Abstract
The usage of orange peel wastes for solid biofuel production can provide a sustainable solution for solid waste management while also reducing the negative effects of fossil fuels. The aim of this study is to compare the combustion properties of orange peel wastes and solid biofuels obtained from them. In this context, the combustion behaviour of orange peel wastes and hydrochar and biochar samples obtained as a result of their hydrothermal carbonization and pyrolysis were investigated by the thermogravimetric method in this study. In addition, the combustion kinetics of these fuels were determined using the Kissinger-Akahira-Sunosa (KAS) and Flynn–Wall–Ozawa (FWO) methods. As a result of the thermogravimetric analysis, it is seen that the combustion behavior of raw biomass and hydrochar are quite similar, but the combustion of biochar is different from them. The combustion of raw waste and its hydrochar took place in multiple steps, the combustion of biochar took place in a single step. While the average combustion activation energy values of orange peel, hydrochar and biochar were calculated by KAS method as 159.876, 208.561 and 77.656 kJ/mol, respectively; they were calculated by the FWO method as 161.717, 208.600 and 85.209 kJ/mol, respectively. According to these results, the production of biochar from orange peel wastes by pyrolysis and the use of the obtained biochars in combustion systems are more suitable in terms of energy efficiency. Future research of the combustion of solid biofuels from orange peel will be very important to determine their potential for use in combustion systems, either directly or as an additive, and to increase energy efficiency.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The use of biofuels as an alternative to fossil fuels has gained significant attention in recent years due to concerns about climate change and the depletion of non-renewable resources [1]. Orange peel is a waste product generated by the food industry and its use as a biofuel can provide a sustainable solution for waste management while also reducing greenhouse gas emissions. Orange is the most produced and traded citrus fruit in the world because it is the most preferred citrus fruit in the juice industry. According to Food and Agriculture Organization of the United Nations data, the orange production in the world has increased from 63.85 million tons to 75.57 million tons in the last ten years [2]. Assuming that 30% of the fruit weight is peel, this would result in approximately 22.8 million metric tons of orange peel produced worldwide [3]. With such a large amount of orange peel being produced, finding sustainable uses for this waste product can provide significant environmental and economic benefits. Utilization of orange peel for different usage areas such as the production of valuable chemicals, biofuel and animal feed has been suggested by scientists. For example, Otieno et al. [4] used different fruit wastes, including orange peel, in mushroom cultivation and reported that they produced antioxidant-rich mushrooms. Kotsampasi et al. [5] added essential oils obtained from orange peel to the diets of dairy sheep and reported that these oils increased the performance, health, and milk quality and quantity of animals. While Xiao et al. [6] evaluated the orange peel wastes in the production of hydrochar by hydrothermal carbonization; Santos et al. [7] reported that orange peel wastes can be used as solid biofuel and biosorbent.
Hydrothermal carbonization (HTC) and pyrolysis processes are effective methods for evaluating waste biomass and obtaining high value-added products. While HTC is a thermochemical process that takes place at temperatures of 180 – 250 °C and at autogenous pressures in an aqueous medium [6]; pyrolysis is a thermochemical conversion process that takes place in oxygen-free environments and at higher temperatures [8]. At the end of the HTC process, a solid main product called hydrochar and a liquid by-product called process water are released. Hydrochars are a solid biofuel that can be an alternative to fossil fuels due to their high carbon content and calorific values [9, 10]. The liquid product obtained as a result of this reaction is called bio-oil and is an interesting research and study area for many researchers because it contains amino acids, phenolic compounds, furans, furfural, etc. [11]. As a result of the pyrolysis process, solid, liquid and gaseous products called biochar, bio-oil, and syngas are released, respectively, and the yields and quality of these products vary depending on parameters such as temperature, retention time, heating rate, etc. Like hydrochar, biochar can be used as an alternative to coal or co-combustion systems because it is rich in carbon [12]. In addition to these, it is possible to use biochar and similar hydrochar in the fields of environment, agriculture and animal husbandry [13]. There are many studies in which many types of biomass such as domestic wastes, agricultural organic wastes and aquatic plants are used for both biochar production and hydrochar production [8, 11].
One of the most important areas of use of carbon-rich products such as hydrochar and biochar is to obtain energy by using them in combustion systems. The combustion process refers to the thermal degradation of fuels by reacting with oxygen at high temperature [14]. When raw biomass is burned directly, less energy is released compared to biofuels because biomass has several limitations such as high moisture, low atomic O/C and H/C ratios, low calorific value and poor grindability. All of these limitations of raw biomass reduce combustion efficiency and accordingly, less energy is obtained [15]. For this reason, the production of biofuels from biomass and their use in combustion systems are very important in terms of energy efficiency. Scientists have agreed that it is necessary to determine the combustion mechanisms and kinetics of biofuels such as biochar and hydrochar in this context. When the studies in the literature are examined, it is seen that there are many studies on the determination of the combustion mechanism and kinetics of various biomass kinds and hydrochar and biochars produced from these biomass. For example, in previous study, the combustion behavior of Ulva lactuca and biochar obtained from this macroalgae was determined and it was reported that biochar is a more suitable source than raw biomass for use in combustion systems [14]. Fan et al. [16] examined the combustion of macadamia shell and their biochars and stated that biochars burned more easily according to the obtained combustion activation energy values. Arauzo et al. [17] found that biochar and hydrochar are better fuels than raw material in their study examining the combustion of raw, hydrochar and biochar forms of sewage sludge. Zhu et al. [18], Lang et al. [19] and Ro et al. [20] obtained similar results in their studies.
