Abstract
Over the past years, uncontrolled population explosion coupled with an upsurge in the living standard of the people has led to a rise in the generation of municipal solid waste and energy consumption per capita. This has also increased the energy crisis and carbon footprint on the environment. Anaerobic digestion is a mature and sustainable technology for recovering energy from biomass, which can reduce the burden on municipal communities. Hydrolysis and acidogenesis are the rate-limiting steps in anaerobic digestion (AD), which needs to be improved to facilitate quick digestion of the highly biodegradable organic waste such as and fruit and vegetable waste (FVW) and food waste. Optimization of the operating parameters of hydrolysis and acidogensis, particularly for the treatment of FVW, has not been studied in detail so far. Therefore, in our present study, an attempt has been made to optimize the influence of environmental parameters such as pH and solid-to-liquid (S/L) ratio on VFA and chemical oxygen demand of the leachate through batch test. Influence of adding waste activated sludge toward enzymatic hydrolysis was also studied. The optimum pH and S/L ratio were found to be 6 and 7%, respectively, for the first phase of AD. Solubilization rate constant (k) for hydrolysis and acidification of FVW was found as 0.1785 d−1. Improving the degree of hydrolysis will enhance the VFA production, which is a precursor of the methanogenesis step, thus enhancing the overall process efficiency of AD. Hydrolysis rate was found to increase from 44 to 83%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Energy security and waste management are the two major concerns faced globally. In current era, energy security is becoming more important due to its crucial role in today’s technology-dependent societies. In general, over 87% of the world’s population still relies on energy produced from fossil fuels like coal and natural gas [1]. According to current estimates based on remaining fossil fuel reserves and rates of world consumption, it can last for another 30 to 50 years, which calls for a vigorous push toward the development of alternative energy sources [2]. In this regard, biofuel generation via anaerobic digestion (AD) in the form of the biogas, which mainly consists of methane and carbon dioxide, offers a highly attractive substitute to conventional energy sources. In current years, much attention is paid to utilize organic waste resources for the production of the renewable energy through AD of the organic biomass. Agrawal et al. [3] reported that the current generation of municipal solid waste (MSW) is around two billion tons annually, anticipated to increase multifold and by the end of 2025, it will reach three billion tons. This huge quantum of the waste holds strong potential to produce up to 4000 mm3 of biogas annually and an energy potential of roughly 86,000 TJ per year. This will help in mitigating energy crisis and to provide an eco-friendly solution to waste management [4]. Food waste (FW) is an integral part of the MSW and almost 40% of the fraction of the MSW is FW which ends up at sanitary landfill in most of the developing and developed countries. In addition, fruit and vegetable waste (FVW) is the highest in FW fractions in every country and every season [4,5,6]. Almost 15% of fruit and 25% of vegetables produced are being lost at the bottom of the supply chain [7]. India is the second largest producer of the FVW contributing to approximately 1748 million tons of FVW annually [8], out of which approximately 33% ends up in landfill [9]. FVW is characterized by the presence of highly biodegradable organic matter (volatile solids 85–95%, moisture content 80–85%) [10]. Furthermore, due to high biodegradability of FVW, its disposal to sanitary landfill is highly discouraged as it emits toxic gases, and contributes to global warming through methane generation [10, 11]. Nevertheless, AD has been emerged as environmental friendly and sustainable technology for the utilization and valorization of the FVW for the production of the biogas. AD is a sequence of biological pathways, where complex organic matters are solubilized by different microbes to methane and carbon dioxide. Furthermore, the digestate produced at the end of the AD can be utilized as organic manure or natural soil conditioner after treatment [12]. Three key stages of AD for stabilizing organic matter are hydrolysis, acidogenesis, and methanogenesis to produce methane and carbon dioxide with a trace amount of other gases (viz. H2, NH3, H2S, and N2, etc.) [13]. However, the methane yield of the substrate will heavily rely on the type of the feedstock, digester configuration, and various environmental parameters associated with AD. Past researchers show that the single-stage digester treating substrates which are rich in biopolymer content, like FVW, often suffers VFA inhibition due to accumulation of the organic acids. Hence, to overcome this shortcoming, stage separation has been studied by various researchers [14,15,16]. AD can be conveniently performed in two-stage anaerobic digestion process in order to optimally satisfy the physiology requirements of the distinct microbial populations and therefore improves the reaction kinetics [17]. In the two-stage system, hydrolysis and acidogenesis of the substrate take place in the first phase, and the product of the first phase, i.e., organic acids, is used as precursor for the methanogenesis, which is the energy yielding phase.
