Abstract
In the present work, we used an eco-friendly, simple, and selective nitrogen (N2) activation strategy for the preparation of hierarchical porous activated carbon (HPAC) derived from abundant and low-cost Terminalia catappa shell (TCS) agro-waste. The effect of N2 activation strategy compared to air activation is studied where the N2 activated carbon (HPAC) showed enhancement in the specific surface area (SSA) (616.04 m2 g−1) as compared to air activated carbon (HPC) which showed 243.97 m2 g−1. Both activated carbons were further explored for CO2 capture studies and showed a relatively high capacity (2.73 mmol g−1) for HPAC than HPC (1.70 mmol g−1) at 288 K. The low N2 capture capacity (0.22 mmol g−1) of HPAC suggests its selectivity toward CO2 (41.80 at 288 K) is useful for gas separation. Obtained HPAC was successfully applied for adsorption removal of methyl orange (MO) dye with detailed kinetics studies resulting in 99.50% removal efficiency. The optimized adsorption conditions were further used for adsorption removal studies of methylene blue (MB), malachite green (MG), and bromophenol blue (BPB) with 75.30, 82.88, and 52.40% removal efficiency respectively. The superior CO2 capture and potential dye removal property of obtained HPAC suggests its applicability in environmental remediation.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hierarchical porous activated carbons (HPACs) have been enormously applied in various research areas including catalysis, CO2 capture, heavy metal ion and dye removal by adsorption, energy applications such as supercapacitor and battery [1,2,3,4,5,6]. Among these areas, HPACs have become one of the largest applicative adsorptive materials that showed potential for environmental applications viz. air and water remediation [7, 8]. Meanwhile they have been considered efficient in improving respective application performances by enhancing specific surface area (SSA) and total pore volume (Vt) which drastically increases the adsorption capacities of the HPACs. Therefore, optimizing these properties has always been a promising approach in recent years. The activation strategy at different temperatures and under different environments plays an important role in improving HPAC properties and adsorption [9]. It is well proven that activation of HPACs results in larger SSA which provide large active sites, offering high adsorption capacity for adsorption applications. Similarly, rich ordered meso and micropores as well as increasing amounts of nitrogen are very much applicable for the capture capacities. Excessive meso and micro pores contribute to an increment in Vt and a decrease in the density of HPACs [10]. In this regards, HPACs which possesses high SSA and Vt are appreciable candidates for potential adsorptive applications such as CO2 capture and dyes removal [11, 12].
The accumulation of gas and dyes in the environmental aspects is mainly responsible for pollution, which is a severe environmental issue. The CO2 capture technology exhibits a substantial reduction in the emission level of gas from the atmosphere and dye adsorption removes carcinogenic dyes from aqueous media [13]. Therefore, researchers are focused on developing HPAC as an efficient material for CO2 capture and dye removal applications by varying synthetic paths such as carbonization and activation. The first step is to prepare carbonized samples by pyrolysis or using chemical carbonizing agents (H2SO4, HNO3, H3PO4 etc.), which often give raw carbon material. The next step is to activate raw carbon with the help of activating agents (KOH, NaOH, ZnCl2 etc.) or inert gases (N2, CO2, Ar etc.) [14, 15]. The chemical activation process facilitates the formation and release of carbon monoxide results in to porous carbon matrix however inert gas activation enhances porosity by direct pyrolysis preventing oxidation [3]. Still, there are numerous challenges for preparing HPACs because of limited precursor availability and high price for large scale applications [16]. To overcome these problems and challenges, natural precursors such as agro-wastes are proven to be a promising source for HPACs [17]. The availability of agro-waste from different natural sources increases every year worldwide [18]. Combusting these agro-wastes leads to dangerous effects on the natural environment [5]. By keeping in front the above discussion, the developing eco-friendly and facile synthesis approach to prepare HPACs from such agro-wastes instead of burning and utilizing them for environmental remediation is the best alternative [19].
In continuation of our precious work [20], herein we used easily available and low cost Terminalia catappa shell (TCS) agro-waste as a source for HPACs. The air and N2 activation strategy was employed to study the effect of the activation strategy on the HPAC properties. Further, both air activated carbon (HPC) and N2 activated carbon (HPAC) were thoroughly characterized and employed for the CO2 capture study. Further, the HPAC studied for dye adsorption using methyl orange (MO) as model dye and the optimised conditions were employed for methylene blue (MB), Malachite green (MG) and Bromophenol blue (BPB) adsorption to investigate environmental remediation applicability.
