Abstract
Laccases from white rot fungi, Perenniporia subtephropora, were collected and studied for the production of the enzyme. In order to increase the efficacy of the enzyme laccase, optimization studies were carried out. Perenniporia subtephropora was optimized by the statistical method Box-Behnken design with four variables (carbon, nitrogen, pH, and temperature) at a time. The higher laccase activity was found to be pH 6.68, temperature 26.49 ºC, sucrose content 34.54 g/L, and yeast extract content 4.07 g/L. A mean value of 2423.4 ± 2.18 (U/L) (N = 5) for laccase acquired from real trials validated the RSM model, indicating that the model was satisfactory. Based on the findings, the crude laccase was tested for decolorization activity against five different textile colors. Direct blue 1 exhibited a maximum decolorization yield of 99%. Further, UV–VIS spectral analysis showed that direct blue 1 was completely degraded by the laccase over a 24-h time period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Laccases are the most important subgroup of the multicopper oxidase protein subfamily and the active site of laccases normally contains four copper atoms and is located on a glycoprotein. They are also known as blue copper oxidases (EC 1.10.3.2) [1]. These enzymes are able to simultaneously reduce di-oxygen to two water molecules and mono-electronic oxidize substituted phenols [2]. According to Mayolo-Deloisa et al. [3], laccases are prevalent in bacteria, plants, insects, and fungi. Enzymes catalyze the oxidation of an extensive range of inorganic and organic substrates, such as ketones, phosphates, amines, ascorbate, phenols, and lignin. Regarding their industrial applications, laccases are particularly beneficial enzymes. So, the production methods of laccase enzyme are a new development in biotechnology. Laccase enzymes have wide applications in a variety of environmental situations as a result of their extremophilic characteristics. An essential component of the bioremediation of industrial waste is the laccase enzyme [4].
The ultimate goal of fundamental research is to lower the cost of enzyme synthesis by employing less expensive raw materials and refining the fermentation process for industrial applications. The traditional classical optimization method of changing one component at a time takes time and cannot ensure the best possible physical and dietary circumstances since it ignores interactions between various variables/parameters. Numerous biochemical and biotechnological processes have effectively employed RSM (Response Surface Methodology) for modelling and optimization studies. RSM is an advanced set of statistical and mathematical methods that are used to investigate the interaction between various components at various levels and the creation of suitable models for problem-solving.
To identify the interactions among the elements, response surface modelling can explore many levels of more than one component at once. According to Demirel et al. [5], the testing methodology can produce accurate optimization findings, and their interactions with other factors that affect manufacturing processes in general, like the synthesis of microbial enzymes, can be significant [6].
Wood decay fungi, which are Basidiomycetes, owns the capability to decolorize toxic chemicals by using extracellular ligninolytic enzymes. While laccases mostly adhere to the extracellular hyphal sheaths that encircle the hyphae expanding in cells, additionally, they have been found to be connected to the cell plate (rich in lignin), which breaks down first in specific white rot. In the textile sector, there are more than 10,000 chemical dyes, 60 to 70% are azo dyes, the most frequent type. According to estimates, azo dyes are lost during the manufacturing and dyeing processes by roughly 2% and 10% to 15%, respectively. They make up the largest family and produce 70% of the synthetic dyes produced globally each year [7]. They can be identified by the presence of azo groups (-N1–4N-) coupled into auxochromes of the -OH/-NH2 type. These dyes are hazardous to aquatic life and have been linked to human cancer, which has a negative effect on various ecosystems when they are released into the environment [8]. White rot fungi, which are effective at removing color, have an extracellular laccase that enables them to break down pollutants rapidly. They can degrade dye since they are not substrate-specific. For the aim of recycling clothing, higher amount of extracellular laccase made by fungi help remove colors from hazardous waste, textile waste materials in cases of rough staining [9].
The current research of extracellular laccase production was optimized from Perenniporia subtephropora using response surface methods (RSM). It was assessed whether laccase could be used to slow dye deterioration. The capacity for degradation of several dyes was examined and assessed. This is helpful if the application in the future doesn't necessitate using many pure laccases, such as when decolorizing textile colors.
