Abstract
The present study deals with the preparation and characterization of porous activated carbon from Cassia fistula seed shell (CFSS), an agriculture solid waste (byproducts) for the removal synthetic anionic Acid Violet (AV17) dye by batch adsorption technique, and the results were compared with commercial activated carbon (CAC). BET surface area and point zero charge of the CFSSC is 485.5 m2/g and 6.5. The as prepared CFSSC structural and morphological features were confirmed using XRD, FITR, TGA, and SEM analysis. The effect of diverse experimental parameters such as dosage of adsorbent, contact time, initial concentration of AV17 dye, and pH on the removal of dye by adsorption using the adsorbents (CFSSC and CAC) has been studied in room temperature. The Langmuir and Freundlich isotherm models were applied. The adsorption capacities of the CAC and CFSSC on AV17 dye was found to be 290.90 and 132.45 mg/g, respectively. The adsorption kinetics initiates to be first order with respect to intra-particle diffusion as rate determining step. The process of removal of dyes by adsorption on CAC and CFSSC was found to be highly pH dependent. The maximum adsorption observed at pH 2. The result in the present study indicates that CFSSC could be used as a cost-effective alternative adsorbent for the removal of dilute acidic dyes from wastewater instead of CAC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Dyes and pigments used in many industries pose a significant environmental threat by being released into wastewater. The effluent from these industries is highly colored, primarily due to different types of dyes, including anionic, cationic, and non-ionic dyes [1,2,3,4]. Acid dyes, for example, are commonly used with specific types of fibers and consist of organic sulfonic acids available as sodium salts. However, wastewater containing acid dyes can negatively impact aquatic life and human health due to its highly colored effluent [3]. The colored effluent water can lead to carcinogenesis, chromosomal fractures, mutagenesis, teratogenicity, respiratory toxicity, and other harmful side effects [5,6,7]. Studies have indicated that industrial activities, such as dye manufacturing and textile finishing, significantly contribute to releasing substantial amounts of dyes into the environment. Approximately 10 to 15% of dyes are lost in the effluent during dyeing, exacerbating environmental contamination [2].
Various techniques are available for treating wastewater contaminated with metals, including chemical precipitation, membrane filtration, reverse osmosis, ion exchange, and adsorption. Chemical precipitation involves adding chemicals to wastewater to create solid particles that can be removed through sedimentation or filtration. Membrane filtration passes wastewater through a membrane that selectively removes contaminants. In reverse osmosis, pressure removes contaminants by forcing water through a semipermeable membrane. Ion exchange involves exchanging metal ions in wastewater for other ions, while adsorption uses materials that adsorb and remove contaminants from the wastewater. The selection of the most appropriate method depends on several factors, such as the nature and extent of the contamination, the volume of wastewater to be treated, and the desired level of treatment [8,9,10,11,12]. However, many treatment methods used for dye wastewater require significant financial investment and incur recurring expenses, such as the cost of chemicals, making them unsuitable for small-scale industries. Adsorption is a cost-effective method that is versatile and practical for removing dye pollutants due to its simple design, ease of operation, high efficiency, and low cost. As such, there have been ongoing efforts to develop more efficient and environmentally friendly adsorbents that can further improve the performance of this method. The search for better adsorbents is an essential area of research that aims to provide a more affordable and sustainable solution to the problem of dye wastewater contamination [3].
The utilization of agricultural waste as an alternative source for producing low-cost and highly effective adsorbents has emerged as a promising solution. However, due to the high production costs, researchers have been exploring various agricultural solid wastes as potential sources for generating affordable activated carbon (AC). The conversion of waste materials into activated carbon can enhance the economic value and reduce waste disposal expenses, providing a potentially more cost-effective solution than commercial alternatives. This approach holds considerable promise for fighting water pollution as these raw materials can be transformed into valuable adsorbents for removing pollutants from aqueous systems. Using agricultural waste for this purpose can facilitate the development of a sustainable and environmentally friendly solution for effectively eliminating contaminants from water sources [4, 5].
C. fistula is a flowering plant belonging to the Fabaceae family and is also known as Indian laburnum and purging cassia. The plant’s natural habitat is the Indian subcontinent and other parts of Southeast Asia, and it can be found worldwide, from India and Sri Lanka to Bangladesh, Myanmar, Thailand, and even Thailand. The plant is widely grown for its beauty and medicinal uses, and the seed pods of C. fistula are shiny, black, approximately 30–60-cm long and 1.5–2 centimeters in width. Researchers have also explored agricultural waste as a source for generating low-cost and highly effective adsorbents. Agricultural waste includes a wide range of organic materials such as rice husks, coconut shells, sawdust, and sugarcane bagasse. These materials are rich in carbon and can be transformed into activated carbon through various processes, including physical activation, chemical activation, and pyrolysis. Among the various agricultural wastes, C. fistula has emerged as a promising alternative for producing activated carbon. This flowering plant belongs to the Fabaceae family and is commonly found in the Indian subcontinent and other parts of Southeast Asia. C. fistula seed pods, in particular, have been found to be an excellent source of activated carbon due to their high carbon content and cellulosic fibers.
Researchers have developed activated carbon from C. fistula seed pods using different activation methods, including phosphoric acid and physical activation. The resulting activated carbon exhibited a high surface area and pore volume, making them efficient adsorbents for various pollutants. In one study, researchers used activated carbon prepared from C. fistula seed pods to remove Congo red dye from aqueous solutions. The adsorption process was optimized by evaluating the effect of several operational parameters such as dye concentration, adsorbent dose, contact time, and solution pH. The results demonstrated that C. fistula seed pod activated carbon has high efficiency in removing Congo red dye, with a 90% and 99% removal rate.
