rate of 61 scans per minute using a Bruker FTIR spectrometer. In the range of wave numbers between 500 and 4000 cm−1, it exhibited a resolution of 3 cm−1.
2.2.6 Mechanical testing
Alkali treatment is applied to the Portulaca quadrifida fiber reinforcement at 5%, 10%, and 15%, respectively. On an Instron universal testing machine of type 5500 R, with composite samples of 50-mm gauge length, the tensile properties of Portulaca quadrifida fibers reinforced with epoxy composites were assessed according to the ASTM D 3379 standard [31]. The test conditions were as follows: cross head speed of 0.1 mm/min, an ambient temperature of 21 °C, and a relative humidity of roughly 61%. A pneumatic gripper with an applied pressure of 0.4 MPa was employed in a load cell with a weight of 1.0 kN. An ASTM D790 three-point bending test was conducted using Instron 5500R universal testing equipment with a maximum capacity of 150KN/15Ton to assess the flexural strength of the composite [6]. The test conditions were as follows: cross head speed of 0.1 mm/min, ambient temperature of 21 °C, and a relative humidity of roughly 61%. In accordance with ASTM D256, the impact energy absorption of the composite was measured using a versatile impact testing machine (Werkstoff Pruf Maschine Leipzig (WPM), Germany) with a maximum load capacity of 30 kph [8]. Five samples were examined under each condition to account for the diversity of natural fibers, and an average result was given.
2.2.7 Thermogravimetric analysis
On both treated and untreated Portulaca quadrifida fiber-reinforced composites, TG analysis was carried out using METTLER-2, Germany. The test was performed using a sample mass of approximately 2.5 mg [30]. The range of temperatures was 30 to 600 °C. Additionally, a 10 K/min heating rate was maintained. A nitrogen environment with a flow rate of 60 mL/min was used to conduct the TG analysis.
2.2.8 Water absorption test
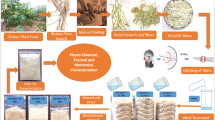
The Portulaca quadrifida composite sample is placed into 100 mL of water in accordance with ASTM D570 [35]. Continuous measurements are performed on the composite sample for a period of 72 h. Every 12 h, the composite sample’s water absorption measurements are observed. Figure 2 illustrates the methodology of the Portulaca quadrifida fiber-reinforced composite.
3 Result and discussion
3.1 Single fiber tensile strength of Portulaca quadrifida fiber
Specimens of Portulaca quadrifida natural fiber are selected at 5%, 10%, and 15% from the alkali treatment, and Fig. 3 provides a good explanation of the tensile strength. The percentage of alkali treatment had a significant impact on the tensile strength in this case [36]. The maximum tensile strength of Portulaca quadrifida fiber treated with 15% alkali is 5.45 MPa, whereas the untreated fiber is 2.70 MPa.
3.2 Chemical composition of Portulaca quadrifida fiber
The chemical composition in terms of the quantity of untreated and treated fiber is displayed in Table 2. Contrarily, untreated fiber had a cellulose content of 73.92%, whereas 15% alkali-treated fiber had the lowest cellulose content at 64.06%. As can be observed, the 10% and 5% have been exposed to 69.37% and 71.19%, respectively. This could be because non-cellulosic fiber surface components have been removed [33]. In terms of fiber tensile strength, the cellulose content is quite important. The crystallinity values determined by X-ray diffraction examinations support the measured cellulose content of the various percentages of alkali-treated fibers. Additionally, the untreated fiber’s hemicellulose level was found at 14.24%. Hemicellulose values declined after alkali treatment and the following treatments. As compared with untreated fibers, all types of treated fibers experienced a significant drop in lignin content [37]. This indicates that lignin and some contaminants are removed from the fiber through alkali treatment using various percentage. The removal of substances from the fiber surface causes wax content to drop as well as alkali treatment of the fibers.