2 Materials and methods
Perenniporia subtephropora used in this work was obtained from the Culture Collection Center, Center for Advanced Studies in Botany, Guindy Campus, University of Madras. The culture was maintained on plates and slants of potato dextrose agar. The basal medium for production of laccase consisted of g/L glucose—10, peptone – 1, yeast extract–3, K2HPO4.3H2O – 1, MgSO4.7H2O—1, pH -6.5. In 250-mL Erlenmeyer flasks, 100 mL of medium was sterilized by autoclaving at 121 °C and 15 min in 15 lbs pressure.
2.1 Laccase activity
The laccase activity was measured by oxidation of ABTS (2, 2`-azinobis-3-ethylbenzthiazoline-6-sulfonate) as substrate at 25 ºC. The reaction mixture (200 µL) containing 10 µL of 10 mM/L ABTS, 180 µL of 50 mM /L sodium acetate buffer solution (pH-4.5), and 10 µL enzyme sample and the laccase activity was determined at 420 nm. One unit of enzyme activity was defined as the amount of enzyme that oxidation of 1 µmol of substrate per minute. The results are the average of the three values across all trials, which were all performed in triplicate.
2.2 Design for optimization
An optimal concentration of the factors for the highest production of mycelial growth and laccase was established using statistical method. The most important culture conditions influencing P. subtephortephora's laccase production were screened using the Box Behnken Method (BBD). The Box-Behnken design purposes to investigate and optimize the levels of the significant parameters and their interactions with other factors that affect the extra-cellular laccase concentration [10].
The experimental design for this study included 29 runs, with each variable being examined by one of three levels. Experiments were performed in the manner presented in Table 1 throughout 29 scheduled runs. The average extracellular laccase concentration was measured in each experiment in triplicate, and this value served as the dependent variable or response (YLaccase).
Variable associations were determined by modelling a second-order polynomial equation using data from the 29 runs. The equation for fitting the three variables is:
where, X represents the response variable, C is model constant, linear coefficients are y1,y2 and y3 are y11A2, y22B2and y33C2, quadratic coefficientsare y11, y22 and y33,coded levels of variables are A, B, and C.
The coefficient of determination R2 was used to represent the degree of fitting by the polynomial model equation. The aforementioned software was also used to provide the response surface graphs. Therefore, the regression equation was solved in order to determine the perfect conditions for increased laccase yield.
2.3 Decolorization experiment
Five different synthetic textile dyes, including Acid orange 52 (465 nm), Disperse red 13(206 nm) Acid black 1(622 nm), Acid orange (488 nm), and direct blue 1 (618 nm) were bought from Sigma Chemical Co. Ltd. USA. The structures of the above dyes were represented in Fig. 1. Each dye was produced in a sterile stock solution.
2.4 Screening of dye decolourization using laccase from Perenniporia subtephropora
The crude laccase was used to investigate the decolorization of numerous textile dyes. The reaction mixture (2 mL) contained sodium acetate buffer (100 mM, pH 5), 50 mg/L of dye, and 30 U/L of laccase. At every 2-h intervals, samples were taken out and examined. Testing was done to determine the laccase percentage of decolorization. As a control, enzyme-free dyes were utilized. The rate of decolorization was monitored at particular dye range (nm) with spectrophotometer and calculated to the following formula:
2.5 UV–VIS spectrophotometer analysis
After the screening of dyes, a best decolorization rate of dye was further studied by UV–visible spectrophotometer. To measure the degree of color loss at the dye’s maximal wavelength (200–800 nm), decolorization was evident by a drop in absorbance, which was also recorded as a percentage in the control group without any enzyme. Three distinct sets of treatments were carried out, and the data collected were expressed as mean of the triplicate sample. The amount of color removal was calculated.
3 Results
The influence of concentrations of carbon and nitrogen sources, pH, and temperature on laccase production was investigated using the Box-Behnken methodology. The predicted yield of the laccase from the quadratic model and their corresponding experimentally observed values were given in Table 2. Simultaneous evaluation of these results showed that strong correlation noted between the predicted yield by the model and experimental values.