Overall, using agricultural waste, particularly C. fistula, as a source for generating activated carbon offers a sustainable and cost-effective solution for removing pollutants from aqueous systems. Developing environmentally friendly and cost-effective adsorbents could potentially contribute to developing new strategies for treating wastewater containing dyes, ultimately leading to a cleaner and healthier environment.
2 Materials and methods
2.1 Chemicals and reagents
The commercial activated carbon (CAC) adsorbent was provided by E. Merck, India, and BDH supplied Acid Violet 17 (AV 17) as an adsorbate without purification. Table 1 provides the physico-chemical properties, applications, toxicity, and chemical structure of the CR dye. The study utilized chemicals of analytical grade, which were used in their original form without any further purification. The experiments were conducted at room temperature, and double-distilled water was used throughout the investigation.
2.2 Preparation of Cassia fistula seed shell carbon
The adsorbent source material used in this study was locally collected Cassia fistula seed shells (CFSS), which were dried without exposure to sunlight. To activate the CFSS, a physical carbonization process was utilized, where the sample was placed in a stainless-steel tube and heated to 400 °C for 45 minutes in the absence of air. The resulting carbonized raw materials were then ground and sieved with molecular sieves. The carbonized CFSSC materials were further subjected to acid activation and steam digestion processes. The CFSSC was rinsed with distilled water until it was acid-free, then dried in a hot air oven at 120 °C, later powdered, and sieved through molecular sieves with a 90-micron size. The experimental procedure is illustrated in Fig. 1 with a schematic diagram [7, 13].
2.3 Instrumental studies
The CFSSC, which was prepared as described in the previous section, was mechanically ground and sieved using a Jayanth brand mechanical sieve to produce particles of consistent size (90 microns). A Systronics digital pH monitor (model: 335) was used to measure pH, and FTIR spectra of the adsorbent material were obtained using the KBr pellet technique. The analysis of the adsorbent’s X-ray diffraction was carried out using a RICH SIEFRT & CO model that was equipped with Cu-Kα radiation. Furthermore, the surface microstructure of the adsorbent material was examined using a transmission electron microscope (Hitachi H-800 model) and an atomic force microscope (Nanosurf AG), while a Perkin Elmer Thermal Analyzer was used to conduct the thermogravimetry (TGA) analysis under an inert atmosphere.
2.4 Batch adsorption studies and experiments
The adsorption experiments were carried out using a batch process, where a fixed amount of the adsorbent was added to stopper flasks with a capacity of 250 mL, containing 100 mL of dye solution with different initial concentrations. The experiments were conducted at a temperature of 30 ± 1 °C. The flasks were then agitated using a mechanical shaker at 130 rpm until equilibrium was reached. The adsorption capacity of the Cassia fistula seed shell (CFSS) sample was determined using Eqs. (1) and (2) to determine the equilibrium and instantaneous adsorption capacity. The experiments were carried out in duplicate to ensure data accuracy and reliability. To understand the adsorbate–adsorbent interaction and the time dependence of the adsorption system, the study included an adsorption isotherm study and a kinetics study.
The Langmuir isotherm model was utilized to analyze the experimental data, and it demonstrated that the maximum adsorption capacity of the CFSS sample for Acid Violet 17 dye was 54.95 mg/g. The kinetics study of the adsorption process indicated that chemical adsorption occurred, involving the formation of a chemical bond between the adsorbate and adsorbent. This was confirmed by using the pseudo-second-order kinetic model (Table 2). These results suggest that CFSS is an effective adsorbent with high adsorption capacity and strong affinity towards Acid Violet 17 dye. The use of a nonlinear isotherm and kinetics model in this study can aid in optimizing the adsorption process and exploring the potential of CFSS as an efficient adsorbent for removing dyes from wastewater.
2.5 Statistical analysis
The trials’ outcomes were reported as the mean value plus the standard deviation of three distinct observations. The statistical study was carried out using Excel, a Microsoft software.
3 Results and discussion
3.1 Physico-chemical characterization and BET surface area analysis of CFSSC
Physico-chemical characterization and BET surface area analysis are widely used methods for investigating the properties of materials, including seed shells. Physico-chemical characterization involves examining the physical and chemical characteristics of the material, such as particle size distribution, pore size distribution, surface area, and chemical composition [5,6,7, 13]. A variety of techniques, including X-ray diffraction, scanning electron microscopy, Fourier transform infrared spectroscopy, and thermogravimetric analysis, can be utilized for this purpose. BET surface area analysis is a specialized method for determining the surface area of a material, which is based on the physical adsorption of gas molecules onto the material's surface. The BET analysis technique is often used for measuring the specific surface area of seed shells, providing valuable information about their porosity and other properties, including their adsorption capacity [7].
The research conducted proximate analysis of optimized activated carbon (AC), which involved evaluating the physical and chemical properties of the material, according to the ASTM D 3172-3175 standard method. Additionally, the study examined the proximate and ultimate analysis of CFSSC, a type of agricultural byproduct, and presented the results in Table 3. The proximate analysis of CFSSC revealed that it contains 4.70% moisture, 24.1% volatile content, 2.3% ash content, and 68.90% fixed carbon. These results indicate that CFSSC has low ash and volatile matter content and high fixed carbon amount, which are essential factors in determining its suitability as a precursor for AC preparation. The high fixed carbon content of CFSSC enhances its potential for AC preparation, as it can increase the surface area and adsorption capacity of the final product. Conversely, high ash content can decrease the adsorption capacity of the final product. Therefore, the low ash content of CFSSC is an essential factor that contributes to its suitability as a precursor for AC preparation. Overall, the proximate analysis of CFSSC demonstrates its potential as a suitable precursor for AC production, particularly when compared to other types of biomass or agricultural byproducts. The findings emphasize the importance of proximate analysis in determining the properties of materials for specific applications, particularly for materials intended for use in adsorption processes such as AC.