FVW contains cellulose and hemicellulose in their skin, seed, and thus, requires more time to undergo hydrolysis. This prolongs the entire digestion period. Therefore, in order to break down the bond between the cellulose, strong enzymatic actions are necessary. This enzymes are secreted by the fermentive bacteria, and possess unique ability to speed up the entire hydrolysis process [18]. The enzymatic hydrolysis of the FVW progresses in the subsequent phases. During the first step, the enzymes secreted by the hydrolytic bacteria are transported from the aqueous phase to the surface of the organic matter followed by the adsorption of the enzyme and formation of the enzyme-substrate complex. This carries out the liquefaction of the cellulose, hemicellulose, and lignin [19, 18]. The effectiveness of the hydrolysis is governed by the physical, chemical, and morphological structure of the substrate. Hydrolysis of the polysaccharides into fermentable monosaccharides depends upon the digestibility of the cellulose and hemicelluloses [20]. Numerous researchers have concentrated on enhancing operational parameters to increase VFA production via AD; for example, temperature affects the development of bacteria, and also the excretion of enzymes which enhances the hydrolysis of organic matter [21]. Furthermore, stage separation also offers better process control and digester stability by maintaining the required environment for the survival of the microbes [16, 22]. The maximum amount of the energy recovered by organic fraction of the MSW relies on the extent of the solublization of organic mass. The degree of solubilization of the organic waste is determined through the hydrolysis process, which is a rate-limiting step in AD [11] that governs the efficiency of the overall AD process. This has a direct correlation with methane yield in AD, although the rate of gas generation relies on the slowest of the three stages of AD, i.e., methanogenesis [23, 24]. In addition, hydrolysis and acidogenesis are influenced by various environmental parameters such as pH, hydraulic retention time, solid-to-liquid ratio, and substrate characteristics. To maintain a constant system performance and maximize the volatile fatty acid (VFA) production, the influence of process parameters on the conversion of FVW to volatile organic acids must be explored. Therefore, in this paper, an attempt has been made to evaluate the optimum parameters for the maximization of the VFA production. Process kinetics of the hydrolysis and acidification of the FVW have also been covered in the present study. The novelty of the work lies in utilization of waste activated sludge (WAS) for facilitating enzymatic hydrolysis. Inclusion of the WAS speeds up the hydrolysis process and, thus, ultimately maximizes the bioenergy generation.
2 Material and methods
2.1 Materials
2.1.1 Fruit and vegetable waste
FVW was procured from the local wholesale vegetable market. The waste comprises of bottle gourd (20%), okra (10%), cauliflower (10%), ridge gourd (10%), banana (10%), pumpkin (10%), brinjal (10%), cabbage leaves (10%), tomato (10%), and papaya (10%). The percentage of the individual component indicates the amount of waste likely to be generated in the market during that particular day. After procuring from the market, the inert material viz. plastic plates, paper, pebbles, etc. are removed and then, FVW was washed to get rid of the impurities present. Physical characterization of FVW was done and several parameters such as specific gravity, total solids, volatile solids, and moisture content were determined as per APHA 2005 [8]. All the characterization was done in triplicate to avoid the chances of possible errors. The procured FVW was chopped into 5–6-cm-thick pieces using a kitchen knife to reduce the size of the FVW.