The effect of component variables on the Perenniporia subtephropora laccase is presented in Table 2. It is obvious that the yield of extracellular laccase varied significantly depending on the fermentation conditions. The results near the zero level (mid-point) shows higher yield of extracellular laccase when compared to the other levels. By using multiple regression analysis, the equation for calculating the predicted yield of extracellular laccase (YLaccase) was obtained and written as,
where YLaccase was predicted extracellular laccase yield (U/L) and the test variables pH, fermentation temperature (ºC), carbohydrate content (g/L) and nitrogen source (g/L), are represented in coded values as A, B, C, and D respectively. The statistical significance of Eq. (2) was verified using ANOVA and F-test and the parameter of quadratic model analysis using Box Behnken design was tabulated in Table 3.
The results of ANOVA for quadratic model prove the experimental design was significant (P model > F) = 0.0001. The value of adj-R2 (0.9984) proposes that the total variation of 99.84% noted for the laccase yield might be due to independent variables and only 0.16% of the total variation was excluded by the model. Here, the value of R2 (0.9992) indicates that the predicted, experimental values of the yield of laccase were in agreement with each other. For regression Eq. (2), the value of lack-of-fit is not significant (P = 0.1382) and hence it explains the model equation is adequate for prediction the laccase production for different set of variables values. The results show that the adequate precision value is greater than 4 and indicates an adequate signal for the prediction. The coefficient factors of regression analysis and their corresponding P-values, laccase yield during submerged fermentation from Perenniporia subtephropora was tabulated in Table 4. The coefficient arrived from Eq. (2), and their P-values are found to be significant.
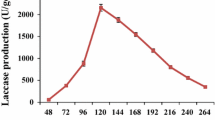
The graphical illustrations of the regression Eq. (2) such as the 2D contour and 3D response surface plots are presented in Fig. 2(a–f). From the 3D plots, it is established that the optimal values of the fermentation conditions required for maximum extracellular laccase production were obtained in ranges of pH 4.59–8.66, temperature 20.28–34.7 ºC, sucrose content 18.17–49.17 g/L, and yeast extract content 2.89–9.6 g/L.
3-D response surface plot and 2-D contour plot indicating the effect of an independent factor on laccase production a pH and Temperature interaction b Carbon source and pH interaction c Nitrogen source and pH interaction d Carbon source and Temperature interaction e Nitrogen source and Temperature interaction f Carbon source and Nitrogen source interaction
The optimal values of the test variables in uncoded (natural) units obtained by solving the inverse matrix (Eq. (2)) were as follows: pH 6.68, temperature 26.49 ºC, sucrose content 34.54 g/L, and yeast extract content 4.07 g/L. The maximal predicted by the inverse square matrix for yield of extracellular laccase in the above condition was 2391 U/L of the fermentation liquor was obtained through the submerged fermentation of Perenniporia subtephropora.
3.1 Validation of the model
The optimal set of conditions produced by the RSM optimization approach were used to validate experimentally and verify the expected values of the model equation responses. The validation of the RSM model was proved by a mean value of 2423.4 ± 2.18 (U/L) (N = 5) for laccase obtained from the real trials (Table 5), suggesting that the model was acceptable and trustworthy for forecasting the production of extracellular laccase by Perenniporia subtephropora.
3.2 Dyes decolorization
The results of decolorization studies carried out by using laccase from Perenniporia subtephropora are shown in Fig. 3. The laccase showed enhanced decolorization in all the dyes at initial hour except Acid Orange 52 and Disperse Red 13 (12% and 18%). Among the dyes tested, the laccase decolorized Direct Blue 1 to a higher extent, nearly 60% in the first 4 h, and enhanced to complete decolorization (99%) within 12 h of incubation (Fig. 3). It was also moderately efficient in decolorization of Acid Black 1 (95% decolorization), Acid orange 8 (92% decolorization), and Disperse red 13 (90% decolorization) after 12 h of incubation. However, the decolorization potential of laccase on Acid Orange 52 was low and showed only 64% of decolorization after 12 h. The percentage of decolorization of Direct Blue 1 was found to be higher by converting the blue colored dye to pinkish then colorless at the end.