Additionally, for fibers treated with NaOH at 5%, 10%, and 15%, the corresponding moisture contents were 8.09%, 8.82%, 8.87%, and 9.54%. A further improvement in wax content contributed to the improvement in moisture content. Due to the wax’s ability to absorb water, the moisture content of the fiber rose after treatment. The ash content of fibers treated with 10% was the lowest, being nearly 0.82% smaller than that of untreated fibers and 2.14% smaller than that of the alkali treatment used in this study. On the other hand, the ash content of fibers treated at 5% and 15% was less than that of untreated fibers. The fiber has greater fire resistance with a lower ash content. For applications requiring fire resistance, 15% treated fibers might be preferred. The untreated fiber density was recorded at 1.32 g/cc. Following 5%, 10%, and 15% treatments, it rose to 1.28 g/cc, 1.24 g/cc, and 1.16 g/cc, respectively. This might be brought on by the denaturation of cell walls and an increase in moisture content [26]. Densification of the cell wall shows that depolymerization and damage to the cell wall are not present. As a result, cellulose, hemicellulose, and lignin in cell components decreased. Table 3 provides a detailed comparison of the many features of several natural fibers that have been explored by previous researchers.
3.3 Morphological analysis of Portulaca quadrifida fiber
Fibers from Portulaca quadrifida, the surface structure of both treated and untreated, underwent morphological investigation, as seen in Fig. 4. The fibrils were aligned along the fiber axis length-wise, and the fiber structure was frequently perceived as cylindrical. On the surface of the untreated fiber, one can see several imperfections and a very rough surface (Fig. 4a). The fiber structure also significantly changed after the alkali treatment [18]. The removal of contaminants, oil, particulates, and waxes caused the 5% modified fiber surfaces to be rough (Fig. 4b). The surfaces of the other two treated ones, 10% treated (Fig. 4c) and 15% treated (Fig. 4d), were contrasted with this. The fiber surface also seemed to be spongy. This study’s findings led to the conclusion that alkalization had a severe impact on the fiber surface compared to untreated fiber.
3.4 X-ray diffraction analysis of Portulaca quadrifida fiber composites
Figure 5 depicts the XRD spectra of the amorphous and crystalline structures of untreated and treated Portulaca quadrifida fiber composites, showing their characteristics.
The Segal empirical method equation was used to get the crystallinity index:
Iam is the intensity of the amorphous material, while I002 is the highest intensity of the crystalline material. As a result of the hemicellulose present in the sample, all C = O groups have been removed [30]. In the high intensity samples, there was a significant improvement in the amount of cellulose present in the reinforcement fibers. Thus, the CI values for untreated, alkali treated with 5%, 10%, and 15% of Portulaca quadrifida composite were 40.02% 56.25%, 84.31%, and 90.06%, respectively. It is evident that fiber treatments with alkali at 5%, 10%, and 15% had an impact on fiber differences and geographical location [13]. The first peaks of 15% and 5% are located at locations 2θ = 15.47˚ and 15.17˚ on the crystallographic plane [002]. In the crystallographic plane [110], there is a second peak that is located at 2θ = 21.85˚ and 22.57˚, respectively. Because these fibers’ structure was more orderly after NaOH treatment, a higher degree of crystallinity index was seen in comparison. Due to the increased proportion of amorphous components in 15% treated fibers, the ordered structure was found to be lower. As a result, the fiber’s crystallinity improved after alkali treatment. Additionally, the rise in hemicellulose and lignin values may have contributed to the decline in crystallinity. The mechanical strength of the composite samples increases as a result of these increased crystallinity index values.
3.5 FTIR of Portulaca quadrifida fiber composites
The FTIR spectra of Portulaca quadrifida fiber composites that have been untreated and alkali treated with 5%, 10%, and 15% are shown in Fig. 6. The figure shows that the spectrum for the untreated fiber composite exhibits absorption bands between 1297 and 976 cm−1, which is comparable to that seen for other treated fibers. The hydrophilic properties of the fiber cause this band to appear [50]. Alkyl chains are indicated by these, which are related to aliphatic C–H stretching vibrations. Due to the acid carbonyl’s absorption, the band was visible at 1625 cm−1. The non-aromatic C–H stretching in all of the treated fibers is confirmed by the absorption bands at 3700–2950 cm−1 in the case of NaOH with 5%, 10%, and 15% treated composites. In NaOH-treated fiber composites, the strong absorption band, which is 1425 cm−1 in untreated fiber composite, is moved to 1127 cm−1 [31]. This might be a result of the sodium carboxylate group forming a salt. However, fibers that had been exposed to alkalization did not exhibit any alteration in this band. This shows that, despite what has been seen by others, the organic portion of the fibers of Portulaca quadrifida did not undergo any structural changes after being exposed to sodium hydroxide. In contrast, hemicellulose dissolves the C = O groups in Portulaca quadrifida fiber-reinforced composites. When the reinforcement fiber’s cellulose content peaks, peak intensities in composite samples increase. These remaining peaks were observed in the raw fibers of Portulaca quadrifida.