Figure 2 presents the N2 adsorption/desorption isotherms for activated CFSS carbon. The amount of N2 adsorbed per unit mass of sample is plotted as a function of relative pressure (p/p0), where p represents the equilibrium pressure and p0 is the saturation pressure of the adsorbate at – 196 °C. The adsorption isotherms closely resemble the type IV isotherm (according to IUPAC classification) to a significant extent. This indicates that the adsorbent has primarily a microporous structure with only a limited number of mesopores. As illustrated in the Fig. 2, a hysteresis loop is evident at relative pressures ranging from 0.4 to 0.9. This phenomenon is related to capillary condensation that occurs in slit-shaped mesopores. Additionally, a more pronounced hysteresis loop suggests opening previously inaccessible pores during activation or the enlargement of microspores. The surface area, average pore volume, average pore size, and point zero charge of CFSSC were 485.5 m2/g, 1.059 cm3/g, 4.3 nm, and 6.5 nm, respectively.
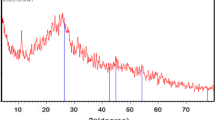
3.2 X-ray diffraction (XRD) studies of CFSSC
X-ray diffraction (XRD) is a technique that can identify a material’s crystal structure, including the unit cell’s size and shape, the atomic arrangement within the unit cell, and the orientation of the atoms within the crystal lattice. XRD analysis was used to determine its crystal structure in the case of as-prepared seed cell carbon. By analyzing the diffraction pattern produced by the scattered X-rays, it is possible to identify the type of crystal structure present in the sample. XRD analysis is a crucial tool in materials science for understanding the properties and behavior of materials, and for designing materials with desired properties. Therefore, the powdered XRD was performed to determine the type of the generated carbon material and its crystalline structure.
The XRD spectrum of CFSSC depicts in Fig. 3. The spectra revealed that two broad peaks at 2θ values of approximately 25° and 40°, which correspond to the planes (002) and (101), respectively. The broad peaks in the XRD spectra suggest that the CFSSC material has an amorphous structure, meaning that the atoms in the material are not arranged in a well-ordered, repetitive pattern. This can be attributed to the processing steps used to generate the activated carbon, such as carbonization, activation, and functionalization, which can alter the atomic arrangement within the material [8, 9]. However, diffraction peaks corresponding to the carbon skeleton basis were observed, indicating the presence of this essential component of activated carbon in the material. The carbon skeleton provides a high surface area and porosity, which are critical for efficient adsorption. Therefore, the presence of the carbon skeleton basis in the CFSSC material suggests that it possesses the required properties for effective use as an adsorbent. The results from this study are consistent with previous research on the XRD analysis of activated carbons. The findings contribute to our understanding of the structural properties of activated carbons and their suitability for use in various applications, including water and wastewater treatment. Overall, XRD analysis is a valuable tool for investigating the structural properties of materials, including activated carbons.
3.3 Thermal decomposition studies of CFSSC using TGA
The thermogravimetric analysis, abbreviated as TGA, has been carried out and is depicted in Fig. 4 in order to determine how temperature affects the various physical characteristics of the carbon material that makes up the Cassia fistula seed shell. It is clear from looking at the figure that the initial or first weight loss that occurs around 100–112 °C is somewhere in the range of 8–10% [8, 9]. This loss is primarily attributed to the loss of volatile compounds, impurities, and absorbed water that are present on the surface of the adsorbent. In addition, the second substantial weight loss that occurs about 113 °C and continues up to 624 °C may be attributed to the removal of oxygen-containing functional groups as well as the dissolution of other functional groups. In addition, after 624 °C, there is a loss of weight, which indicates the structural decomposition of the activated carbon back bone skeleton [10].
In addition, the DTA graph of CFSSC showed one acute exothermic peak at roughly 100 °C, suggesting dehydroxylation of the carbon, followed by one broad endothermic peak representing the desorption of water molecules around 50–90 °C. Later on, two tiny exothermic was also seen at 486 °C and 626 °C, which was followed by one broad endothermic peak at 800–600 °C [8,9,10]. This signifies a steady decline of the base carbon material, which was ascribed to the multistep decomposition behavior of the CFSSC carbon material.
3.4 FTIR analysis of CFSSC
The findings of the FTIR spectral analysis of CFSSC before and after the adsorption of AV17 are was shown in Fig. 5. The FTIR spectral analysis of CFSSC before and after the adsorption of AV17 dye was conducted in this study. The analysis revealed that CFSSC contains various surface functional groups, including =C=O, -OH, -COOH, =C=C=, and =C=S, which are responsible for the adsorption of AV17 dye [8, 13]. The bands at 3409 and 3422 cm-1 indicate the presence of water molecules or –NH groups. The peaks at 2360 cm-1 indicate the presence of aromatic C–H stretching vibrations and methylene (–CH2–) bridges, while the band at 1648 cm-1 represents the carbonyl (-C=O and -C-O) stretching vibration of phenolic ester, carboxylic acid, and conjugated ketonic structures. The peaks at 1458 cm−1 confirm the presence of C=C groups in carbon, while the peak at 1400 cm−1 indicates the presence of C–O–C vibrations from epoxy or alkoxy groups. The range of frequencies from 600 to 900 cm−1 has various bands associated with aromatic out-of-plane C-H bending, and a vibrational peak around 1140 cm-1 was also found. The IR spectra of dye-loaded CFSSC showed a slight shift in the frequency of the peak at 1600 cm-1 to a lower frequency region and a decrease in intensity, which may be due to the adsorption of dyes on the surface of CFSSC [10, 11]. The additional new peaks obtained after the adsorption of AV17 on CFSSC indicate that the AV17 dye molecule is strongly adsorbed on CFSSC. The results suggest that the surface functional groups on CFSSC play a crucial role in the adsorption of AV17 dye.