3.3 UV–VIS spectrum analysis
The absorption spectra of Direct blue 1 control dye and dye with enzymatic treatment were studied in the UV–Visible range (Fig. 4). The reduction of the peak at 618 nm was noted at 12 h. A second peak, with a maximum absorbance at 320 nm also disappeared was evident from Fig. 4. The peak observed at 618 nm in control was decreased without any shift in the new peak. As the time increases, there was a loss of corresponding peak at 618 and 320 nm at 24 h. Thus UV–VIS spectroscopy indicates that completes decolorization of was achieved.
4 Discussion
Industrial use of laccase necessitates its overproduction, which can be accomplished through genetic manipulation and altering the medium composition. The optimization of growth medium is the good method for the maximizing enzyme production as the secretion of the lignolytic enzyme is influenced by the medium components and culture conditions[11]. Many classical optimization research uses one-dimensional research like the one factor at a time (OFAT) technique. OFAT was performed by changing only one independent variable at a time and keeping all the others factors as constant. Even today, it is widely used in optimization as the go-to technique for selecting optimal conditions. The main limitations of OFAT are the insufficient description of the influence of the factors on the response and the inability to account for interaction effects between the variables. Due to the high number of tests needed, this technique is time-consuming and labor-intensive. Furthermore, it may cause inaccurate conclusions to be drawn [12].
Table 2 illustrates the results of a Box Behnken experiment in which each variable was tested at three different levels and corresponding laccase yield was expressed in units per liter of fermentation media was calculated across 29 trials. The significance of an associated coefficient increases as its associated P value decreases [13]. In this RSM optimization, the P values [(P model > F) = 0.0001] of the extracellular laccase production represent significance of the model for the prediction of laccase yield. Multiple correlation co-efficient (R) and determination coefficient (R2) represent the perfectness of the model. The R2value 0.9958for laccase production was found to be closer to 1, which indicates the better correlation between predicted values and experimental values [14]. Moreover in the present study, there is strong correlation noted between experimental values and values predicted by the final factorial design in Table 2, ANOVA of second-order polynomial model, their lack of fit value in Table 3 represents the polynomial model was precise and very substantial [15].
The “Pred R-Squared” and the “Adj R-Squared” agreed reasonably good. The signal to noise ratio must typically be more than 4, was measured with sufficient precision. The value of this ratio in the current investigation indicated that the second order polynomial model had an appropriate signal and could be used to navigate the design space [10]. In the present study, lack of fit ANOVA value for regression Eq. (2) was found to be not significant (P = 0.1382); hence, it represents the model equation was suitable for forecasting laccase in any combinational variable values [16].
The regression function was represented graphically by the 2D contour plots and the 3D response surface plots. To establish the optimal conditions, the pictorial representation illustrates the association between the experimental levels of each variable, and response as well as the types of interactions between test variables. The Design Expert Software (version 13) was used in the current study to construct the contour plots and response surface plots, from the degree of interaction between the variables could be seen, as shown in Fig. 2a–f. They indicate the types of interactions that occurs when two variables were put together and their correlation between the predicted and experiment level of the individual variables. Different surface plot patterns represented various interactions between the factors. Therefore, in present investigation, 3D surface plot of laccase production was represented by a small pattern in the contour plot. The elliptical contour plots represent that the results were extremely significant, whereas contour plots in circular shape show the insignificant interactions of variables. Because of this, the elliptical contours obtained here demonstrate that the independent variables perfectly interact [17], and represent the importance of the interaction of corresponding variables [18].
Considering a large number of variables simultaneously, RSM under box Behnken design optimizes for laccase enzyme activity and predicts a tenfold increase in enzyme yield attained after a series of modifications to the variables. The current investigations of Perenniporia subtephropora were discovered to be higher, and similarly good results were previously reported [19,20,21].