2.1.2 Inoculum
Anaerobic sludge used in this study was procured from anaerobic digester having capacity 1000 m3 of Vachan dairy and Food Products Ltd., Kharora district, Raipur, Chhattisgarh, India. The sludge procured was acclimatized in the laboratory using 10-l bucket; the biomass was cultured in the laboratory by addition of the synthetic feed. The synthetic feed mainly consists of 10 g of anhydrous dextrose-D, 0.43 g of potassium di-hydrogen phosphate, and 2.85 g of ammonium nitrate. Before adding synthetic feed, 1 l of the supernatant was removed from the bucket. The cultured biomass was also supplemented with trace solution of phosphate buffer, ferric chloride, magnesium sulfate, and calcium chloride. The sludge obtained after the inoculation was named as waste activated sludge. The inoculum plays a crucial role in AD. The absence of the inoculum delays the digestion period and reduces biomethane production. It is the main source of the microorganism which carries out biodegradation. Anaerobic sludge was subjected to degasification for 5 days before the experiment to deplete the residual biodegradable organic matter present.
2.1.3 Chemicals
Laboratory-grade sodium bicarbonate was procured from Avantor, Rankem, India (97% purity). Dilute solutions of different concentrations were prepared by dissolving stoichiometric amounts of NaHCO3 in deionized water to maintain the pH. Hydrochloric acid was also obtained from Avantor, Rankem, India (35% assay). All other chemicals used were of AR grade. Deionized ultrapure water (Merck Millipore, Germany) was used throughout all the experiments wherever needed.
2.1.4 Characteristics of the substrate and inoculum
Parameter | Units | Inoculum | FVW |
|---|---|---|---|
pH | – | 7.2 | 5.2 |
Moisture content | % | 90 | 92% |
Total solids (TS) | % | 6.56 | 4.56 |
Volatile solids (VS) | % of TS | 72 | 92 |
Alkalinity | mg/L of CaCo3 | 4100 | 3700 |
Total VFA | mg/L | 105 | 80 |
VFA/alkalinity | – | 0.015 | 0.021 |
Carbon | % | 32 | 44 |
Nitrogen | % | 2.1 | 2.23 |
C/N ratio | – | 14.35 | 19.73 |
2.2 Methods
2.2.1 Experimental setup
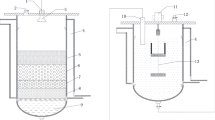
The hydrolysis of the FVW was performed in an acrylic cylinder of capacity 5 l with a working volume of 3 l. One liter volume was kept idle at the top and bottom for feeding of the fresh water and storage of leachate generated, respectively. At the height of 20 cm, a circular plate screen having 10 holes of average diameter 5 mm size was placed inside the reactor to prevent a solid fraction of FVW from sliding down. At the bottom of the reactor, plastic tube of diameter 5cm was provided for the collection of leachate. The schematic diagram of the reactor is illustrated in Fig. 1.
2.2.2 Hydrolysis study
Batch studies were conducted on the raw FVW procured from the local market and after physical sorting, the waste was chopped into 5- to 6-cm-thick pieces at ambient temperature. Reactor was fed with 150 g of the composite waste having total solids (TS) content 6.84% along with the predetermined quantity of the inoculum. The inoculum-to-substrate ratio was fixed as 25:75. The study was undertaken to optimize various environmental parameters like pH, and solid-to-liquid ratio for maximizing VFA and COD yield. The impact of adding WAS on the enzymatic hydrolysis was studied. Furthermore, the kinetic study of hydrolysis and acidogenesis was also conducted to evaluate the solubilization rate, degree of hydrolysis, and hydrolysis yield.
2.2.3 Analytical methods
Leachate collected at the bottom of the reactor was withdrawn periodically and analyzed daily to evaluate the influence of the environmental parameters such as pH and VFA as per the methods suggested in Standard Methods of Water and Wastewater Examination, APHA 2005. The VFA was measured by the ferric hydroxamate method reported by Chatterjee et al. [20], using a single-beam spectrophotometer. The COD of the leachate obtained from different operating conditions was analyzed as per closed reflux method as per APHA 2002 [25].
3 Result and discussion
3.1 Characterization of FVW
The suitability of FVW as a potent substrate to undergo hydrolysis can be evaluated from the characterization of the waste indicated in Table 1. From the table, it is evident that the average moisture content of the FVW is 86.14%, whereas VS content of the waste is mostly above 90% confirming the suitability of the substrate for the AD [26]. An increase in the moisture content of the FVW increases the bulk density of the waste. The bulk density of the FVW was found mostly above 1000 kg/m3. Furthermore, bulk density and moisture content of the substrate depend on the nature of the substrate which changes according to seasonal variation in the feedstock.