One of the most effective techniques used to determine the combustion characteristics and kinetics of solid fuels such as biochar, hydrochar and raw biomass is the thermogravimetric method. With this method, combustion activation energy, reaction degree, reaction mechanism models and thermodynamic parameters can be easily calculated using various mathematical approaches such as model-free and model-fitting methods [21]. Model-fitting methods were developed to calculate a single set of kinetic parameters for the entire combustion process. Although this method provides excellent fit for both isothermal and nonisothermal data, it gives uncertain values of some Arrhenius parameters. Because the values obtained in this manner are averages, they do not accurately reflect how the temperature and the degree of conversion affect the mechanism and kinetics. Since model-free methods calculate the kinetic parameters in a transformation-dependent manner, multi-step kinetics can be determined without error. In this way, it contributes both to the explanation of the complexity of the process and to the determination of the kinetic scheme [22]. The most commonly used mathematical approaches in studies in the literature are Kissenger-Akahira-Sunosa (KAS), Flynn–Wall–Ozawa (FWO), Starink, distribution activation energy model (DAEM), Coats-Redfern (CR) and Friedman methods [21]. Tariq et al. [23] determined the co-combustion kinetics of orange peel – biomass blends using various model-free and model-fitting methods and found their activation energy values between 82–120 kJ/mol. Sobek et al. [24] calculated the combustion activation energy values of the hydrochar obtained as a result of hydrothermal carbonization of the waste straw between 45.10 and 724.08 kJ/mol. Barbanera et al. [25] calculated the co-combustion kinetics and thermodynamic parameters of gasification char and solid digestate with the OFW model-free method.
The research question of this study is what are the combustion characteristics and kinetics of solid biofuels made from orange peel and what are the differences between them. Considering this question, the objectives of this study are to determine the physicochemical properties of orange peel-based solid biofuels, to investigate the combustion behaviour of orange peel-based solid biofuels and to determine the combustion kinetics parameters of these biofuels. In this context, the properties of the biofuels were analyzed using standard methods and the combustion kinetic parameters of raw biomass waste, biochar produced as a result of pyrolysis and hydrochar obtained as a result of HTC were estimated using KAS and FWO methods. The study of the combustion behaviour and kinetics of orange peel-based solid biofuels is an innovative area of research that has the potential to contribute to the development of sustainable energy sources. It will be feasible to maximize the use of these biofuels in energy production and evaluate their potential as a sustainable alternative to fossil fuels by comprehending the combustion behaviour and kinetics of these fuels. This research has the potential to contribute to the development of a circular economy, where waste products are repurposed to create value and reduce environmental impact.
2 Materials and methods
2.1 Sample preparation
The orange peel wastes used in this study were obtained from a local market in France. After the collected orange peels were cleaned with distilled water, they were dried in a furnace at 70 °C for 18 h. Hydrochars and biochars were produced from orange peel wastes according to the procedures specified in Tarhan et al. [10] and Koçer and Özçimen [14], respectively. Hydrochars were obtained as a result of hydrothermal carbonization of 30 g of orange peel and 180 g of distilled water in a 300 mL nickel-base alloy batch reactor at 200 °C for 1 h. After HTC reaction, the hydrochar and process water were separated via Büchner filtration and hydrochar was dried at temperature of 105 °C for 18 h. Biochars were obtained as a result of pyrolysis of dry orange peel wastes in a split-pipe furnace (Proterm ASP 11/100/500) in a nitrogen environment at the temperature of 400 °C and at the heating rate of 20 °C/min. Approximately 10 g dry orange peel was positioned in this furnace and nitrogen gas was sent to the furnace for 15 min to provide an oxygen-free environment. Biochar samples formed after the carbonization reaction were kept in an oxygen-free environment until the furnace cooled down and then removed from the furnace and weighed. Raw orange peel, hydrochar and biochar samples were stored in a desiccator for characterization and combustion experiments.
2.2 Characterization analyses
In this study, thermal, structural and proximate analyses were carried out. To the thermal analyzes, the TA Instruments SDT Q600 thermogravimetric analysis (TGA) device was used. In this analysis, approximately 5 mg of dried samples were heated up from the temperature of 25 °C to 600 °C with the heating rates of 10, 20 and 40 °C/min. These analyses were carried out in dry air at a flow rate of 40 ml/min. The ignition temperature (Ti), burnout temperature (Tb), maximum combustion rate temperature (Tmax) and maximum combustion rate (Rmax) were calculated using the tangent method specified in Liu et al. [26]
The FTIR spectra of solid biofuels used in this study were obtained using Bruker Alpha FTIR spectrometer. The moisture, volatile matter (VM) and ash content of samples were determined according to the ASTM standards E 871, E872 and E 1755, respectively. Fixed carbon (FC) content of samples was calculated by difference [27]. The percentages of key elements (C, H, O and N) of the samples used in this study were determined using the equations given below [28]:
The higher heating values (HHV) of orange peel, biochar and hydrochar were calculated using the following Eqs. (5) given in Parikh et al. [29]:
2.3 Combustion kinetic
The model-free kinetic methods are most commonly used for the determination of thermal degradation kinetics because they provide a certain guess of activation energy values from isothermal and non-isothermal measurements [30]. For the calculation of the activation energy values of the combustion process of solid fuels, the model-free kinetic methods are based on the following four equations [12]:
where α, Wi, Wf and WT, refer to the degree of conversion, the sample weight at the beginning, at the end and at a temperature of T, respectively.
where k is the rate constant and f(α) is the reaction mechanism function.
where E, A, T and R express the activation energy value (J/mol), the frequency factor (1/s), temperature (K) and universal gas constant (8.314 J/mol K), respectively.
where Ti and β are the initial temperature (K), the linear heating rate (K/min), respectively.