Azo dyes are man-made organic substances that are frequently employed in the dyeing of textiles. The chemical family of dyes, defined by the presence of minimum of one azo bond aromatic ring(N = N), accounts for over 70% of the global market for dyestuffs [22].The majority of the highly adaptable colorants known as azo dyes and their pigments are released into the environment during the dying procedure. Because bioremediation techniques generate more of the enzyme, laccase-based decolorization techniques may be effective [23].
Water resources are seriously threatened by dye runoff from textile industry. Chemical and physical procedures, including adsorption, coagulation-flocculation, oxidation, ion-exchange, and electrochemical techniques, can be applied to remove color from effluent. The cost of the aforementioned cleanup methods restricts their use. In contrast, the efficient use of microbial enzymes for dye decolorization has attracted a lot of attention recently [24]. Current findings suggest that the laccase enzyme from the fungus Perenniporia subtephropora can be exploited for bioremediation of toxic industrial dye effluent due to its high capacity for dye degradation.
The current study demonstrated the value of evaluating a wide range of dyes from various effluents in an effort to determine the most effective laccase decolorization. Most of the dyes exhibited consistent decolorization abilities and were able to remove colour from treatments that were identical [25]. However, in this investigation, the fungal laccase had specific decolorizing activity on Direct Blue 1, and the laccase decolorized the majority of the dyes with specific series. Similar behavior has been observed in many fungal laccases, hence the laccase’s ability to immediately decolorize certain synthetic colors makes sense [26]. The capability of laccase to decolorize an extensive range of synthetic dyes quickly and effectively has increased interest in this process [27]. Laccase improves the range and rate of decolorization. In this investigation, laccase effectively decolourised all of the tested dyes, with Direct Blue 1 showing the highest decolorization rate.
The dye’s toxicity to the laccases or an inadequate enzyme/reactants ratio likely contributed to the drastic slowing of the decolorization rate with increasing dye concentration. A UV–Vis spectrum of dyes in aqueous solution showing corresponding troughs and peaks in wide range wave length reveals the primary color, maximum absorption, etc. Fastness behavior may be related to the dye's UV–Vis spectral peaks, which reveal the dye's properties under UV light [28], and demonstrated that UV–VIS spectroscopy is the most effective tool for investigating decolorization. Analysis of UV–Vis spectrum in this study exposed the peak responsible for absorption maxima of parent dye (618 nm) was nearly absent in the sample produced after dye decolorization. According to UV–Vis spectral analyses, direct blue 1 is the most amenable substrate to breakdown by laccase from Perenniporia subtephropora, as evidenced by its decolorization. Furthermore, spectral analyses show that the total removal of the peak at 618 nm in the treated dye was due to the azo group degradation by laccase. Under experimental conditions, the azo dye peaks faded entirely, suggesting complete azo bond conversion [29]. The UV-absorbers with faster retention times and the visible-absorbers with slower retention times are the intermediates [30].
5 Conclusion
In conclusion, laccases have received more attention than any other family of oxidases. Optimizing one’s diet through experimentation necessitates research on a narrow subfield of personal elements. Changing a medium component led to a rise in laccase production. Statistical optimization by Box Behnken Design -Response Surface Method was a high model of accuracy with tenfold increase in yield of laccase. The results of this study suggest Perenniporia subtephropora an environmentally friendly, efficient, and low-cost biological product for degradation of the dye direct blue 1. The laccase isolated from Perenniporia subtephropora is a potential strain for the degradation of dyes from environmental effluents. However, this study has few limitations such as stability, efficiency, time duration of the enzyme in industrial dye effluents needs to be evaluated. Though these limitations can be overcome by immobilization and stabilization techniques and have real chances in future perspectives such as cost-effective industrial dye effluent treatment methods and development of modern sewage treatment plants (STP).
Data availability
All data generated or analyzed during this study are included in this published article.