3.2 Influence of operational parameters on hydrolysis and acidification
VFA is the key product of hydrolysis and acidogenesis, which determines the biomethane yield of the waste. The production of the VFA is majorly dependent on several environmental parameters viz. pH, digestion time, organic loading rate, solid-to-liquid ratio, inoculum, nature of substrate and temperature, etc. In this context, it becomes essential to evaluate the influence of these environmental parameters on hydrolysis and acidogenesis, for effectively designing the engineering system.
3.2.1 Influence of pH on VFA production
The nature of a substrate significantly affects the digester’s pH. Anaerobic digestion is pH sensitive process, and the performance of the AD is dictated by the pH of the digester. The drastic change in pH is detrimental for the smooth operation of AD [3]. pH also influences the extent of solubilization and VFA production [27]. Therefore, the present study is aimed at determining the effect of pH variation (2, 4, and 6) on VFA and COD yield. The pH of raw FVW is mostly below 5.5 [28]. Due to the acidic nature of the substrate and high biodegradability, it results in VFA accumulation in the digester. Also owing to the inadequate buffering capacity of the digester, it becomes difficult to maintain pH, during the initial stage of operation. Therefore, it necessitates the addition of a strong alkali for the smooth operation of the digester. Hence, the pH of the digester was adjusted with the help of strong acid (5N HCL) or alkali (6N NaHCO3). The pH of the digesters was monitored regularly at an interval of 6 h. The influence of the pH on VFA production and COD of the leachate was as depicted in Figs. 2 and 3. It is clear from the graph that regardless of how the pH of the leachate changes, the VFA production rises along with the increase in the digestion time [27]. This is mainly due to the conversion of the easily biodegradable organics into volatile organic acids during initial stage [29, 27]. Furthermore, VFA production reached to its peak value, when the pH of the reactor was maintained at 6 which lies in the optimal range of pH required for acidogenesis [29]. Highest VFA was found to be 5750 mg/L at pH 6, followed by pH 4 and then pH 2. This is in agreement with the results obtained by other researchers [29]. At pH 4, the highest VFA concentration was noticed as 3100 mg/L, after which it started to decline. This is due to utilization of the organic acid by microbes for conversion into gas [18]. In addition, at pH 2, the highest VFA produced was only 1200 mg/L. Thus, it can be concluded that at lower pH, the VFA production decreases due to the inactivation of enzymes secreted by acidogenic microbes and less degradation of the substrate [30, 27, 31]. As the microbes are pH sensitive and requires optimal pH range to function, their efficiency to convert the complex organic matter to VFA drops down at lower pH. Our study concludes that optimal pH for the maximization of VFA as 6, which is in agreement with the various studies conducted in the past [27, 32, 33, 30]. Zhang et al. [27] pointed out that optimal pH for the maximization of VFA and solublization is at pH 7.
3.2.2 Influence of pH on COD
The variation in soluble COD concentration of leachate obtained at different pH is indicated in Fig. 3. From the graph, it is seen that during the initial stage, the soluble COD (sCOD) values go on increasing during the initial period of operation (1–4 days), which is due to the solublization of the easily biodegradable fraction of the organic matter present in the substrate [29, 19] regardless of the pH in the system. Highest sCOD concentration was noted as 5750 mg/L at pH 6, followed by 4700 mg/L at pH 4 and 4000 mg/L at pH 2. This is in co-relation with the amount of the VFA generated in the reactor; the higher the % of solublization, the higher will be the amount of the VFA generated. Furthermore, fourth day onwards, sharp decrease in the sCOD concentration was noticed which is due to the utilization of the organics by the hydrolytic microbes for their growth [18]. However, after this, again sharp increase in the COD values was noted on day 8; this is mainly because of the breakdown of the highly complex organic matter which requires strong enzymatic reactions for their breakdown [34, 18]. Therefore, it can be concluded that at lower pH, the amount of COD produced is less, which confirms the inability of the enzymes to degrade the organic matter. Enzymes are secreted by the acidogenic bacteria as they flourish well when the optimal pH (5.5 to 6.5) is maintained [3]. The results obtained are in accordance with the literature [18, 29].