2 Experimental
2.1 Materials and methods
Terminalia catappa was collected from the Botanical garden, Jaysingpur College, Jaysingpur. Terminalia Catappa shell (TCS) material was prepared by bursting the Terminalia catappa fruit followed by washing to remove dust and drying under sunlight for several days. AR grade H2SO4 (Loba-Chem, India.), CO2 and N2 gas (purity > 99.9%), Methyl orange (Sigma Aldrich), Methylene blue (Merck, India), Malachite green G (Fischer Scientific India), and Bromophenol blue (Sd-Fine Chemicals, India) were used are of AR grade; however, distilled deionized (DI) water was used throughout the experiment.
The carbonization of TCS material, its activation and CO2 capture and dye removal kinetics were studied by following our previous work [20]. Briefly, the TCS material was carbonized by soaking in concentrated H2SO4 (1:1 mass ratio with precursor) for 24 h with intimate stirring followed by washing with deionized water and vacuum drying at 373 K for 2 h to obtain raw carbon. The obtained raw carbon material was further activated at 773 K for 2 h under air (named HPC) and N2 gas (named HPAC) separately in tube furnace and the resulting activated carbons, HPC and HPAC were crushed using agate mortar to a finer size for using them for capture applications.
2.2 Characterization
A thorough spectroscopic and microscopic analysis of obtained HPC and HPAC was carried out to get structural, morphological, and elemental properties. The powder X-ray diffraction (PXRD) patterns were examined on a Philips X-ray diffractometer employing nickel-filtered Cu Kα radiation. The Fourier Transform Infrared (FTIR) spectra were recorded in the range of 400–4000 cm−1 on FTIR-4600, Jasco, Japan model. The images of field emission scanning electron microscopy (FESEM) micrographs were obtained using FESEM, Jeol, JSM-6360. The elemental analysis (C, H, and N) was carried out using Elemental Analyzer Thermo, Finnigan and the mass percentage of oxygen was determined by subtraction method. The X-ray photoelectron spectroscopy (XPS) was performed using a Shimadzu ESCA 3400 instrument. The determinations of surface area and pore volume were carried out from N2 adsorption–desorption isotherms at relative pressure (p/po) 0.99 and 77 K using BET analyzer, Bell sorp mini II, Japan. The Barret-Joyner-Halanda (BJH) method was employed for the determination of pore size distributions from adsorption branches of isotherm.
2.3 CO2 adsorption studies
The HPC and HPAC samples were activated by degassed at 573 K for 5 h under a constant vacuum (~ 10–6 to 10–7 mbar) to remove the guest molecules from the carbon pores. The adsorption tests were conducted at constant temperature 288 K, 298 K and 308 K under 1 atm pressure range using gas adsorption analyzer (Bellsorp mini II). In order to complement the CO2 adsorption studies, the isosteric heat of adsorption was determined based on the Clausius–Clapeyron equation using CO2 adsorption isotherms at different temperatures. These adsorption isotherms were fitted with Dual-site Langmuir models to determine the adsorption correlation. In addition, the prediction of binary mixtures CO2/N2 selectivity was done by employing Henry’s Law.
2.4 Dye removal study of HPAC
The dye removal efficiency of HPAC was determined by batch equilibrium adsorption experiments. The experiments were carried out in a series of solutions with 0.1 g HPAC and 50 mL MO dye solution in the concentration range of 20–400 mgL−1. The dye and HPAC were continuously mixed with shaking on an orbital shaker at 200 rpm to ensure complete mixing and to attain equilibrium. The 2 mL dye solution fractions were collected at incremental time intervals and the adsorbent was separated from the dye solution using a filter membrane (0.22 μm). The absorbance of MO, MG, MB and BPB dye was measured by monitoring the absorbance respectively at 464, 617, 664, and 590 nm with a UV–Vis spectrophotometer (UV-3600, Lab India). The percentage dye removal (E %) and adsorption kinetics was determined from 20 mgL−1 MO dye experiment, while the equilibrium adsorption capacity (qe) of the HPAC for MO was determined. Further, the optimized adsorption condition was employed for the adsorption removal study of MB, MG and BPB using 20 mgL−1 dye concentration and their percentage removal (E%) was determined.