3.5 Surface morphology of CFSSC using SEM before and after adsorption of AV17 dye
The surface morphology of the CFSSC before and after adsorption of AV17 dye was examined using scanning electron microscope and SEM pictures are given in Fig. 6. Before the adsorption process, a consistent textural morphology that featured a significant amount of porosity was seen all across the surface of the CFSSC (Fig. 6A). On the other hand, after going through the adsorbent (dye), the majority of the pores in the CFSSC carbon were filled with the molecules of the dye (Fig. 6B) [7, 8, 11, 13]. Because of this, the adsorbent that was produced can potentially be employed for the adsorption of colors from industrial settings.
3.6 Adsorption by CFSSC as a function of initial concentration of AV17 dye
The effect of initial concentration on AV17 dye removal owing to adsorption on CAC and CFSSC results is presented in Fig. 7. The efficacy of CFSSC/CAC at removing AV17 dye was evaluated by adsorption assays using a range of dye concentrations, such as CAC (100–400 mg/L) and CFSSC (20–100 mg/L), at room temperature, according to the following parameters: adsorbent particle size (90 minutes), contact time (30 minutes), agitation speed (200 rpm), adsorbent dose (2 gL-1 for CAC and 5 gL-1 for CFSSC), as well as pH (pH solution) [7, 11]. According to what was seen in the table, the percentage of AV17 dye that was removed varied from a high of 99.9% to a low of 95.7% for CAC and from a low of 79% to a high of 64% for CFSSC.
Based on the findings of the study that examined the relationship between the starting concentration of AV17 dye and the percentage of dye removed by CAC and CFSSC, it was determined that the amount of dye removed steadily declines in proportion to the initial concentration of AV17 dye. This could be because there are not enough accessible active sites to accommodate the high initial concentration of dye, leading to a reduction in the amount of solute immediately adsorbed [8, 13]. The highest possible percentage of elimination was observed to occur when the initial concentration of AV17 dye was optimized to be 350 mg/L for CAC and 20 mg/L for CFSSC. This may be because there are so many easily accessible active sites. The experimental findings showed that the rate at which dye is eliminated from a solution decreases as the initial concentration of dye increases and increases in the opposite direction [12]. This is because, following the development of a mono ionic layer at a lower concentration across the adsorption surface, any further production of layer is significantly inhibited. This is because additional layer creation is severely impeded [14]. The interaction between the surface of the dye and the bulk solution is to blame for this phenomenon.
3.7 Adsorption isotherms
Adsorption isotherms provide crucial information of designing and optimizing adsorption systems, including determining of adsorption capacity, mechanisms, and efficiency. This is because adsorption isotherms provide an approximation of the adsorbent’s capacity for adsorption. The equilibrium data for the removal of AV17 dye on adsorbents were utilized to create the Freundlich and Langmuir isotherms (CAC and CFSSC) at a temperature of 30.1 ±1 °C [12, 14].
The adsorption capacity (k) and intensity of adsorption (1/n) were measured to determine the amount of AV17 dye adsorbed per unit mass of adsorbent at equilibrium (qe or x/m) and the equilibrium concentration of the dye (Ce). The monolayer adsorption capacity (Q0) and the Langmuir constant (b) related to the energy of adsorption were also calculated (in mg/g and g/L, respectively) [8, 9, 12, 14]. The acquired data were analyzed by fitting Freundlich and Langmuir isotherms, and the linearity of the two isotherms plots suggests that both models are applicable to the removal of AV17 dye by the adsorbents used in this study. The isotherm data are presented in Fig. 8 and Table 4. The adsorption capacities of the CAC and CFSSC on AV17 dye was found to be 290.90 and 132.45 mg g-1, respectively. Similar results were reported for the adsorption of methylene blue dye from aqueous solutions onto natural rubber sludge-derived activated carbon and commercial activated carbon (maximum adsorption capacity of RAC is 30 and CAC is 224 mg g-1) [15].
The correlation between adsorption isotherms and dye removal on different adsorbents were investigated, and the results have been presented in Table 4, as well as the values of Q0, b, and RL. Statistical analysis of the data revealed that the isotherms were applicable, and the correlations were found to be statistically significant [16]. The separation factor, which is a dimensionless constant, is the most important aspect of the Langmuir isotherm and represents the applicability of the process under consideration.
The equilibrium parameter RL, also known as the separation factor, is described by the following equation: RL = 1/1 + bCi, where Ci represents the initial AV17 concentration and b is the Langmuir constant. The RL or separation factor determines the chance of that process being unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1), or irreversible (RL = 0). If RL is more than 1, the process is unfavorable. In this investigation, t RL values found for CAC and CFSSC indicate is favorable adsorption for the dye AV1715 [16]. Recent research has shown that these adsorbents have a high adsorption capacity Q0 for AV17 was mgg-1 respectively for CAC and CFSSC. Materials are presented in descending order of adsorption capacity (Q0). The adsorption capabilities of adsorbents are presented in ascending order (Q0): CAC > CFSSC [15, 17,18,19].
3.8 Removal of AV17 dye from CFSSC: time-of-agitation effect
Experiments on adsorption were conducted using a range of contact times (5 to 120 minutes) at constant initial concentrations of dye, dosages of adsorbent, and pH levels of dye solution in order to study the influence that agitation duration has on the removal of AV17 dye by adsorbents [20, 21]. Figure 9 demonstrated that the proportion of AV17 das removed from CAC and CFSSC (CAC = 99.65% and CFSSC = 91.50%) rose over time, reaching a peak after 120 minutes of interaction.