References
Prajapati HV, Minocheherhomji FP (2021) Optimization of laccase enzymes production through response surface methodology by trametes elegans H6. J Adv Sci Res 12:301–308
Janusz G, Pawlik A, Świderska-Burek U et al (2020) Laccase properties, physiological functions, and evolution. Int J Mol Sci 21. https://doi.org/10.3390/ijms21030966
Mayolo-Deloisa K, González-González M, Rito-Palomares M (2020) Laccases in food industry: bioprocessing, potential industrial and biotechnological applications. Front Bioeng Biotechnol 8:1–8. https://doi.org/10.3389/fbioe.2020.00222
Neifar M, Sci JT, Bacteria A (2020) International Journal of Textile Science & Engineering Modeling and Optimization of Textile Dye Decolourization Us-. 1–8. https://doi.org/10.29011/TSE-107
Demirel C, Kabutey A, Herák D et al (2022) Using Box-Behnken Design coupled with response surface methodology for optimizing rapeseed oil expression parameters under heating and freezing conditions. Processes 10:490. https://doi.org/10.3390/PR10030490
Bagewadi ZK, Mulla SI, Ninnekar HZ (2017) Optimization of laccase production and its application in delignification of biomass. Int J Recycl Org Waste Agric 6:351–365. https://doi.org/10.1007/s40093-017-0184-4
Sun S, Liu P, Ullah M (2023) Efficient azo dye biodecolorization system using lignin-co-cultured white-rot fungus. J Fungi 9:91. https://doi.org/10.3390/jof9010091
Ikram M, Naeem M, Zahoor M et al (2022) Biological degradation of the Azo dye basic orange 2 by Escherichia coli: a sustainable and ecofriendly approach for the treatment of textile wastewater. Water 14:2063. https://doi.org/10.3390/w14132063
Latif Z, Shafique J, Summuna B et al (2021) Development of efficient strain of Ganoderma lucidum for biological stripping of cotton fabric dyed Reactive Blue 21. Saudi J Biol Sci 28:7550–7560. https://doi.org/10.1016/j.sjbs.2021.09.038
El Hazzam K, Mhada M, Metougui ML et al (2022) Box-Behnken Design: wet process optimization for saponins removal from chenopodium quinoa seeds and the study of its effect on nutritional properties. Front Nutr 9:1–16. https://doi.org/10.3389/fnut.2022.906592
Rajeswari M, Bhuvaneswari V (2017) Optimization of laccase production from Bacillus sp. PK4 through statistical design of experiments. Microbiol Biotechnol Lett 45:330–342. https://doi.org/10.4014/mbl.1709.09003
Ali NS, Huang F, Qin W, Yang TC (2022) Identification and characterization of a new serratia proteamaculans strain that naturally produces significant amount of extracellular laccase. Front Microbiol 13:1–12. https://doi.org/10.3389/fmicb.2022.878360
Nazim M, Khan AAP, Asiri AM, Kim JH (2021) Exploring rapid photocatalytic degradation of organic pollutants with porous CuO nanosheets: synthesis, dye removal, and kinetic studies at room temperature. ACS Omega 6:2601–2612. https://doi.org/10.1021/acsomega.0c04747
Sondhi S, Saini K (2019) Response surface based optimization of laccase production from Bacillus sp. MSK-01 using fruit juice waste as an effective substrate. Heliyon 5:e01718. https://doi.org/10.1016/j.heliyon.2019.e01718
Liu C, Feng J, Wang S, Cao Y, Shen Y (2022) Enhancement of hyphae growth and medium optimization for Pleurotus eryngii-3 under submerged fermentation. Agronomy 12(10):2413. https://doi.org/10.3390/agronomy12102413
Kumar G, Mullick P, Nandakumar K et al (2022) Box–behnken design-based development and validation of a reverse-phase HPLC analytical method for the estimation of paclitaxel in cationic liposomes. Chromatographia 85:629–642. https://doi.org/10.1007/s10337-022-04172-w
Yang J, Zhou GY, Liu J, Zuo J (2013) Optimization of fermentation conditions by Trametes versicolor FG-97 using response surface methodology for laccase production. J Chem Pharm Res 5:221–226