3 Results and discussion
The specific surface area (SSA), porosity and pore size distribution (PSD) of obtained samples were determined from the N2 adsorption/desorption isotherms, BET and BJH plot. The isotherms (Fig. 1a) showed that both HPC and HPAC exhibit type I isotherm, capillary condensation in relative pressure (p/p0) below 0.1, and a very small hysteresis loop at 0.4, which possess the characteristics of monolayer adsorption in micropores and mesopores, respectively. It means that both HPC and HPAC showed a hierarchically porous nature, which creates larger space for trapping sorbates in that pores; however, from PSD (Fig. 1b), it was observed that the N2 activation increases microporosity below 1 nm along with mesoporosity in HPAC. In other words, although there were two kinds of pores (micro- and meso-) for HPAC, the volume of micropores below ∼1 nm is more than HPC, which is the size limit establishing the volume-filling mechanism for adsorption [21]. When adsorption occurs at narrow pores, there is a strong interaction between sorbate and sorbent, ascribed to the overlapping of the potential fields from the side walls could be beneficial for CO2 capture and dye adsorption. The textural parameters are summarized in Table 1, which shows that the HPC and HPAC exhibits 243.97 and 616.04 m2 g−1 of SSA 0.13 and 0.26 cm3 g−1 of Vt respectively. Obviously, the SSA and Vt of HPAC drastically increased over HPC due to the unblocking of the pore walls during the activation. Therefore, the nitrogen activation at 773 K can lead to the maximum SSA and Vt.
The PXRD technique is used for the determination of crystal structure, phase purity, and the nature of the material. The PXRD patterns of HPC and HPAC samples obtained are given in Fig. 1c. The broad peaks in the 2θ range of 23.82O to 25.03O and 42.57O to 43.52O respectively corresponding to the (002) planes and (101) planes revealed typical lattice planes of the amorphous carbon material [22]. The interplaner distance, d(002) in inner layers of graphite is 3.35 Å, wherein, this distance in the synthesized HPC and HPAC was found to be 3.86 and 3.64 Å respectively, additionally the relative intensity for the broad peak 43.52O in HPC is slightly weaker than HPAC, revels that N2 activation can moderately shrinks the inner layer distance or increases the degree of graphitization in HPAC. The surface functional groups of HPC and HPAC were confirmed by FT–IR analysis (Fig. 1d). The broad band between 2900 cm−1 to 3500 cm−1 was assigned to hydrogen-bonded –OH functional groups. According to peak analysis, two bands observed at ~ 1154 cm−1 and 1590 cm−1 are associated with –C–O stretching in either alcohol, ether, phenol or lactones and stretching of –C = C– bond in aromatic compounds, respectively. The sharp peak at 1707 cm−1 demonstrated the presence of –C = O functional group along with a high intense peak in HPAC [23, 24].
The FE-SEM images (Fig. 2) reveal that the HPAC material constructed by TCS shows a fully developed array of the honeycomb structure with hierarchical pores, which were connected with each other and contribute to micropores along with mesopores offering larger adsorption opportunities for dye and gas trap. In contrast HPC displays an irregular porous surface and slightly close-packed structure, which is in good agreement with XRD results confirming graphitization in HPAC as shown in Fig. 1c.
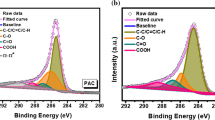
The XPS full survey spectrum of HPC and HPAC shows the presence of C1s peak at 283.6 eV, N1s peak at 400.2 eV and O1s peak at 531 eV (Fig. 3a and 3b). The high resolution XPS spectra of C1s and N1s are shown in Fig. 3c–d and 3e–f, respectively, for HPC and HPAC. The three deconvoluted peaks located at 284.7 (C1), 285.9 (C2) and 290.5 eV (C3) are assigned to C = C/C–C, C-N/C-O and O-C = O, respectively [25]. Meanwhile, the N1s peak of the HPAC can be resolved into three peaks centered at binding energies of 398.8 eV, 399.9 eV and 400.4 eV, representing pyridinic-(N2), quaternary-(N3) and pyrrolic-(N4) N atoms, respectively [26, 27]. This is beneficial for the enhancement in capture capacities. In contrast, air-activated HPC showed one peak centered at 398.7 eV attributed to pyridinic-N (N1). The peak intensities of C1s in HPAC are observed to be greater than HPC, confirming the graphitization under the N2 environment, which supports the XRD results. Further, the nitrogen (N) content of the HPC and HPAC was characterized by elemental analysis (Table 2). The N content in HPAC was found to be 0.90 wt% and 2.8 w% based on the elemental and XPS analysis, respectively which is little higher than HPC. From the results, it can be attributed that the N2 activation prevents the removal of elemental nitrogen compared to air activation.