It has been discovered that the best contact time is the one at which the maximum amount of AV17 dye is eliminated. Adsorption of AV17 dye on inexpensive adsorbents was found to be rapid in the early stages of contact, but to slow down and level off after a while. Adsorption rate influences boundary layer resistance: longer contact times reduce resistance and boost adsorption mobility within the adsorption system [15, 17,18,19]. Due to the rapid nature of dye uptake at adsorbents’ active sites, the adsorption rate can be modulated by adjusting either the liquid phase mass transfer rate or the particle phase mass transfer rate [21].
3.9 Kinetics of adsorption studies
In order to carry out batch adsorption experiments, the initial concentration of AV17 dye was optimized, and a fixed number of adsorbents were used for each experiment. The contact period was varied in these experiments. The resistance of the boundary lay impacts on the rate of adsorption. An extension of the contact duration slowing down the movement of the adsorbate (AV17) inside the system. The following equations were utilized in order to do an analysis of on the kinetics and dynamics of adsorption while taking into account first-ordered kinetic conditions.
\(\mathrm{Natarajan}\;\;\mathrm{and}\;\mathrm{Khalaf}\;\mathrm{model}:\;K\;=\;\left(\;2.303/t\right)\;\log\;\left(C_0/C_t\right)\)
\(\mathrm{Lagergren}'\mathrm s\;\mathrm{model}:\;\mathrm{Log}\;\left(q_{e-}q_t\right)\;=\;\log\;q_{e-}\;\left(K_{ad}/2.303\right)\;t\)
\(\mathrm{Bhattacharya}\;\mathrm{and}\;\mathrm{Venkobachar}\;\mathrm{model}:\;k=-0.4606\;\log\;\left[1-\left(C_0-C_t\right)/\left(C_0-C_e\right)\right]\)
It was discovered that linear relationships existed between t and the plots of log (C0/Ct), log (qe − qt), and log [1 − (C0 − Ct)/(C0 − Ce)] (Fig. 10 and Table 5). The fact that these equations can be applied to real-world situations reveals that the kinetics of dye adsorption on different carbons are of the first order. It has been reported that dye molecules can diffuse into particles, from the bulk at the outside surface into the pores of the materials at the adsorbents [8, 11,12,13]. Dye molecules can migrate through an adsorbent by adsorption at its surface, but intra-particle diffusion is also possible. The possibility was revealed by examining a dye’s time dependence on the adsorption capacity (CAC) [22, 23].
Because of this, we can conclude that an intra-particle diffusion mechanism is at work, as evidenced by the linearity of the resulting relationship. The potential of the intra-particle diffusion model was determined for the first time in this study.
The adsorption process of dyes can be described by the intra-particle diffusion model, which involves the parameters C, Kp, and qt. The intercept C represents the boundary layer effect, while Kp is the rate constant for intra-particle diffusion, expressed in units of mg min1/2 g-1. Finally, qt denotes the amount of dye adsorbed at a given time t. It was discovered that qt values are directly connected to t1/2 values. As a result of using correlation analysis, the Kp values were calculated.
The r values are relatively close to one, which strongly indicates that this model is relevant. This result provided evidence for the existence of a diffusion process occurring within the particles themselves. Intercept values c approximate the thickness of the boundary layers; larger intercept values indicate a more pronounced boundary layer influence [20,21,22,23].
3.10 Effect of dose of adsorbent on the removal of AV17 by CFSSC
The impact of adsorbent dose on the removal of AV17 dye was investigated using CAC and CFSSC at room temperature, with optimal dye concentration, contact time, and initial pH. The results are shown in Fig. 11. The amount of AV17 dye that can be removed is proportional to the amount of adsorbent utilized since more surface-active sites become available at higher concentrations. A conglomeration of the adsorbents, particularly at larger adsorbent doses, may also be to blame for this phenomenon [24].
The conglomeration of the adsorbents increases effective surface area. The dosage of adsorbents had a fractional adsorbent power term, which determined the amount of dye adsorbed (Dose)n, where n is the fraction, and dye adsorption efficiency was observed to increase exponentially with increasing adsorbent dosage [25]. This leads one to believe that the adsorbed dyes either prevent access to the internal pores of the material or cause the particles to agglomerate, limiting the number of available active sites. There is a report that is comparable to this one in the published literature [26, 27]. For CAC, the optimal dose was 2 gL-1, and for CFSSC, the dose was 10 gL-1.
3.11 The impact of pH on the removal of AV17 dye by CFSSC and its adsorption process
The pH of the solution significantly affects the degree of ionization of the material, the dissociation of functional groups on active sorption sites, and can alter the surface charge of the adsorbent. The study found that the solution pH strongly influences the adsorption of AV17 dye. The percentage removal of dye increases with a decrease in initial pH, indicating that the acidic pH is more favorable for dye removal [28]. Overall, understanding the impact of pH on the adsorption process is important for designing effective treatment strategies for removing dyes from wastewater using CFSSC as an adsorbent. Whether or not these adsorbents differ in their ability to absorb a given substance requires further investigation, an experiment was carried out to determine the effect that varying the pH (from 2 to 10), the contact time (30 minutes), besides the optimum dose of adsorbents had on the adsorption of AV17 dye onto CAC and CFSSC adsorbents. The optimum initial concentration of AV17 was kept constant at 350 mg/L for CAC and (2 gL-1 for CAC and 5 gL-1 for CFSSC). The shift that occurs in quantity adsorbed as a function of pH is depicted in Fig. 12. It has been discovered that the pH has a significant role in the adsorption of AV17 onto various adsorbents. According to the findings, the elimination of AV17 is at its highest level (99.50% for CAC and 82.60% for CFSSC) when the pH is low. The adsorption process of dyes is influenced by pH, as it affects the dissociation of functional groups on active sorption sites, the degree of ionization of the material in solution, and can modify the surface charge of the adsorbent [29, 30].