3.6 Mechanical properties of Portulaca quadrifida fiber composites
The tensile strength of untreated and alkali treated Portulaca quadrifida composites is displayed in Table 4. The average tensile strength values for each of the five composites were plotted for this purpose. Untreated fiber composites exhibit the characteristic slow increase in strength with modulus, as shown in the Table 4. Portulaca quadrifida fibers exposed to high strength and modulus show lower strength than those that were alkali treated at 15%, followed by 10% and 5%. In comparison to alkali-treated fibers, untreated fibers exhibit the lowest tensile properties across all categories [37]. Thus, 10% treatment might reduce the swelling of fibers and improve strength properties by forming cross-linked networks. Fifteen percent treatments of Portulaca quadrifida fibers may produce high tensile properties as a result of increased load bearing capacity. The produced composites were conducted through flexural testing in accordance with ASTM D790 [51, 52]. The impact of flexural characteristics on alkali-treated epoxy composites reinforced with Portulaca quadrifida. For every combination of composites, a single-axis bending load is applied to the specimen in the traverse direction. The manufactured composites attained a flexural strength ranging from 36.69 to 61.32 MPa, respectively. After the elimination of naturally occurring agents such as lignin, cellulose, the fiber’s strength improved with a 15% alkali treatment and exhibited a 50% improvement [25]. The elimination of contaminants and the waxy coating on the fiber surfaces are the reasons for the improvement in flexural strength. This allows for better bonding between the Portulaca quadrifida and epoxy matrix. Table 4 illustrates the effect of alkali-treated Portulaca quadrifida reinforced epoxy composite on impact strength. For every combination of composites, a sudden load with a pendulum weight of 3.567 kg and a velocity of 2 m/s strikes the specimen in a horizontal direction. The produced composites’ impact strength ranged from 0.235 to 0.572 kJ/m2. A high impact strength composite was achieved for 15% of the treated specimens because sodium (Na) and hydroxide (OH) formed polyfunctional groups bonded with fibers and matrix. Compared to treated composites, untreated Portulaca quadrifida is less resistant to sudden loads acting on the specimen due to its naturally occurring hydroxyl group (waxy, cellulose, lignin, etc.). Table 5 provides a detailed comparison of the many features of several natural fibers that have been explored by previous researchers.
3.7 Thermogravimetric (TG) analysis of Portulaca quadrifida fiber composites
The TG and DTG curves of Portulaca quadrifida fiber-reinforced composites are shown in Figs. 7 and 8. Temperatures between 35 and 130 °C were used to remove moisture and other volatile matter [30]. Additionally, the TG plot revealed that alkali-treated fiber composites displayed higher thermal stability than untreated fiber composites below 220 °C. At 220 °C, the curve’s shape changed. In comparison to 5%, 10% treated, and untreated fibers, the 15% alkali-treated fiber composite showed greater thermal stability. In contrast, cellulose, which forms the majority of fibers, degrades between 130 and 400 °C, peaking at 350 °C. When lignin thermally decomposes, various flammable organic compounds are created between 260 and 400 °C. Except for the 5% treated composite, where 70% of the fibers were degraded at roughly 324 °C, 60% of the components were degraded in every fiber, as seen in Fig. 7. Up until a maximum of 80% of the total weight is absorbed, a very slow decline is observed [13], except for fibers treated with 15%, where residual sodium weight must have contributed to its weight in some way. As a result of the reaction between residual sodium hydroxide and an unknown substance, either sourced from the atmosphere or from the fiber, an unusual exothermic reaction occurred at 580 °C in a sodium hydroxide-treated composite with a slim gain in weight, which eventually drained out according to the TGA analysis. Since it is difficult to differentiate cellulose degradation products from hemicellulose degradation products, hemicellulose is often partially removed by 5% and 10% at temperatures between 125 and 195 °C. DTG curves indicates the 15% treated composite shows a higher peak value when compared to the 5%, 10% treated, and untreated composites.