Tamilarasan N, Yasmin BM, Anitha P et al (2022) Box–Behnken design: optimization of proanthocyanidin-loaded transferosomes as an effective therapeutic approach for osteoarthritis. Nanomaterials 12. https://doi.org/10.3390/nano12172954
Swapnil K, Amit D, Mahendra D, Aditya P (2018) Isolation of potential laccase producing strain Lenzites sp. DK14 and laccase production using response surface methodology. Int J Curr Microbiol App Sci 6:1256–1266
Pratheebaa P, Periasamy R, Palvannan T (2013) Factorial design for optimization of laccase production from Pleurotus ostreatus IMI 395545 and laccase mediated synthetic dye decolorization. Indian J Biotechnol 12:236–245
Chenthamarakshan A, Parambayil N, Miziriya N et al (2017) Optimization of laccase production from Marasmiellus palmivorus LA1 by Taguchi method of Design of experiments. BMC Biotechnol 17:1–10. https://doi.org/10.1186/s12896-017-0333-x
Kamal IM, Abdeltawab NF, Ragab YM et al (2022) Biodegradation, decolorization, and detoxification of Di-Azo Dye Direct Red 81 by halotolerant, alkali-thermo-tolerant bacterial mixed cultures. Microorganisms 10. https://doi.org/10.3390/microorganisms10050994
Sridharan R, Gayathri V, Archana KKM, Rajagopal R (2021) Integrated approach on azo dyes degradation using laccase enzyme and Cul nanoparticle. SN Appl Sci 3:1–12. https://doi.org/10.1007/s42452-021-04164-9
Alsaiari NS, Amari A, Katubi KM et al (2021) The biocatalytic degradation of organic dyes using laccase immobilized magnetic nanoparticles. Appl Sci 11. https://doi.org/10.3390/app11178216
Chakravarthi B, Mathkala V, Palempalli UMD (2021) Degradation and detoxification of congo red azo dye by immobilized laccase of streptomyces sviceus. J Pure Appl Microbiol 15:864–876. https://doi.org/10.22207/JPAM.15.2.41
Kunjadia PD, Sanghvi G V., Kunjadia AP et al (2016) Role of ligninolytic enzymes of white rot fungi (Pleurotus spp.) grown with azo dyes. Springerplus 5. https://doi.org/10.1186/s40064-016-3156-7
Mani P, Fidal VT, Keshavarz T et al (2021) Laccase immobilization strategies for application as a cathode catalyst in microbial fuel cells for azo dye decolourization. Front Microbiol 11:1–11. https://doi.org/10.3389/fmicb.2020.620075
Samanta AK, Konar A (2011) Dyeing of textiles with natural dyes. Natural Dyes 3:30–56. https://www.intechopen.com/chapters/23051
Thampraphaphon B, Phosri C, Pisutpaisal N et al (2022) High potential decolourisation of textile dyes from wastewater by manganese peroxidase production of newly immobilised Trametes hirsuta PW17-41 and FTIR analysis. Microorganisms 10:992. https://doi.org/10.3390/MICROORGANISMS10050992/S1
Srinivasan S, Bankole PO, Sadasivam SK (2022) Biodecolorization and degradation of textile azo dyes using Lysinibacillus sphaericus MTCC 9523. Front Environ Sci 10:1–13. https://doi.org/10.3389/fenvs.2022.990855
Acknowledgements
The authors thank the Researchers Supporting Project Number (RSP2023R414), King Saud University, Riyadh, Saudi Arabia. The authors also thank the Department of Science and Technology, Government of India (PDF/2022/001253).
Author information
Authors and Affiliations
Contributions
Sudarson Jenefar: Experimental work, Field collection, Writing—original draft. Venkatesan Kaviyarasan: Validation, Formal analysis, Supervision. Jayaraman Narenkumar: Validation, Result Interpretation. Supervision. Bader O. Almutairi: Validation, Formal analysis, Writing—review & editing. Paulraj Arunkumar: Validation. Shenbhagaraman Ramalingam: Project administration, Supervision, Validation, Writing—review & editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jenefar, S., Kaviyarasan, V., Narenkumar, J. et al. Response surface based optimization of laccase production from Perenniporia subtephropora and its application in decolorization of dyes. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04517-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04517-x