3.1 CO2 capture capacity of HPC and HPAC samples
The CO2 adsorption experiments were carried out using a Belsorp mini II at 288, 298, and 308 K (Fig. 4a) and the results are tabulated in Table 3. The results showed that the CO2 capture capacity of HPC is less (1.70 mmol g−1) than that of HPAC (2.73 mmol g−1) at 288 K. The hierarchical porosity along with more micropore character and high SSA (616.04 m2 g−1) of HPAC than HPC enhances CO2 capture capacity. The low CO2 capture capacity of HPC may be ascribed to the poor pore structure due to obstruction by the tar and impurities into the developed pores, which reduces its SSA and active site for adsorption. However, the N2 activation helps to generate of well-defined pores in HPAC with high SSA with more microporous character, and provides strong interaction between sorbate and sorbent which eventually boosts CO2 capture capacity. Herein, it is observed that, HPAC exhibits CO2 adsorption capacity of 2.73, 2.30, and 1.82 mmol g−1 at 288, 298, and 308 K, respectively. CO2 adsorption is an exothermic process and temperature greatly influences the amount of CO2 adsorption. Based on the volume-filling mechanism, the volume of micropores, especially (< 1 nm) is most responsible for CO2 adsorption [20]. Additionally, the presence of pyrrolic nitrogen in HPAC is strongly bonded with active sites of acidic CO2 because functional nitrogen serves as Lewis-base contributing to high CO2 capture. The N2 capture on HPAC shows a small capture capacity of 0.22 mmol g−1 at 288 K (Fig. 4b), indicating that HPAC could be used for potential selective adsorbent for CO2 and N2 separation.
The Henry’s law is used to determine the CO2/N2 selectivity (Sij) and calculated by applying Eq. 1 and the Isosteric heat of adsorption (Qst) is determined using Clausius–Clapeyron equation (Eq. 2) by fitting the CO2 adsorption data using dual site Langmuir model, Eq. 3
where, KH is Henry’s Law constant; i for CO2 and j for N2, q is amount of CO2 adsorbed (mmol g−1), qs is saturation adsorption capacity (mmol g−1), and b is equilibrium constants (Pa−1) [28].
The selectivity of CO2 over N2 acquired by the ratio of Henry’s law constant [29] (Table 4) from pure gas isotherms was found to be 41.80 at 288 K (Fig. 4b), which signifies the more selectivity of HPAC towards the CO2 than N2 which can be beneficial for CO2 and N2 separation.
The Qst values of CO2 adsorption in HPAC are found to be 26–29 kJ mol−1 (Fig. 4c), as calculated by the Clausius–Clapeyron equation Eq. 2 [30, 31] using dual-site Langmuir (Fig. 5 and Eq. 3) parameters at 288 and 298 K, which indicate that the HPAC interacts strongly with the CO2 molecules. Therefore, higher C content, electron rich N atoms, and more microporosity due to N2 activation enhances CO2 capture capacities of HPAC than air activated HPC. The literature reveals that sugarcane pith [20], rice husk [32], peanut shell [33], rubber seed shell [34] and Nauclea diderrichii [35] agro-waste were successfully used for the CO2 capture and dye removal applications.
3.2 Application of HPAC for adsorptive removal of organic dyes (methyl orange, methylene blue, malachite green, and bromophenol blue)
The adsorption by ACs is a very attractive method for environmental remediation because of its porous nature, ease of preparation and low cost of raw materials. Here, HPAC was studied as a vessel of adsorption for MO dye. Percentage dye removal (E%) (Eq. 4), adsorption capacity (qt) (Eq. 5) and equilibrium adsorption capacity (qe) (Eq. 6) of HPAC for MO are calculated using following equations:
where, C0, Ct, and Ce are initial after time t (min) and equilibrium concentration (mg L−1) of the dye in aqueous medium respectively, m is the mass of adsorbent (g) and V is the volume of solution (L) [36].