The combination of Eqs. (6)-(9) gives the Eqs. (10) as follow:
Integration of this equation between the limits: α = 0 at T = T0 and α = α at T = Tα gives Eqs. (11) as follow:
Since Eq. (11) expressed here does not have an exact solution [31], some integral iso-transformation methods have been proposed by some researchers [32]. Among these, KAS method [33] and FWO method [34, 35] are expressed as Eqs. (12) and Eqs. (13) as follow, respectively:
where the activation energy (E) value is determined from the slope of a plot of ln(β/T2) against 1/T.
where the activation energy (E) value is determined from the slope of a plot of ln(β) against 1/T.
Pre-exponential factor (A) in Arrhenius equation was calculated by:
where Tm is the maximum temperature at which maximum decomposition occurs. To estimate the pre-exponential factor, the presented study used an intermediate value of β (20 °C min−1).
3 Results and discussion
3.1 The results of characterization analyses
The proximate and elemental composition values of orange peel, hydrochar and biochar samples are summarized in Table 1. As shown in this table, the VM contents of raw biomass, hydrochar and biochar were calculated as 70.8%, 54.34% and 10.77%, respectively. According to these results, while approximately 15% of the volatile substances in the raw biomass are degraded in the HTC process, this rate is approximately 58% in the thermal carbonization process. When the FC content values are examined, it is seen that the biofuel with the highest fixed carbon is biochar and the FC value of biochar is approximately 2 times that of hydrochar. While the highest moisture content was found in raw biomass; the sample with the highest ash content is biochar as expected. Because, the amount of ash in the biomass does not change with the thermochemical processes, but the ash content mathematically increases as moisture and other volatiles are removed from the biomass by these processes. The elemental analysis results in this table show that the ratio of C and N in the structure increased with carbonization and hydrothermal carbonization processess, but the ratio of H and O decreased. The variations in these contents depend on the characteristics of the feedstock or the process variables, like temperature [36]. According to the calculations, the HHV of raw biomass, hydrochar and biochar were found as 18.28 MJ/kg, 21.65 MJ/kg and 29.92 MJ/kg, respectively. Since the fuel with the highest fixed carbon content is biochar, the biofuel with the highest combustion value was determined as biochar [37]. Ortiz-Sanchez et al. [38] calculated the contents of VM, FC, ash and HHV of orange peel wastes as 80.49%, 16.82%, 2.69% and 18.28 MJ/kg, respectively. Alvarez et al. [39] stated that these values were 74.1%, 23.6%, 2.3% and 19.4 MJ/kg, respectively. When compared with these studies, it can be said that the results obtained are similar to those in the literature. The atomic ratios of H/C and O/C are examined, it was seen that the atomic ratios of H/C and O/C of all samples were in the range of 0.37–1.43 and 0.06–0.61, respectively. These data were analyzed using the Van Krevelen diagram, it was seen that the biochar fell in the anthracite region and the hydrochar in the lignite region.
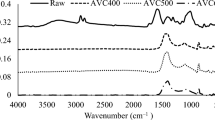
FTIR spectra of orange peel, biochar and biochar were shown in Fig. 1. When the FTIR spectrum of the orange peel waste is examined, the presence of five peaks draws attention. The peak at 3268 1/cm represents the OH stretch in alcohols [42]. The absorbtion band at 2920 1/cm was assigned to the C-H stretches of CH2 and CH3 which represents the aliphatic structures of orange peels [8]. The weak peak at 1729 1/cm shows the entity of C = O vibrations, which is referred to C = O groups of carboxylic acids, aldehydes and ketones. While the strong peak at 1605 1/cm was due to NH stretches in amines or amides; the weak peaks between 1400 and 1200 1/cm were due to vibration at C-O bonds [43]. The peak at 1012 1/cm is due to the C–O–C stretches in the carbohydrate structures in the orange peel [10]. The FTIR spectra of hydrochar and biochar were examined, some shrinkage and shifts were observed in some peaks due to thermochemical processes, unlike that of biomass. For example, the peaks around 3300 and 2900 1/cm expressing OH stretch and CH stretches have almost completely disappeared with thermochemical processes. Similarly, it was observed that the peaks originating from amines or amides structures observed in the 1600 cm-1 peak region also decreased. This is because thermochemical processes such as carbonization and hydrothermal carbonization involve the removal of non-carbon atoms from biomass, resulting in a highly carbon-rich solid structure. Unlike the other spectra, the peaks at 1562 1/cm and 1375 1/cm seen in the biochar’s spectrum indicate C = C ring stretches and symmetrical dimethyl bonding, respectively [14].