Here, the DTG results of Portulaca quadrifida fiber that had been both untreated and treated composite were compared to the degradation processes. Figure 8 shows this phenomenon in untreated and sodium hydroxide-treated composites, as well as to a lesser extent, in 15% treated fiber [48]. It overlaps with other peaks in the sodium hydroxide-treated fiber case. The degradation of cellulose can be seen at 315 °C in all spectra, as previously mentioned. In addition, a broad peak around 160–405 °C was visible, which is probably the result of lignin component decomposition. A 3–6% mass loss was seen at the beginning of the process, which was likely brought on by the fiber’s wetness, primarily from the hemicellulose components or the leftover chemicals used for the treatment. Except for the 15% treated composite, all other composites revealed very slight variations, according to the DTG analysis. Hydrogen bonding during treatment may have prevented residual sodium hydroxide from being absorbed by cellulose [14]. Ten percent treated fiber composite has a strong natural ability to repel water, which results in less moisture content being visible. With small peaks at 220 °C, substantial organic breakdown in the majority of composites began at 160 °C and finished between 405 and 450 °C.
3.8 Water absorption of Portulaca quadrifida fiber composites
A water absorption test is performed on a Portulaca quadrifida fiber composite sample using the appropriate ASTM D570 standard. Using Portulaca quadrifida agricultural waste to create an effective commercial product with increased mechanical strength is the major goal of this project. The water absorption test of various treated samples, such as 5–15%, is explained in detail in Fig. 9. The absorption tests suggest that water is not having a major impact on the composite [8]. A further observation was made by the results that untreated fiber composites absorbed significantly more water than their treated composites. As a result of the hydrophilic nature of the fiber, the Portulaca quadrifida composite sample initially absorbs very little water, and the continuous monitoring results confirm the samples are treated after 12 h. A plot shows that the composite absorbs less water initially but gradually increases over time to become constant. Moreover, the composite attempted to reach the saturation condition at the lateral stage (after 60 h). Also, after reaching saturation, the treated fiber composites did not change [18]. Finally, the tests result showed that the Portulaca quadrifida fiber-reinforced composite samples did not absorb large amounts of water. As compared to 5% treated and untreated fibers, the 15% alkali fiber absorbs less moisture. A clear understanding of the water absorption behavior of the Portulaca quadrifida composite sample is plotted in Fig. 9.
4 Conclusion
This study looked at the physical, chemical, mechanical, and thermal characteristics of Portulaca quadrifida’s fiber composite. The effects of alkali treatment on fiber characteristics were investigated after treating fibers at 5%, 10%, and 15%.
-
As a result of cell wall densification, the results indicated that cell elements increased after alkali treatment, which is a better option than using a better reinforcement of the matrix material. Further, the density and moisture absorption of treated fibers also increased.
-
From the element analysis, Portulaca quadrifida fiber crystallized at 56.25%, due to the addition of lignin and hemicellulose. The alkali treatment (NaOH) also decreased the fiber’s crystallinity.
-
The fiber structure also significantly changed after alkali treatment. The fiber’s cylindrical and spongy shape was discovered through morphological research.
-
In comparison to 5%, 10%, and untreated fibers, the 15% treated fiber showed greater thermal stability. In comparison to untreated fibers (23.16 MPa), 15% treated Portulaca quadrifida’s fibers had the highest tensile strength (39.14 MPa), followed by 10% treated fibers (32.28 MPa), and then fibers treated with 5% (27.25 MPa). It is the best reason to be used in structural and other engineering composites with polymer matrixes.