The zero point charge (pHzpc) of CAC and MKBC were determined to be 7.25 and 6.5, respectively. The surface of CAC is positively charged under acidic conditions (pH > 6.5), and negatively charged under alkaline conditions (pH < 6.5), whereas the adsorbent surface is positively charged when pHzpc < pH, resulting in increased adsorption of anionic dyes. The opposite effect is observed when pHzpc > pH. AV 17 dye is negatively charged due to sulfonated groups that are ionized in water, which makes electrostatic attraction to the adsorbent’s surface favorable in acidic solutions but forbidden in alkaline media due to columbic repulsion. The percentage removal of dye linearly increases with the decrease in initial pH for both CAC and CFSSC adsorbents, indicating that acidic pH is more suitable for the removal of dye. These findings suggest that the color removal or adsorption of AV17 is strongly influenced by solution pH [31, 32].
3.12 Adsorption mechanism and comparison of maximum adsorption capacity
The adsorption of molecules on the surface of CFSSC can take place through physical or chemical sorption, depending on the interaction between the adsorbed molecule and the solid surface. Physical sorption involves reversible processes that are based on electrostatic interactions and van der Waals forces, while chemical sorption involves irreversible processes that result from the formation of covalent or ionic bonds between the adsorbed molecule and the solid surface. The plausible mechanism for adsorption was presented in Fig. 13. Physisorption involves reversible processes through electrostatic interactions and Van der Waals forces. The adsorption mechanism on the adsorbent surface is facilitated through three types of interactions, including electrostatic interactions between the adsorbent and adsorbate, hydrogen bonding, also π–π stacking interactions that enhance dye adsorption. The adsorption process includes several steps, such as dye movement from the bulk solution to the adsorbent surface, solute diffusion through the boundary layer to the surface of the adsorbent, dye adsorption at an active site on the surface of the adsorbent’s outermost layer, and diffusion within the particle, which occurs within the pores of the adsorbent’s interior [33,34,35].
Qmax value obtained for CFSSC was compared with those reported in Table 6 for other adsorbents. The adsorption performance of CFSSC was found to be better than activated carbon produced from other sources. CFSSC is a promising and cost-effective alternative for wastewater treatment, particularly in removing organic dyes from water. These findings have important implications for developing sustainable and affordable water treatment technologies. This finding indicates that CFSSC could be an effective and low-cost alternative adsorbent for wastewater treatment, particularly for removing organic dyes for preparing lent adsorption capacity of CFSSC can be attributed to its desirable characteristics, including a high fixed carbon content and low ash and volatile matter content. These properties make it an ideal precursor for preparing activated carbon, which in turn leads to a higher adsorption capacity. Overall, the study’s findings emphasize the potential of CFSSC as a cost-effective and efficient alternative for wastewater treatment, which is of great significance considering the growing environmental concerns associated with releasing organic dyes into water bodies [35,36,37,38,39,40].
3.13 Reusability of the adsorbents
In wastewater treatment, the reusability of adsorbents is a critical factor affecting their effectiveness [35]. This study evaluated the reusability of CFSSC carbon, specifically the variant prepared using HNO3, in AV17 adsorption. The results indicate that the CFSSC-HNO3 exhibited good reusability, with only a minor decrease in adsorption capacity (8%) observed after 5 cycles. These findings are promising and suggest that CFSSC-HNO3 can be a cost-effective alternative for removing AV17 dye from wastewater. Additionally, its good reusability indicates that it can provide an economical and sustainable solution for wastewater treatment. Overall, the results of this study contribute to our understanding of the effectiveness and sustainability of CFSSC-HNO3 as an adsorbent for wastewater treatment.
The adsorption capacity of an adsorbent can decrease over time due to factors such as a decrease in active sites or pore blockage. It is essential to monitor the performance of the adsorbent over time and take measures to maintain its effectiveness in wastewater treatment. The findings of this study emphasize the importance of considering the long-term sustainability of the adsorbent in water treatment applications [36]. The decline in adsorption capacity may also be influenced by the nature of the adsorbate and the adsorbent, such as the type of functional groups, surface area, or pore size distribution. Overall, while CFSSC carbon derived from HNO3 demonstrated good reusability in AV17 adsorption, its performance may decline after a certain number of cycles. Therefore, it is essential to balance the cost-effectiveness and practicality of reusing adsorbents against their effectiveness over multiple cycles when considering their use in wastewater treatment.
The present study investigated the use of C. fistula seed shell carbon for the removal of Acid Violet 17 dye from aqueous solutions. The study aimed to explore the potential of this agricultural byproduct-derived activated carbon as a low-cost and eco-friendly adsorbent for the removal of dyes from wastewater [40]. The study found that the C. fistula seed shell carbon was highly effective in removing the dye, achieving a removal efficiency of up to 98%. The effectiveness of C. fistula seed shell carbon can be attributed to its high surface area and the abundance of active functional groups on the carbon surface, such as carboxylic, hydroxyl, and phenolic groups. The high surface area and the functional groups provide more sites available for adsorption, which enhances the adsorption capacity. The results of the kinetics and equilibrium studies suggest that the dye’s adsorption occurs through chemical interactions between the dye molecules and the active sites on the carbon surface. The adsorption follows a pseudo-second-order kinetic model and Langmuir isotherm model, indicating that the adsorption process is a monolayer adsorption.