3.2 Thermogravimetric analysis
The thermal behaviors of orange peel wastes and hydrochar and biochar produced from them at different heating rates in a dry air environment are shown in Fig. 2. Figure 2a and b are examined, it can be said that the combustion of raw biomass takes place in three temperature regions and the degradation continues up to a temperature of approximately 550 °C. In the first temperature region (approximately < 170 °C), the moisture content and volatile/semi-volatile compounds in the biomass were removed from the structure [14]. In the second temperature zone (between 170 and 450 °C), thermal degradation of the cellulose, hemicellulose and lignin contents of the biomass took place [44]. In the third temperature zone, the remaining lignin structures and char remaining after devolatilization of the samples were burned. Biomass degradation rates in these three regions were calculated as 10.31 ± 2.46%, 63.58% ± 1.14 and 23.63 ± 3.15%, respectively. Zapata et al. [44] reported that during the orange peel burning process, there was a mass loss of 2.73% up to 100 °C, a mass loss of 58.07% between 150 and 360 °C, and the mass loss of 36.09% between 360 and 585 °C. The three basic components found in the structure of orange peel waste and removed from the structure in the second stage of the combustion process (between 150 – 400 °C) are hemicellulose, cellulose and lignin [45]. Hemicellulose is a mixture of various polymerized monosaccharides with lower stability and molecular mass than cellulose. Cellulose is more resistant to thermal degradation due to its long hydrogen bond network and the crystal structures formed accordingly. Lignin, on the other hand, is an amorphous cross-linked resin and highly resistant to thermal degradation [46]. The studies in the literature are examined, the thermal degradation temperature ranges of these three structures are approximately 200 – 260 °C, 260 – 360 °C and 280 – 500 °C, respectively [44, 46].
When Fig. 2c and d are examined, it can be said that the combustion of hydrochars is similar to the combustion of raw biomass. Although the combustion of hydrochars takes place in three temperature ranges such as the combustion of raw biomass, the amounts of decomposition rates differ. The mass loss amounts in the first, second and third temperature zones of the hydrochars were found to be 5.45 ± 0.3%, 48.14 ± 3.62 and 44.67 ± 3.58%, respectively. According to these results, it can be interpreted that some of the cellulose, hemicellulose and lignin content in the raw biomass is charred during the hydrothermal carbonization process. To increase the charring rate of these ingredients, it is necessary to increase the levels of hydrothermal carbonization conditions such as temperature and processing time [18, 47]. The studies in the literature are examined, it is seen that the combustion processes of hydrochars occur in similar stages. Espro et al. [47] investigated the combustion behavior of orange peels-derived hydrochars produced at different hydrothermal temperatures and stated that the combustion of hydrochars generally occurs in three stages. Li et al. [48] reported that the temperature ranges of the combustion of volatile components and char contents in the structure of hydrochars produced in lettuce wastes are 160 – 419 °C and 419 – 521 °C, respectively. As stated in these studies, in the course of the combustion processes of hydrochars, the moisture is removed from the structure up to 200 °C, the cellulose and lignin structure remaining from charring at 200 – 450 °C decomposes, and the burning of fixed carbon and charred structures between 450 – 550 °C.
When the thermogram in Fig. 2e and f are examined, it is seen that the combustion of biochar behaves differently from the combustion of raw biomass and hydrochar. The combustion of the biochar took place in 2 stages and there was a total mass loss of 90.80 ± 1.14%. About 85% of the total mass was burned in the second temperature zone. The main reason for this situation is the removal of hemicellulose content from the structure as volatile matter during the biochar production process and the charring of most of the cellulose and lignin content [14, 16, 44]. When compared with other studies, it is seen that the results related to the combustion behavior of the biochars obtained in this study are in parallel with the literature. Fan et al. [16] reported that the main mass loss in the combustion of macadamia shells biochars occurred in a single step between approximately 300 °C and 550 °C. Koçer and Özçimen [14] reported that approximately 45% of the total mass was burned between 573 and 873 K in their study examining the combustion behavior of chars obtained from Ulva lactuca macroalgae. Wang et al. [45], on the other hand, reported that the greatest mass losses during the combustion of wood and coal chars occur between approximately 450 – 600 °C, and these mass losses are measured between 70 and 90%.
The Ti, Tmax, Rmax and Tb values determined based on the TG and DTG graphs in Fig. 2 are shown in Table 2.
3.3 Kinetic analysis
3.3.1 Raw orange peel combustion kinetics
The non-isothermal curves of KAS and FWO methods at heating rates of 10 °C/min, 20 °C/min, and 40 °C/min for conversions ranging from 0.1 to 0.9 were shown in Fig. 3. Combustion activation energy values and regression coefficients calculated with the help of the curves in this figure are summarized in Table 3. According to these results, it was monitored that the combustion activation energy calculated by both methods ranged between 92.592 kJ/mol and 234.057 kJ/mol, and the regression coefficients altered between 0.897 and 0.999. In addition, it was determined that there was less than 4 kJ/mol difference between the activation energy values calculated by both methods. The fact that the regression coefficients obtained are very close to 1 and the values of activation energy calculated by the two methods are close to each other shows that the results presented in this study can be accepted as correct [14].