Thus, to testify the adsorption kinetics of MO on HPAC typically 0.1 g of adsorbent was added into the solution of the MO (20 mgL−1, 50 mL) at room temperature. The capability of HPAC for MO was quantitatively evaluated using UV/Vis spectrophotometer, which showed that the concentration of MO significantly decreased with the passage of time (60 min) and removal percentage of MO was found to be 99.5% (Fig. 6a).
For the dye adsorption kinetics study, two kinetic models pseudo-first order and pseudo-second order [37], were used to understand the adsorption rate of MO on the HPAC using Eq. 7 and Eq. 8.
To describe the kinetic process of MO adsorbed on the HPAC, the pseudo-first-order kinetic model (Fig. 7a), and pseudo-second order kinetic model (Fig. 7b), are used to analyze the experimental data quantitatively and the fitted results are given in Table 5. The validity of good fitting is evaluated by the high correlation coefficient (R2). The high R2 value indicates that the MO adsorption process of the HPAC is mainly controlled by the rate-determining step by following pseudo-second-order kinetic model.
The equilibrium adsorption capacity of a series of solutions within 20–400 mgL−1 was carried out as the adsorption capacity is greatly influenced by the concentration of the dye solutions. The adsorption MO removal ratio decreased with increasing initial concentration, indicating that the number of the adsorption sites are blocked by upcoming MO. The maximum adsorption capacities of HPAC for the MO is reached to 866.4 mg g−1 (Fig. 6b) when C0 is 200 mg L−1.
In most cases, the adsorption data will be confirmed using isothermal adsorption models, namely the Freundlich (Eq. 9), Langmuir (Eq. 10), and Temkin (Eq. 11) adsorption isotherm models. The linear forms of isotherms are given as
The Langmuir (Fig. 8a), Freundlich (Fig. 8b), and Temkin (Fig. 8c) models are used to describe the adsorption equilibrium of MO onto the HPAC by fitting equilibrium adsorption data quantitatively [38]. The fitted parameters given in Table 6 show that the adsorption isotherm of MO could be described better by the Langmuir model because of the higher R2, indicating single layer adsorption. The presence of hetero-atoms along with large active sites in HPAC attributed to high adsorption capacity for MO.
The results showed that, the HPAC is highly efficient for the removal of MO as a model dye from an aqueous solution at optimized conditions, but it is a major challenge to remove other organic dyes in the same way. Keeping this in front, HPAC is employed for the adsorption of other organic dyes, particularly MB, MG, and BPB. The same experimental conditions of MO adsorption were employed for all these dyes. During this experiment, the amount of HPAC, the contact time and the concentration of dyes were fixed at 0.1 g, 60 min and 20 mg L−1, respectively. Figure 9 (a–d) displayed UV–Visible spectra of MO, MG, MB, and BPB dyes showing removal of dyes with respect to time and the results showed that the removal of MB is 75.30%, MG is 82.88%, and BPB is 52.40%. The CO2 capture and dye removal capacity of reported activated carbons are summarised and compared with HPAC (Table 7).
3.3 Recyclability study of HPAC for CO2 capture and MO dye
To explore the recyclability of HPAC for CO2 capture, the continuous adsorption–desorption experiments were carried out. At each progression, HPAC was degassed at 300 C for 5 h under vacuum for total three cycles. The recyclability results showed that HPAC is highly efficient for CO2 capture and shows only 1% decrease after each cycle where the CO2 capture capacity decreases from 2.73 to 2.71 mmol g−1 for third cycle (Fig. 10).
On the other hand, the recyclability of HPAC for dye removal capacity was carried out using MO as model dye. A minute quantity of HPAC was lost after every cycle due to course and lighter particles which make the recovery quite difficult. To overcome this problem, the adsorbent was recovered using a 0.22-µm nylon syringe septum and recovered successfully using thermal treatment (773 K for 60 min). Still some amount of HPAC was lost after every cycle and hence we have opted for only three cycles of recyclability. The recyclability result shows 97%, 88.5%, and 78.5% (± 1%) recovery for 1st, 2nd, and 3rd cycle which indicates decrease in dye removal percentage (Fig. 11). This decrease is due to the loss of HPAC as well as the blockage of active sites after thermal treatment. However, by considering the loss of material and its subsequent removal efficiency %, recyclability of HPAC is appreciable.