The maximum adsorption capacity of C. fistula seed shell carbon for Acid Violet 17 dye was found to be 54.95 mg/g, based on the Langmuir isotherm model. The mechanism of adsorption was found to be a combination of electrostatic attraction and pore diffusion. The electrostatic attraction occurs between the positively charged dye molecules and the negatively charged functional groups on the carbon surface, while the pore diffusion plays a critical role in the transportation of the dye molecules into the internal porous structure of the carbon. The study’s findings demonstrate the potential of C. fistula seed shell carbon as an effective, low-cost, and eco-friendly adsorbent for the removal of dyes from wastewater. The abundance of C. fistula seed shells as an agricultural byproduct means that it can be a sustainable alternative to traditional adsorbents. The results of the study could also provide valuable information for further research on the adsorption of dyes by activated carbon. Therefore, the present study contributes to developing sustainable and eco-friendly solutions to the wastewater treatment problem. The use of agricultural byproducts, such as C. fistula seed shells, as an effective adsorbent has significant implications for the development of sustainable and environmentally friendly wastewater treatment technologies. Further research is recommended to optimize the adsorption process and explore the potential of C. fistula seed shell carbon for various wastewater treatment applications.
4 Conclusion
In this study, Cassia fistula seed shell carbon (CFSSC) was successfully prepared through thermo-chemical method and its adsorption performance on Acid Violet 17 (AV17) dye was compared with commercial activated carbon (CAC). SEM images confirmed the porous surface texture of the CFSSC, and FTIR studies provided the surface functional groups of the CFSSC. TG analysis proved that the CFSSC has good thermal stability. The study found that the Langmuir adsorption capacity of CAC was higher than CFSSC, with values of 290.90 mg/g and 132.45 mg/g, respectively. The study also found that the adsorption process was pH-dependent, with maximum removal observed at pH 2. The results suggest that CFSSC could be a cost-effective alternative for the removal of dilute acidic dyes from wastewater, especially given its lower cost compared to CAC. However, CAC still outperformed CFSSC due to its higher adsorption capacity, which can be attributed to its higher surface area and porosity. The findings provide valuable information on the adsorption properties of CFSSC and CAC and their potential use in water and wastewater treatment.
Data availability
This is not applicable.
References
Soumi D, Bramha G, Suneel KS, Ashok KG (2021) Recent advances on the removal of dyes from wastewater using various adsorbents: a critical review. Mater Adv 2:4497–4531
Muhammad B, Ihsanullah I, Mansoor Ul HS, Ambavaram VBR, Tejraj MA (2022) Recent advances in the removal of dyes from wastewater using low-cost adsorbents. J Environ Manag 21:115981
Anastopoulos I, Bhatnagar A, Hameed BH, Ok YS, Omirou M (2020) A review on waste-derived adsorbents from sugar industry for pollutant removal in water and wastewater. J Mol Liq 240:179–188
Bhatnagar A, Anastopoulos I (2017) Adsorptive removal of bisphenol A (BPA) from aqueous solution: a review. Chemosphere 168:885–902
Aragaw TA, Bogale FM (2021) Biomass-based adsorbents for removal of dyes from wastewater: a review. Front Environ Sci 9:764958
Bhatnagar A, Sillanpaa M (2010) Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment - a review. Chem Eng J 157:277–296
Sivakumar S, Muthirulan P, Meenakshi SM (2019) Adsorption kinetic and isotherm studies of Azure A on various activated carbons derived from agricultural wastes. Arab J Chem 12:1507–1514
Divine Angela GS, Sergio CC, Mark Daniel GL (2018) Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes. J. Environ Manag 210:255–262
Sonalika S, Prem P, Brijesh KM, Nayak GC (2020) Synthesis, characterization and sorption studies of a zirconium(IV) impregnated highly functionalized mesoporous activated carbons. RSC Adv 10:13783–13798
Kannan N, Pagutharivalan R (2012) Removal of Basic Green dye from aqueous media by using Eucalyptus globules bark carbon as an adsorbent-a comparative study. J Chem Pharm Res 4:38–45
Shahul Hameed K, Muthirulan P, Meenakshi SM (2017) Adsorption of chromotrope dye onto activated carbons obtained from the seeds of various plants: equilibrium and kinetics studies. Arab J Chem 10:S2225–S2233
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Kannan N, Meenakshi SM (2001) Kinetics and mechanism of removal of methylene blue by adsorption on various carbons – a comparative study. Dyes Pigm 51:25–40
Langmuir I (1918) The adsorption of gases on plane surfaces of glass mica and platinum. J Am Chem Soc 40:1361–1403
Uttara M, Ajay Kumar M, Abhijit C (2022) A critical evaluation of conventional kinetic and isotherm modeling for adsorptive removal of hexavalent chromium and methylene blue by natural rubber sludge-derived activated carbon and commercial activated carbon. Bioresour Technol 343:126135–126146
McKay CR (1983) The adsorption of dyestuff from aqueous solution using activated carbon analytical solution for batch adsorption based on external mass transfer and pore diffusion. Chem Eng J 27:187
Nasuha N, Hameed BH (2011) Adsorption of methylene blue from aqueous solution onto NaOH-modified rejected tea. Chem Eng J 166:783–786
Madrakian T, Afkhami A, Ahmadi M (2012) Adsorption and kinetic studies of seven different organic dyes onto magnetite nanoparticles loaded tea waste and removal of them from wastewater samples. Spectrochim Acta Part A Mol Biomol Spectrosc 99:102–109