-
The application of chemical modification techniques to natural fiber results in the production of superior fibers, which are then utilized to construct innovative green composite materials for light-weight applications.
Data availability
All relevant data to the study are included in the article.
References
Karimah A, Ridho MR, Munawar SS, Adi DS, Ismadi DR, Subiyanto B, Fatriasari W, Fudholi A (2021) A review on natural fibers for development of eco-friendly bio-composite: characteristics, and utilizations. J Mater Res Technol 13:2442–2458
Daud MAM, Ghani AFA, Zakaria KA, Selamat MZ, Dharmalingam S, Thirukumaran M (2021) The effect of pineapple leaf fiber as a filler in polymer matrix composite for interior part in automotive. Int J Nanoelectron Mater 14:363–372
Selvaraj M, Pannirselvam N, Ravichandran PT, Mylsamy B, Samson S (2023) Extraction and characterization of a new natural cellulosic fiber from bark of Ficus carica plant as potential reinforcement for polymer composites. J Nat Fibers. https://doi.org/10.1080/15440478.2023.2194699
Thyavihalli Girijappa YG, Mavinkere Rangappa S, Parameswaranpillai J, Siengchin S (2019) Natural fibers as sustainable and renewable resource for development of eco-friendly composites: a comprehensive review. Front Mater 6:1–14
Savio L, Pennacchio R, Patrucco A, Manni V, Bosia D (2022) Natural fibre insulation materials: use of textile and agri-food waste in a circular economy perspective. Mater Circ Econ 4:1–13
Latif R, Wakeel S, Khan NZ, Noor Siddiquee A, Lal Verma S, Akhtar Khan Z (2019) Surface treatments of plant fibers and their effects on mechanical properties of fiber-reinforced composites: a review. J Reinf Plast Compos 38:15–30
Mohammed M, Rahman R, Mohammed AM, Adam T, Betar BO, Osman AF, Dahham OS (2022) Surface treatment to improve water repellence and compatibility of natural fiber with polymer matrix: recent advancement. Polym Test 115:107707
Sanjeevi S, Shanmugam V, Kumar S et al (2021) Effects of water absorption on the mechanical properties of hybrid natural fibre/phenol formaldehyde composites. Sci Rep 11:1–11
Madueke CI, Mbah OM, Umunakwe R (2023) A review on the limitations of natural fibres and natural fibre composites with emphasis on tensile strength using coir as a case study. Polym Bull 80:3489–3506
Gopinath R, Billigraham P, Sathishkumar TP (2023) Characterization studies on new cellulosic fiber extracted from Leucaena leucocephala tree. J Nat Fibers. https://doi.org/10.1080/15440478.2022.2157922
Selvaraj M, Chapagain P, Mylsamy B (2023) Characterization studies on new natural cellulosic fiber extracted from the stem of Ageratina adenophora plant. J Nat Fibers. https://doi.org/10.1080/15440478.2022.2156019
Huang S, Fu Q, Yan L, Kasal B (2021) Characterization of interfacial properties between fibre and polymer matrix in composite materials—a critical review. J Mater Res Technol 13:1441–1484
de Neuba LM, Junio RFP, Souza AT, Chaves YS, Tavares S, Palmeira AA, Monteiro SN, Pereira AC (2023) Alkaline treatment investigation for sedge fibers (Cyperus malaccensis): A promising enhancement. Polymers (Basel). https://doi.org/10.3390/polym15092153
Etale A, Onyianta AJ, Turner SR, Eichhorn SJ (2023) Cellulose: a review of water interactions, applications in composites, and water treatment. Chem Rev 123:2016–2048
Kabir MM, Alhaik MY, Aldajah SH, Lau KT, Wang H, Islam MM (2021) Effect of hemp fibre surface treatment on the fibre-matrix interface and the influence of cellulose, hemicellulose, and lignin contents on composite strength properties. Adv Mater Sci Eng 2021:9753779