4 Conclusion
In conclusion, the abundant and low-cost Terminalia catappa shell (TCS) agro-waste is used to prepare hierarchical porous activated carbons and is successfully employed for CO2 capture and dye removal applications. The N2 activation strategy used here is proved to be superior over air activation which enhanced SSA, PSD, meso-micro-pores and contribute to the nitrogen content as a CO2 binding sites. Thus, N2 activation allows complete manipulation of activated carbons for effective and selective CO2 capture, and fast removal of dyes with appreciable capacities. A resulting HPAC achieved good selectivity (41.8:1) for CO2 over N2 and exhibited an optimal CO2 capture of 2.73 mmol g−1 at 288 K. The sample also showed low Qst between 26 to 29 kJmol−1. The HPAC was found more efficient for MO and MG with 95.5 and 82.8% removal capability respectively, while it showed comparatively low removal capacity for MB (75.3%) and BPB (52.4%). Overall the prepared HPAC obtained from waste is a good candidate applicable for efficient CO2 capture and environmental remediation applications.
Data availability
All relevant data are included in the paper.
References
Sathish S, Nirmala R, Kim HY, Navamathavan R (2022) Deriving activated carbon using microwave combustion technique and its energy storage applications: a topical review. Carbon Lett 32:1151–1171. https://doi.org/10.1007/s42823-022-00348-4
Wang J, Pu Q, Ning P, Lu S (2021) Activated carbon-based composites for capturing CO2: a review. Greenh Gases Sci Technol 11:377–393. https://doi.org/10.1002/ghg.2051
Thines KR, Abdullah EC, Mubarak NM, Ruthiraan M (2017) Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review. Renew Sustain Energy Rev 67:257–276. https://doi.org/10.1016/j.rser.2016.09.057
Patil CS, Gunjal DB, Naik VM et al (2022) Sustainable conversion of waste tea biomass into versatile activated carbon: application in quick, continuous, and pressure filtration of miscellaneous pollutants. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02125-1
Lee S-Y, Park S-J (2015) A review on solid adsorbents for carbon dioxide capture. J Ind Eng Chem 23:1–11. https://doi.org/10.1016/j.jiec.2014.09.001
Wang S, Zhang T, Li J et al (2023) Potassium citrate-derived porous carbon with high CO2 capture and Congo red adsorption performance. Environ Sci Eur 35:1–13
Iwuozor KO, Ighalo JO, Emenike EC et al (2021) Adsorption of methyl orange: A review on adsorbent performance. Curr Res Green Sustain Chem 4:100179. https://doi.org/10.1016/j.crgsc.2021.100179
Rashidi NA, Yusup S (2017) A review on recent technological advancement in the activated carbon production from oil palm wastes. Chem Eng J 314:277–290. https://doi.org/10.1016/j.cej.2016.11.059
Estevez L, Barpaga D, Zheng J et al (2018) Hierarchically Porous Carbon Materials for CO2 Capture: The Role of Pore Structure. Ind Eng Chem Res 57:1262–1268. https://doi.org/10.1021/acs.iecr.7b03879
Reddy MSB, Ponnamma D, Sadasivuni KK et al (2021) Carbon dioxide adsorption based on porous materials. RSC Adv 11:12658–12681. https://doi.org/10.1039/D0RA10902A
Rattanapan S, Srikram J (2017) Kongsune P (2017) Adsorption of Methyl Orange on Coffee grounds Activated Carbon. Int Conf Altern Energy Dev Ctries Emerg Econ 138:949–954. https://doi.org/10.1016/j.egypro.2017.10.064
Oschatz M, Antonietti M (2018) A search for selectivity to enable CO 2 capture with porous adsorbents. Energy Environ Sci 11:57–70. https://doi.org/10.1039/c7ee02110k
Acevedo S, Giraldo L, Moreno-Piraján JC (2020) Adsorption of CO2 on activated carbons prepared by chemical activation with cupric nitrate. ACS Omega 5:10423–10432. https://doi.org/10.1021/acsomega.0c00342
Singh G, Lee J, Karakoti A et al (2020) Emerging trends in porous materials for CO2 capture and conversion. Chem Soc Rev 49:4360–4404. https://doi.org/10.1039/D0CS00075B