The values of average activation energy of the orange peel were calculated as 159.876 kJ/mol and 161.717 kJ/mol for the KAS and FWO methods, respectively. The behavior of the activation energy values is investigated, it is seen that the values of activation energy increase between the conversion of 0.1 – 0.40 and between the conversion of 0.60 – 0.80; it is seen that the values of activation energy decrease between the conversion of 0.40 – 0.60 and conversion of 0.80 – 0.90. According to these results, it can be interpreted that the protein, lipid and carbohydrate structures of orange peel wastes are burned up to 60% conversion, and after 60% conversion, the char structure formed after devolatilization is burned. Since the char structure is relatively more difficult to burn, the activation energy values tended to increase after 60% conversion. After 80% conversion, the activation energy has decreased since there is no more complex structure that can burn towards the end of the combustion process. The reason for the decrease between 40 and 60% conversion is that a different structure from the decomposed structures at this stage does not begin to decompose thermally. The studies in the literature are investigated, examining the combustion behavior of biomass wastes and determining the combustion kinetics has been one of the subjects focused on by researchers for many years. In these studies, the combustion activation energies of many different biomass wastes were determined using different methods. For example, Zapata et al. [44] stated that they calculated the values of combustion activation energy of hemicellulose, cellulose and lignin structures in the orange peel structure as 90 – 100 kJ/mol, 120 – 190 kJ/mol and 115 – 140 kJ/mol, respectively. Boumanchar et al. [49] calculated the combustion activation energies of biomass wastes such as kernel olive, sugar cane, manure, eucalyptus sawdust, alpha (Macrochloa tenacissima) and wood sawdust using the Coats-Redfern method, and found that these values were calculated as 3.14 kJ/mol – 53.13 kJ/mol. Koçer and Özçimen [14] reported that the combustion of Ulva lactuca macroalgae waste takes place in two stages and the activation energies in these stages are approximately 295 kJ/mol and 225 kJ/mol.
When the A values shown in Table 3 are examined, it is seen that these values vary between 106 1/min and 1016 1/min. The large variation of the degree of conversion and A values as in this result indicates the presence of a complex multi-step reaction during combustion [25]. A values less than 105 1/min indicate low activation energy values and therefore show that the combustion reaction is easier and faster. As the A value increases, the complexity of the material structure increases and the combustion reaction becomes more difficult. The obtained results indicate that the raw orange peel is complex, and all the degradation stages can occur during the combustion process [50].
3.3.2 Hydrochar combustion kinetics
The non-isothermal curves drawn to calculate the combustion activation energy values of hydrochars obtained as a result of hydrothermal carbonization of orange peel wastes by KAS and FWO method, and the activation energy and regression case values calculated with the help of these curves are shown in Fig. 4 and Table 4, respectively. According to this table and figure, the activation energy values of hydrochar calculated by the KAS method range between 137.678 kJ/mol and 254.829 kJ/mol, and the average combustion activation energy value was calculated as 208.561 kJ/mol. The lowest, highest and average activation energies calculated by the FWO method were found to be 142.902 kJ/mol, 252.214 kJ/mol and 208.600 kJ/mol, respectively. The regression coefficients calculated by both methods also vary between 0.895 and 0.984. As seen in Table 4, the A values of hydrochar varied between 109 1/min and 1018 1/min. These values are relatively higher compared to those of the orange peel, which shows that the hydrochar burns more difficult than the orange peel. Zhu et al. [18] determined the combustion behavior and kinetics of cotton stalk hydrochars and calculated the combustion activation energy values of hydrochars obtained under different temperature and reaction time conditions in the range of 55.95 – 108.64 kJ/mol. Yang et al. [51] calculated the combustion activation energy values of hydrochars produced from bamboo under different temperature conditions between 52.5 kJ/mol and 130.2 kJ/mol. The fact that the data obtained in this study is higher than those in the literature can be explained by the differences in the structure of the raw material used in the production of hydrochar and the content of the obtained hydrochar. When the biomass constituents are not completely decomposed during the hydrothermal carbonization process, the combustion activation energy values of the obtained hydrochar will be higher. By increasing the temperature and time parameters of the hydrothermal carbonization process, these materials can be completely decomposed and thus the combustion activation energy of the hydrochars obtained will decrease [18, 51].
When the variation of the activation energy values calculated by KAS and FWO methods depending on the conversion rate is examined, it is seen that the activation energy value increased between the conversion of 0.1 and 0.2 and reached the highest level. Between the conversions of 0.2 and 0.5 conversion, the activation energy value decreased by about 95 kJ/mol. In this range, it can be said that lignin, cellulose and hemicellulose remaining in the structure during the hydrochar formation process are degraded. Between the conversions of 0.5 and 0.7, it was observed that the activation energy value increased by about 95 kJ/mol, and it can be interpreted that the third stage of combustion started in this range. After the conversion ratio of 0.7, the activation energy values decreased by approximately 115 kJ/mol to approximately 137 kJ/mol. The change behavior of the activation energy values calculated by the KAS method is similar to that of the values calculated by the FWO method, and studies in the literature support this situation [18].
3.3.3 Biochar combustion kinetics
The non-isothermal curves drawn to determine the combustion kinetics of biochars produced in orange peel wastes by KAS and FWO methods and the activation energy and regression coefficient values calculated with the help of these curves are shown in Fig. 5 and Table 5, respectively. When the data in this table and figure are examined, it is seen that the combustion activation energy values calculated with FWO are 6 – 9 kJ mol higher than the values calculated with the KAS method. Average activation energy values of biochar calculated by KAS and FWO method were found as 77.656 kJ/mol and 85.209 kJ/mol, respectively. A values ranged from 104 1/min to 106 1/min for both methods. When the changes in the activation energy values and pre-exponential factor values with the conversion rate are examined, it is seen that there is not much change between the initial and final values. This can be explained by the fact that orange peel biochar has a more homogeneous structure compared to orange peel hydrochar and raw biomass. The highest activation energy value calculated by KAS method was calculated as 93.159 kJ/mol in the conversion of 0.2, while the lowest activation energy value was calculated as 67.168 kJ/mol at the conversion of 0.7. The highest and lowest activation energy values calculated by the FWO method were calculated as 99.323 kJ/mol and 75.647 kJ/mol, respectively, at the same conversion rates.