Irullappasamy S, Durairaj R, Irulappasamy S, Manoharan T (2018) Investigation on wear behaviors and worn surface morphology of surface treated palmyra fruit fiber/polyester composites to appraise the effects of fiber surface treatments. Polym Compos 39:2029–2035
Vincenzo F, Calabrese L (2019) Effect of stacking sequence and sodium bicarbonate treatment on quasi-static and dynamic mechanical. Materials (Basel) 12:1363
Sahu P, Gupta MK (2020) A review on the properties of natural fibres and its bio-composites: effect of alkali treatment. Proc Inst Mech Eng Part L J Mater Des Appl 234:198–217
Abdullahi SS, Birniwa AH, Chadi AS, Mohammad REA, Mamman S (2020) Effect of fiber surface modification on the mechanical properties of rice husk/glass fiber reinforcement epoxy resin hybrid composite. Niger Res J Chem Sci 8:147–162
Liyanage S, Acharya S, Parajuli P, Shamshina JL, Abidi N (2021) Production and surface modification of cellulose bioproducts. Polymers (Basel). https://doi.org/10.3390/polym13193433
Aziz T, Farid A, Haq F et al (2022) A review on the modification of cellulose and its applications. Polymers (Basel). https://doi.org/10.3390/polym14153206
Ramasamy S, Kandasamy J, Samrot AV, Vijayashree T (2023) Study of various properties of chemically treated lignocellulosic Cissus quadrangularis stem fiber for composite reinforcement. J Nat Fibers. https://doi.org/10.1080/15440478.2022.2161689
Madhu P, Sanjay MR, Jawaid M, Siengchin S, Khan A, Pruncu CI (2020) A new study on effect of various chemical treatments on Agave Americana fiber for composite reinforcement: physico-chemical, thermal, mechanical and morphological properties. Polym Test 85:106437
Peng Q, Ormondroyd G, Spear M, Chang WS (2022) The effect of the changes in chemical composition due to thermal treatment on the mechanical properties of Pinus densiflora. Constr Build Mater 358:129303
Kathiresan S, Meenakshisundaram O (2022) Effect of alkali treated and untreated cellulose fibers and human hair on FTIR and tensile properties for composite material applications. SN Appl Sci. https://doi.org/10.1007/s42452-022-04946-9
Vijay R, Vinod A, Lenin Singaravelu D, Sanjay MR, Siengchin S (2021) Characterization of chemical treated and untreated natural fibers from Pennisetum orientale grass—a potential reinforcement for lightweight polymeric applications. Int J Light Mater Manuf 4:43–49
Jaiswal D, Devnani GL, Rajeshkumar G, Sanjay MR, Siengchin S (2022) Review on extraction, characterization, surface treatment and thermal degradation analysis of new cellulosic fibers as sustainable reinforcement in polymer composites. Curr Res Green Sustain Chem 5:100271
Cruz J, Fangueiro R (2016) Surface modification of natural fibers: a review. Procedia Eng 155:285–288
Zwawi M (2021) A review on natural fiber bio-composites, surface modifications and applications. Molecules. https://doi.org/10.3390/molecules26020404
Rao HJ, Singh S, Janaki Ramulu P (2023) Characterization of a Careya arborea bast fiber as potential reinforcement for light weight polymer biodegradable composites. J Nat Fibers 20:71–87
Bar G, Chaudhary K (2023) Characterization of textile grade novel Bauhinia vahlii fiber. J Nat Fibers. https://doi.org/10.1080/15440478.2022.2143464
Lim TK (2016) Edible medicinal and non-medicinal plants. Edible Med Non-Medicinal Plants. https://doi.org/10.1007/978-94-017-7276-1
Madhu P, Sanjay MR, Senthamaraikannan P, Pradeep S, Saravanakumar SS, Yogesha B (2019) A review on synthesis and characterization of commercially available natural fibers: Part-I. J Nat Fibers 16:1132–1144
Maheshwaran MV, Hyness NRJ, Senthamaraikannan P, Saravanakumar SS, Sanjay MR (2018) Characterization of natural cellulosic fiber from Epipremnum aureum stem. J Nat Fibers 15:789–798