González-García P (2018) Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew Sustain Energy Rev 82:1393–1414. https://doi.org/10.1016/j.rser.2017.04.117
Ratan JK, Kaur M, Adiraju B (2018) Synthesis of activated carbon from agricultural waste using a simple method: Characterization, parametric and isotherms study. 7th Int Conf Mater Process Charact March 17–19 2017 5:3334–3345. https://doi.org/10.1016/j.matpr.2017.11.576
Paul S, Dutta A (2018) Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour Conserv Recycl 130:164–174. https://doi.org/10.1016/j.resconrec.2017.12.005
Dai Y, Sun Q, Wang W et al (2018) Utilizations of agricultural waste as adsorbent for the removal of contaminants: A review. Chemosphere 211:235–253. https://doi.org/10.1016/j.chemosphere.2018.06.179
Ghasemi M, Mashhadi S, Javadian H, et al (2016) Microwave-induced H2SO4 activation of activated carbon derived from rice agricultural wastes for sorption of methylene blue from aqueous solution. Desalination Water Treat 57:. https://doi.org/10.1080/19443994.2015.1119737
Koli A, Battu AK, Motkuri RK, Sabale S (2022) Hierarchical porous activated carbon derived from agro-waste for potential CO2 capture and efficient dye removal applications. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-03067-y
Gao J, Xie J, Liu X, Hu H (2017) Preparation and evaluation of modified cyanobacteria-derived activated carbon for H 2 adsorption. RSC Adv 7:20412–20421. https://doi.org/10.1039/C6RA28660G
Rahman HA, Chin SX (2019) Physical and chemical properties of the rice straw activated carbon produced from carbonization and KOH activation processes. Sains Malays 48:385–391. https://doi.org/10.17576/jsm-2019-4802-16
Rouzitalab Z, Maklavany DM, Rashidi A, Jafarinejad S (2018) Synthesis of N-doped nanoporous carbon from walnut shell for enhancing CO2 adsorption capacity and separation. J Environ Chem Eng 6:6653–6663. https://doi.org/10.1016/j.jece.2018.10.035
Nikonenko N, Buslov D, Sushko N, Zhbankov R (2000) Investigation of stretching vibrations of glycosidic linkages in disaccharides and polysaccarides with use of IR spectra deconvolution. Biopolymers 57:257–262. https://doi.org/10.1002/1097-0282(2000)57
Oswald S, Thoss F, Zier M et al (2018) Binding energy referencing for XPS in alkali metal-based battery materials research (II): application to complex composite electrodes. Batteries 4:36. https://doi.org/10.3390/batteries4030036
Wu C, Yang S, Cai J et al (2016) Activated microporous carbon derived from almond shells for high energy density asymmetric supercapacitors. ACS Appl Mater Interfaces 8:15288–15296. https://doi.org/10.1021/acsami.6b02942
Sodtipinta J, Amornsakchai T, Pakawatpanurut P (2017) Nanoporous carbon derived from agro-waste pineapple leaves for supercapacitor electrode. Adv Nat Sci Nanosci Nanotechnol 8:035017. https://doi.org/10.1088/2043-6254/aa7233
Ma X, Li Y, Cao M, Hu C (2014) A novel activating strategy to achieve highly porous carbon monoliths for CO2 capture. J Mater Chem A 2:4819–4826. https://doi.org/10.1039/C3TA14684G
Wang X-F, Xiong L, Zhong J-J et al (2020) Nitrogen-Containing Porous Carbon Fibers Prepared from Polyimide Fibers for CO2 Capture. Ind Eng Chem Res 59:18106–18114. https://doi.org/10.1021/acs.iecr.0c03318
González-Camuñas N Unusually Low heat of Adsorption of CO2 on AlPO and SAPO Molecular Sieves. https://doi.org/10.3389/fchem.2020.588712
Park HJ, Suh MP (2013) Enhanced isosteric heat, selectivity, and uptake capacity of CO 2 adsorption in a metal-organic framework by impregnated metal ions. Chem Sci 4:685–690. https://doi.org/10.1039/C2SC21253F
Gutiérrez-Bonilla E, Granados-Correa F, Roa-Morales G, Balderas-Hernández P (2022) CO2 capture on an optimally prepared highly microporous KOH-activated carbon from rice husk. Rev Mex Ing Quím 21:Mat2528–Mat2528. https://doi.org/10.24275/rmiq/Mat2528
Sher F, Iqbal SZ, Albazzaz S et al (2020) Development of biomass derived highly porous fast adsorbents for post-combustion CO2 capture. Fuel 282:118506. https://doi.org/10.1016/j.fuel.2020.118506