When the studies in the literature are examined, it is seen that there are many studies related to the determination of the combustion kinetics of biochars produced from various biomass and wastes. For example, Peterson et al. [52] calculated the combustion activation energy values of biochars obtained from 6 different types of woody and herbaceous biomass between 94 kJ/mol and 129 kJ/mol. Lee et al. [53] found the average combustion activation energy values of biochars obtained from spent coffee grounds as 101.72 kJ/mol and 94.58 kJ/mol by KAS and FWO methods, respectively. Koçer and Özçimen [14] studied the combustion behavior and kinetics of biochars produced from Ulva lactuca macroalgae and reported that the combustion activation energy values determined by KAS and FWO methods were 146.606 kJ/mol and 140.806 kJ/mol, respectively. When these and similar studies are investigated, the main conclusion that can be drawn is that biochars show better combustibility and lower energy requirements for the combustion process compared to raw biomass [25, 53, 54].
4 Conclusion
In this study, the combustion behaviours of solid biofuels produced from orange peel were investigated and their combustion kinetics were calculated using KAS and FWO methods. Following conclusions can be drawn from the findings reported in this study:
-
The average combustion activation energy values of the orange peel waste were calculated as 159.876 kJ/mol and 161.717 kJ/mol, using KAS and FWO methods, respectively.
-
Combustion activation energy values of hydrochar obtained as a result of hydrothermal carbonization of orange peel were calculated as 208.561 kJ/mol and 208.600 kJ/mol using KAS and FWO methods, respectively.
-
Combustion activation energy values of biochar obtained as a result of pyrolysis of orange peel were calculated as 77.656 kJ/mol and 85.209 kJ/mol using KAS and FWO methods, respectively.
-
It has been understood that the most suitable material for combustion is biochar and that the biochar produced from orange peel can be used instead of coal or mixed with coal in combustion systems in the future.
-
According to the results, it is more advantageous to convert orange peel waste into biochar instead of using it directly as fuel, due to reasons such as energy efficiency. However, it is essential to optimize biochar production, evaluate it economically with a circular economy approach, and determine more efficient production processes.
Data availability
Data will be provided upon request.
References
Kumar Sarangi P, Subudhi S, Bhatia L et al (2022) Utilization of agricultural waste biomass and recycling toward circular bioeconomy. Environ Sci Pollut Res 30:8526–8539. https://doi.org/10.1007/s11356-022-20669-1
Perez-Pirotto C, Cozzano S, Hernando I, Arcia P (2023) Different green extraction technologies for soluble dietary fibre extraction from orange by-product. Int J Food Sci Technol 58:2042–2049. https://doi.org/10.1111/ijfs.15756
Su G, Chan C, He J (2022) Enhanced biobutanol production from starch waste via orange peel doping. Renew Energy 193:576–583. https://doi.org/10.1016/j.renene.2022.04.096
Otieno OD, Mulaa FJ, Obiero G, Midiwo J (2022) Utilization of fruit waste substrates in mushroom production and manipulation of chemical composition. Biocatal Agric Biotechnol 39:102250. https://doi.org/10.1016/j.bcab.2021.102250
Kotsampasi B, Tsiplakou E, Christodoulou C et al (2018) Effects of dietary orange peel essential oil supplementation on milk yield and composition, and blood and milk antioxidant status of dairy ewes. Anim Feed Sci Technol 245:20–31. https://doi.org/10.1016/j.anifeedsci.2018.08.007
Xiao K, Liu H, Li Y et al (2018) Correlations between hydrochar properties and chemical constitution of orange peel waste during hydrothermal carbonization. Bioresour Technol 265:432–436. https://doi.org/10.1016/j.biortech.2018.06.014
Santos CM, Dweck J, Viotto RS et al (2015) Application of orange peel waste in the production of solid biofuels and biosorbents. Bioresour Technol 196:469–479. https://doi.org/10.1016/j.biortech.2015.07.114
Koçer AT, Mutlu B, Özçimen D (2020) Investigation of biochar production potential and pyrolysis kinetics characteristics of microalgal biomass. Biomass Convers Biorefinery 10:85–94. https://doi.org/10.1007/s13399-019-00411-7
Li J, Pan L, Suvarna M et al (2020) Fuel properties of hydrochar and pyrochar: Prediction and exploration with machine learning. Appl Energy 269:115166. https://doi.org/10.1016/j.apenergy.2020.115166
Tarhan SZ, Koçer AT, Özçimen D, Gökalp İ (2021) Cultivation of green microalgae by recovering aqueous nutrients in hydrothermal carbonization process water of biomass wastes. J Water Process Eng 40:101783. https://doi.org/10.1016/j.jwpe.2020.101783