Alo OA, Otunniyi IO (2021) Comparative study of flexural and physical properties of graphite-filled immiscible polypropylene/epoxy and high-density polyethylene/epoxy blends. Polym Polym Compos 29:S1103–S1112
Wirawan WA, Choiron MA, Siswanto E, Widodo TD (2022) Morphology, structure, and mechanical properties of new natural cellulose fiber reinforcement from Waru (Hibiscus tiliaceus) bark. J Nat Fibers 19:12385–12397
Bekele AE, Lemu HG, Jiru MG (2023) Study of the effects of alkali treatment and fiber orientation on mechanical properties of enset/sisal polymer hybrid composite. J Compos Sci 7:1–11
Chokshi S, Parmar V, Gohil P, Chaudhary V (2022) Chemical composition and mechanical properties of natural fibers. J Nat Fibers 19:3942–3953
Nguyen TA, Nguyen TH (2022) Study on mechanical properties of banana fiber-reinforced materials poly (lactic acid) composites. Int J Chem Eng. https://doi.org/10.1155/2022/8485038
Madueke CI, Kolawole F, Tile J (2021) Property evaluations of coir fibres for use as reinforcement in composites. SN Appl Sci 3:1–11
Huo S, Ulven CA, Wang H, Wang X (2013) Chemical and mechanical properties studies of chinese linen flax and its composites. Polym Polym Compos 21:275–286
Manaia JP, Manaia AT, Rodriges L (2019) Industrial hemp fibers: an overview Fibers 7:1–16
Wang WM, Cai ZS, Yu JY (2010) Study on the chemical modification process of jute fiber. Tappi J 9:23–29
Yusuff I, Sarifuddin N, Ali AM (2021) A review on kenaf fiber hybrid composites: mechanical properties, potentials, and challenges in engineering applications. Prog Rubber, Plast Recycl Technol 37:66–83
Marinho NP, de Muñiz GIB, Nisgoski S, Venson I, de Cademartori PHG, de Andrade AS (2018) Histochemical analysis of stem and fiber of ramie (Boehmeria nivea (L.) Gaud var. murakami). Acta Sci - Biol Sci 40:1–8
Akram Khan M, Guru S, Padmakaran P, Mishra D, Mudgal M, Dhakad S (2011) Characterisation studies and impact of chemical treatment on mechanical properties of sisal fiber. Compos Interfaces 18:527–541
Saravanakumaar A, Senthilkumar A, Saravanakumar SS, Sanjay MR, Khan A (2018) Impact of alkali treatment on physico-chemical, thermal, structural and tensile properties of Carica papaya bark fibers. Int J Polym Anal Charact 23:529–536
Kumar R, Hynes NRJ, Senthamaraikannan P, Saravanakumar S, Sanjay MR (2018) Physicochemical and thermal properties of Ceiba pentandra bark fiber. J Nat Fibers 15:822–829
Gurukarthik Babu B, Prince Winston D, SenthamaraiKannan P, Saravanakumar SS, Sanjay MR (2019) Study on characterization and physicochemical properties of new natural fiber from Phaseolus vulgaris. J Nat Fibers 16:1035–1042
Sanjay MR, Madhu P, Jawaid M, Senthamaraikannan P, Senthil S, Pradeep S (2018) Characterization and properties of natural fiber polymer composites: a comprehensive review. J Clean Prod. https://doi.org/10.1016/j.jclepro.2017.10.101
Birniwa AH, Abdullahi SS, Ekramul Mahmud HNM (2019) Study on physico-mechanical behaviour of Acacia nilotica (gum tree) and Glass fiber blend reinforced epoxy resin composite. Chem Search Journal 10:46–53
Birniwa AH, Abdullahi SS, Yakasai MY, Ismaila A (2021) Studies on physico-mechanical behaviour of kenaf/glass fiber reinforced epoxy hybrid composites. Bull Chem Soc Ethiop 35:171–184
Author information
Authors and Affiliations
Contributions
YK: experimentation, writing, AM: supervisor, review, and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumarasamy, Y., Muthiah, A. Fiber extraction and enhancement on the physical and chemical properties of Portulaca quadrifida plant fiber-reinforced composite. Biomass Conv. Bioref. 14, 9001–9012 (2024). https://doi.org/10.1007/s13399-024-05357-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-024-05357-z