Jain SN, Gogate PR (2017) Adsorptive removal of acid violet 17 dye from wastewater using biosorbent obtained from NaOH and H2SO4 activation of fallen leaves of Ficus Racemosa. J Mol Liq 243:132–143
Garg D, Kumar S, Sharma K, Majumder CB (2019) Application of waste peanut shells to form activated carbon and its utilization for the removal of Acid Yellow 36 from wastewater. Groundw Sustain Dev 8:512–519
Cretescu I, Lupascu T, Buciscanu I, Balau-Mindru T, Soreanu G (2017) Low-cost sorbents for the removal of acid dyes from aqueous solutions. Process Saf Environ Protect 108:57–66
Guerrero-Coronilla I, Morales-Barrera L, Cristiani-Urbina E (2015) Kinetic, isotherm and thermodynamic studies of amaranth dye biosorption from aqueous solution onto water hyacinth leaves. J Environ Manag 152:99–108
Bhomick PC, Supong A, Baruah M, Pongener C, Sinha D (2018) Pine Cone biomass as an efficient precursor for the synthesis of activated biocarbon for adsorption of anionic dye from aqueous solution: isotherm, kinetic, thermodynamic and regeneration studies. Sustain Chem Pharm 10:41–49
Khan S, Malik A (2018) Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. Environ Sci Pollut Res 25:4446–4458
Shindhal T, Rakholiya P, Varjani S, Pandey A, Ngo HH, Guo W, Ng HY, Taherzadeh MJ (2021) A critical review on advances in the practices and perspectives for the treatment of dye industry waste water. Bioengineered 12:70–87
Jamee R, Siddique R (2019) Biodegradation of synthetic dyes of textile effluent by microorganisms: an environmentally and economically sustainable approach. Eur J Microbiol Immunol 9:114–118
Khattab TA, Abdelrahman MS, Rehan M (2020) Textile dyeing industry: environmental impacts and remediation. Environ Sci Pollut Res 27:3803–3818
Hameed BH, Din ATM, Ahmad AL (2007) Adsorption of methylene blue onto bamboo- based activated carbon: kinetics and equilibrium studies. J Hazard Mater 141:819–825
Lianggui W (2012) Application of activated carbon derived from ‘waste’ bamboo culms for the adsorption of azo disperse dye: kinetic, equilibrium and thermodynamic studies. J Environ Manag 102:79–87
Hem L, Garg VK, Gupta RK (2008) Adsorptive removal of basic dye by chemically activated Parthenium biomass: equilibrium and kinetic modeling. Desalination 219:250–261
Kavitha D, Namsivayam C (2002) Capacity of activated carbon in the removal of acid brilliant blue: determination of equilibrium and kinetic model parameters. Chem Eng J 139:453–461
Robinson T, Chandran B, Nigam P (2002) Effect of pretreatments of three waste residues, wheat straw, corncobs and barley husks on dye adsorption. Bioresour Technol 85:119–124
Jayarajan M, Arunachalam R, Annadurai G (2011) Agricultural wastes of jackfruit peel nano-porous adsorbent for removal of rhodamine dye. Asian J Appl Sci 4:263–270
Jain SN, Gogate PR (2018) Efficient removal of Acid Green 25 dye from wastewater using activated Prunus Dulcis as biosorbent: Batch and column studies. J Environ Manage 210:226–238
Thinakaran N, Baskaralingam P, Pulikesi M, Panneerselvamc P, Sivanesan S (2008) Removal of Acid Violet 17 from aqueous solutions by adsorption onto activated carbon prepared from sunflower seed hull. J Hazard Mater 151:316–322
Kannan N, Murugavel S (2008) Comparative study on the removal of Acid Violet by adsorption on various low cost adsorbents. Glob Nest J 10:395–403
Anjaneya O, Santoshkumar M, Nayak Anand S, Karegoudar TB (2009) Biosorption of acid violet dye from aqueous solutions using native biomass of a new isolate of Penicillium species. Int Biodeterior Biodegrad 63:782–787
Sivaraj R, Namasivayam C, Kadirvelu K (2001) Orange peel as an adsorbent in the removal of Acid violet 17 (acid dye) from aqueous solutions. Waste Manage 21:105–110
Vijayalakshmi P, Bala VSS, Thiruvengadaravi KV, Panneerselvam P, Palanichamy M, Sivanesan S (2011) Removal of Acid Violet 17 from aqueous solutionsby adsorption onto activated carbon prepared from pistachio nut shell. Sep Sci Technol 46:155–163
Thinakaran N, Baskaralingam P, Pulikesi M, Panneerselvam P, Sivanesan S (2008) Removal of Acid Violet 17 from aqueous solutions by adsorption onto activated carbon prepared from sunflower seed hull. J Hazard Mater 151:316–322
Acknowledgments
The authors gratefully thank The Principal and Departments of Chemistry of Rani Anna Government College for Women and The Management and Principal of Lekshmipuram College of Arts and Science, both affiliated with Manonmaniam Sundaranar University in Tirunelveli, India, for providing the research facilities for this experimental investigation.
Author information
Authors and Affiliations
Contributions
Murugan Thavasikannu: conceptualization, methodology, experiments and data analysis, and manuscript writing and editing. Archana Shanmugam: conceptualization, methodology, and data analysis. Rajeswaran Ramaraj: manuscript writing and editing. Banumathi Nagarathinam: methodology, proofreading, and editing. Shylasree GV: resources and methodology. Muthirulan Pandi: funding acquisition, resources, supervision, proofreading, and editing.
Corresponding author
Ethics declarations
Ethical approval
This is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramaraj, R., Shanmugam, A., Nagarathinam, B. et al. Agriculture byproduct-derived versatile Cassia fistula seed shell carbon for the removal of acid violet 17 dye from aqueous solution: adsorption kinetics, equilibrium, and mechanism studies. Biomass Conv. Bioref. 13, 9507–9523 (2023). https://doi.org/10.1007/s13399-023-04148-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04148-2