Wang Q, Wu S, Cui D et al (2022) Co-hydrothermal carbonization of organic solid wastes to hydrochar as potential fuel: A review. Sci Total Environ 850:158034. https://doi.org/10.1016/j.scitotenv.2022.158034
Koçer AT, Özçimen D (2022) Experimental investigation on thermal behavior and thermo-kinetic study on pyrolysis of de-oiled microalgae. Int J Environ Sci Technol 19:12279–12288. https://doi.org/10.1007/s13762-022-03933-2
Chen W, Meng J, Han X et al (2019) Past, present, and future of biochar. Biochar 1:75–87
Koçer AT, Özçimen D (2021) Determination of combustion characteristics and kinetic parameters of Ulva lactuca and its biochar. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01245-4
Riaz S, Al-Abdeli YM, Oluwoye I (2023) Partially Oxidative Torrefaction of Woody Biomass Pellets: Burning Behaviour and Emission Analysis. Bioenergy Res. https://doi.org/10.1007/s12155-023-10572-z
Fan F, Zheng Y, Huang Y et al (2017) Combustion Kinetics of Biochar Prepared by Pyrolysis of Macadamia Shells. Bio Res 12:3918–3932. https://doi.org/10.15376/biores.12.2.3918-3932
Arauzo PJ, Atienza-Martínez M, Ábrego J et al (2020) Combustion characteristics of hydrochar and pyrochar derived from digested sewage sludge. Energies 13:4164. https://doi.org/10.3390/en13164164
Zhu G, Yang L, Gao Y et al (2019) Characterization and pelletization of cotton stalk hydrochar from HTC and combustion kinetics of hydrochar pellets by TGA. Fuel 244:479–491. https://doi.org/10.1016/j.fuel.2019.02.039
Lang Q, Zhang B, Liu Z et al (2019) Co-hydrothermal carbonization of corn stalk and swine manure: Combustion behavior of hydrochar by thermogravimetric analysis. Bioresour Technol 271:75–83. https://doi.org/10.1016/j.biortech.2018.09.100
Ro KS, Libra JA, Bae S et al (2019) Combustion Behavior of Animal-Manure-Based Hydrochar and Pyrochar. ACS Sustain Chem Eng 7:470–478. https://doi.org/10.1021/acssuschemeng.8b03926
Yang X, Wang X, Zhao B, Li Y (2014) Simulation Model of Pyrolysis Biofuel Yield Based on Algal Components and Pyrolysis Kinetics. Bioenergy Res 7:1293–1304. https://doi.org/10.1007/s12155-014-9467-z
Vyazovkin S, Wight CA (1999) Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta 340–341:53–68. https://doi.org/10.1016/S0040-6031(99)00253-1
Tariq R, Mohd Zaifullizan Y, Salema AA et al (2022) Co-pyrolysis and co-combustion of orange peel and biomass blends: Kinetics, thermodynamic, and ANN application. Renew Energy 198:399–414. https://doi.org/10.1016/j.renene.2022.08.049
Sobek S, Tran QK, Junga R, Werle S (2022) Hydrothermal carbonization of the waste straw: A study of the biomass transient heating behavior and solid products combustion kinetics. Fuel 314:122725. https://doi.org/10.1016/j.fuel.2021.122725
Barbanera M, Cotana F, Di Matteo U (2018) Co-combustion performance and kinetic study of solid digestate with gasification biochar. Renew Energy 121:597–605. https://doi.org/10.1016/j.renene.2018.01.076
Liu H, Zhang S, Feng S, Jia C, Guo S, Sun B, Wang Q (2021) Combustion characteristics and typical pollutant emissions of corn stalk blending with municipal sewage sludge. Environ Sci Pollut Res 28:9792–9805. https://doi.org/10.1007/s11356-020-11463-y
García R, Pizarro C, Lavín AG, Bueno JL (2012) Characterization of Spanish biomass wastes for energy use. Bioresour Technol 103:249–258. https://doi.org/10.1016/j.biortech.2011.10.004
Nhuchhen DR (2016) Prediction of carbon, hydrogen, and oxygen compositions of raw and torrefied biomass using proximate analysis. Fuel 180:348–356. https://doi.org/10.1016/j.fuel.2016.04.058
Parikh J, Channiwala SA, Ghosal GK (2005) A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 84:487–494. https://doi.org/10.1016/j.fuel.2004.10.010
Ali I, Bahadar A (2019) Thermogravimetric characteristics and non-isothermal kinetics of macro-algae with an emphasis on the possible partial gasification at higher temperatures. Front Energy Res 7:7. https://doi.org/10.3389/fenrg.2019.00007
Vyazovkin S, Burnham AK, Criado JM et al (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19
Hu Y, Wang Z, Cheng X, Ma C (2018) Non-isothermal TGA study on the combustion reaction kinetics and mechanism of low-rank coal char. RSC Adv 8:22909–22916. https://doi.org/10.1039/C8RA02618A
Kissinger HE (1957) Reaction Kinetics in Differential Thermal Analysis. Anal Chem 29:1702–1706. https://doi.org/10.1021/ac60131a045
Flynn JH, Wall LA (1966) A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Part B Polym Lett 4:323–328. https://doi.org/10.1002/pol.1966.110040504
Ozawa T (1965) A New Method of Analyzing Thermogravimetric Data. Bull Chem Soc Jpn 38:1881–1886. https://doi.org/10.1246/bcsj.38.1881
Yu KL, Show PL, Ong HC et al (2018) Biochar production from microalgae cultivation through pyrolysis as a sustainable carbon sequestration and biorefinery approach. Clean Technol Environ Policy 20:2047–2055. https://doi.org/10.1007/s10098-018-1521-7