Borhan A, Yusup S, Mun YS (2020) Surface modification of rubber seed shell activated carbon with malic acid for high CO2 adsorption. IOP Conf Ser Earth Environ Sci 460:012044. https://doi.org/10.1088/1755-1315/460/1/012044
Omorogie MO, Babalola JO, Ismaeel MO et al (2021) Activated carbon from Nauclea diderrichii agricultural waste–a promising adsorbent for ibuprofen, methylene blue and CO2. Adv Powder Technol 32:866–874. https://doi.org/10.1016/j.apt.2021.01.031
Zambare R, Song X, Bhuvana S et al (2017) Ultrafast dye removal using ionic liquid–graphene oxide sponge. ACS Sustain Chem Eng 5:6026–6035. https://doi.org/10.1021/acssuschemeng.7b00867
Wang J, Guo X (2020) Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 258:127279. https://doi.org/10.1016/j.chemosphere.2020.127279
Jia Y, Ding L, Ren P et al (2020) Performances and Mechanism of Methyl Orange and Congo Red Adsorbed on the Magnetic Ion-Exchange Resin. J Chem Eng Data 65:725–736. https://doi.org/10.1021/acs.jced.9b00951
Qu W, Yuan T, Yin G et al (2019) Effect of properties of activated carbon on malachite green adsorption. Fuel 249:45–53. https://doi.org/10.1016/j.fuel.2019.03.058
Baytar O, Şahin Ö, Saka C (2018) Sequential application of microwave and conventional heating methods for preparation of activated carbon from biomass and its methylene blue adsorption. Appl Therm Eng 138:542–551. https://doi.org/10.1016/j.applthermaleng.2018.04.039
Acknowledgements
We thank the Department of Science and Technology, New Delhi, for the grant under the DST-FIST program (No/SR/FST/College-151/2013(C)) and the Department of Biotechnology for DBT-Star college scheme to Jaysingpur College, Jaysingpur. R.K.M. acknowledge the Laboratory Directed Research and Development (LDRD) program at the Pacific Northwest National Laboratory (PNNL). PNNL is operated by Battelle for the US Department of Energy (DOE) under Contract DE-AC05-76RL01830.
Author information
Authors and Affiliations
Contributions
Dr. Sandip Sabale lead experimental activity and research direction and developed the manuscript. Amruta Koli conducted experimental work under academic direction and drafted manuscript. Dr. R. Motkuri, Dr. Rohant Dhabbe, and Dr. Jian Shen helped with formal analysis and manuscript editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Hierarchical porous activated carbon from Terminalia catappa shell with high SSA (616.04 m2 g−1).
• Enhanced porosity, SSA, pore volume by N2 activation than air activation strategy.
• Facilitates efficient CO2 capture capacity of 2.73 mmol g−1 with 41.80 selectivity at 288 K.
• Efficiently removes methyl orange (99.5%), methylene blue (75.3%), malachite green (82.8%) and bromophenol blue (52.4%).
• Low-cost agro-waste-derived hierarchical porous activated carbon with CO2 capture and environmental remediation potential.
Novelty statement
Activated carbon has been derived from the easily available waste biomass (Terminalia catappa shell) showing enhancement in ordered porosity (Hierarchical) due to N2 activation utilized for bimodal environmental applications viz. CO2 capture and removal of four water soluble dyes (methyl orange, methylene blue, malachite green and bromophenol blue). The chemical carbonization followed by N2 activation is found to be superior over air activation strategy which over all improves structural, morphological, porosity properties for adsorptive environmental applications.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koli, A., Dhabbe, R., Shen, J. et al. Terminalia catappa shell-based activated carbon: N2 activation strategically enhanced hierarchical porosity for CO2 capture and dye removal applications. Biomass Conv. Bioref. 14, 18789–18801 (2024). https://doi.org/10.1007/s13399-023-04140-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04140-w