Demirbaş A (2003) Relationships between heating value and lignin, fixed carbon, and volatile material contents of shells from biomass products. Energy Sources 25:629–635. https://doi.org/10.1080/00908310390212336
Ortiz-Sanchez M, Solarte-Toro JC, Orrego-Alzate CE et al (2021) Integral use of orange peel waste through the biorefinery concept: an experimental, technical, energy, and economic assessment. Biomass Convers Biorefinery 11:645–659. https://doi.org/10.1007/s13399-020-00627-y
Alvarez J, Hooshdaran B, Cortazar M et al (2018) Valorization of citrus wastes by fast pyrolysis in a conical spouted bed reactor. Fuel 224:111–120. https://doi.org/10.1016/j.fuel.2018.03.028
Sial TA, Lan Z, Khan MN et al (2019) Evaluation of orange peel waste and its biochar on greenhouse gas emissions and soil biochemical properties within a loess soil. Waste Manag 87:125–134. https://doi.org/10.1016/j.wasman.2019.01.042
Erdogan E, Atila B, Mumme J et al (2015) Characterization of products from hydrothermal carbonization of orange pomace including anaerobic digestibility of process liquor. Bioresour Technol 196:35–42. https://doi.org/10.1016/j.biortech.2015.06.115
Yaradoddi JS, Banapurmath NR, Ganachari SV et al (2021) Bio-based material from fruit waste of orange peel for industrial applications. J Mater Res Technol. https://doi.org/10.1016/j.jmrt.2021.09.016
Koçer AT, İnan B, Kaptan Usul S et al (2021) Exopolysaccharides from microalgae: production, characterization, optimization and techno-economic assessment. Brazilian J Microbiol 52:1779–1790. https://doi.org/10.1007/s42770-021-00575-3
Zapata B, Balmaseda J, Fregoso-Israel E, Torres-García E (2009) Thermo-kinetics study of orange peel in air. J Therm Anal Calorim 98:309–315. https://doi.org/10.1007/s10973-009-0146-9
Wang X, Hu Z, Deng S et al (2015) Kinetics investigation on the combustion of biochar in O2/CO2 atmosphere. Environ Prog Sustain Energy 34:923–932. https://doi.org/10.1002/ep.12063
Mohan D, Pittman CU, Steele PH (2006) Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 20:848–889
Espro C, Satira A, Mauriello F et al (2021) Orange peels-derived hydrochar for chemical sensing applications. Sensors Actuators B Chem 341:130016. https://doi.org/10.1016/j.snb.2021.130016
Li Y, Liu H, Xiao K et al (2020) Combustion and Pyrolysis Characteristics of Hydrochar Prepared by Hydrothermal Carbonization of Typical Food Waste: Influence of Carbohydrates, Proteins, and Lipids. Energy Fuels 34:430–439. https://doi.org/10.1021/acs.energyfuels.9b02940
Boumanchar I, Chhiti Y, M’hamdiAlaoui FE et al (2019) Investigation of (co)-combustion kinetics of biomass, coal and municipal solid wastes. Waste Manag 97:10–18. https://doi.org/10.1016/j.wasman.2019.07.033
Xiang Y, Xiang Y, Wang L (2016) Thermal decomposition kinetic of hybrid poplar sawdust as biomass to biofuel. J Environ Chem Eng 4:3303–3308. https://doi.org/10.1016/j.jece.2016.07.009
Yang W, Wang H, Zhang M et al (2016) Fuel properties and combustion kinetics of hydrochar prepared by hydrothermal carbonization of bamboo. Bioresour Technol 205:199–204. https://doi.org/10.1016/j.biortech.2016.01.068
Peterson CA, Brown RC (2020) Oxidation kinetics of biochar from woody and herbaceous biomass. Chem Eng J 401:126043. https://doi.org/10.1016/j.cej.2020.126043
Lee XJ, Ong HC, Gao W et al (2021) Solid biofuel production from spent coffee ground wastes: Process optimisation, characterisation and kinetic studies. Fuel 292:120309. https://doi.org/10.1016/j.fuel.2021.120309
Lee XJ, Lee LY, Hiew BYZ et al (2020) Valorisation of oil palm wastes into high yield and energy content biochars via slow pyrolysis: Multivariate process optimisation and combustion kinetic studies. Mater Sci Energy Technol 3:601–610. https://doi.org/10.1016/j.mset.2020.06.006
Acknowledgements
Anıl Tevfik Koçer was supported by The Scientific and Technological Research Council of Turkey (National Scholarship Programme for Doctorate Students) and 100/2000 The Council of Higher Education Doctorate Scholarship Programme. Didem Özçimen was supported by the French Embassy in Turkey and the CNRS for her scholarship at ICARE-CNRS, Orl´eans.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Anıl Tevfik Koçer: Conceptualization, Methodology, Formal analysis, Investigation, Visualization, Writing- Original draft. Didem Özçimen: Funding acquisition, Resources, Supervision, Writing – review & editing. İskender Gökalp: Supervision, Writing – review & editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
All the authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koçer, A.T., Özçimen, D. & Gökalp, İ. An experimental study on the combustion behaviours of orange peel-based solid biofuels. Biomass Conv. Bioref. 14, 22839–22851 (2024). https://doi.org/10.1007/s13399-023-04406-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04406-3