3.2.3 Influence of solid-to-liquid (S/L) ratio on VFA and COD production
Batch studies were conducted at varying (S/L) ratios of 2%, 5%, 7%, and 9%, respectively, in a mesophilic regime. The pH of the reactors was maintained at 6. pH was adjusted with the help of strong acid 5N HCL or strong alkali 6N NaHCO3. pH was monitored regularly at an interval of 6 h. Figures 4 and 5 depict the variation of the VFA and sCOD produced at different solid-to-liquid ratio respectively. From the graphs, it can be interpreted that higher the concentration of the solids will result in higher degree of solubilization. Highest COD was recorded for 7% (S/L) ratio which was equivalent to5950 mg/L, whereas lowest COD was recorded for 2% (S/L) ratio, which was equivalent to 3000 mg/L. It was also noticed that COD production increases with the increase in retention time.
The VFA production was increased linearly for 5%, 7%, and 9% (S/L) ratio and highest VFA was recorded as 5100 mg/L for 7% (S/L) ratio. Furthermore, when (S/L) ratio was increased to 9%, the VFA production was decreased dramatically and reached to 3900 mg/L. Lowest VFA production was noticed for 2% (S/L) ratio, which was almost constant. The rate of solubilization was reported to be higher for 7% (S/L) ratio, beyond which it decreases due to a higher concentration of the soluble solids present [29]. This is due to the fact that higher concentration of the solids will result in quick acidification, which in turn lowers the pH of the system, and ultimately slows down the solubilization process [3]. Therefore, too high concentration of the solids is not desirable and solid-to-liquid ratio of 7% was optimum in our case.
3.2.4 Effect of addition of WAS on the rate of hydrolysis
The reactor was loaded with the optimum (S/L) ratio, i.e., 7% at ambient temperature. The pH of the reactor was maintained at 6. To study the impact of the addition of waste activated sludge, one reactor was inoculated with (25:75) ratio of inoculum to substrate and another reactor was kept un-inoculated. WAS contains the special category of the fermentive bacteria which helps in the liquefaction of the complex and hard to degrade lignocellulose present in the skin and seeds of the FVW [19]. These microbes secrete enzymes and the enzymatic action of the microbes expedite the hydrolysis of the FVW. Therefore, the addition of the WAS plays a crucial role. The impact of addition of WAS on VFA and COD of the leachate is depicted in Fig. 6a and b. From the graphs, it can be inferred that addition of the WAS increases the VFA and COD production. This is attributed to the enzymatic actions of the microbes present in WAS. By the addition of the WAS, breakdown of the complex organic C-Chains gets accelerated which increases the VFA and COD production, thus resulting in the higher degree of hydrolysis [18]. The highest yield of VFA and COD accounts to 5100 mg/L and 5950 mg/L, respectively, for the reactor inoculated with WAS. On the other hand, the reactor devoid of the WAS resulted in the lower yield of VFA and COD equivalent to 3950 mg/L and 4600 mg/L. This is basically due to absence of the microbes carrying out the degradation of the cellulose poor waste (FVW). However, the degradation of the FVW in the absence of WAS got rendered due to the bacteria and microbes present in the tap water and FVW itself [35].
3.2.5 Kinetics of hydrolysis and acidification of FVW
First-order kinetics has been applied by many researchers in the past for studying solid reduction in a single-stage anaerobic digester [36, 29]. Among all the processes of anaerobic digestion, hydrolysis appears to be the limiting step; the overall efficiency of AD depends on the degree of hydrolysis achieved [37]. An attempt has been made to apply first-order kinetic to understand the rate of solids degradation.
3.3 Solubilization rate
A batch study was conducted on a laboratory scale to determine the solubilization rate at different hydraulic retention times. The amount of the FVW (Co) remaining to the initial weight of FVW was plotted against time and is depicted in Fig. 7. From the plot, it is conferred that the rate of solubilization is higher in the initial stage, after which it goes on reducing, which is due to the conversion of the solid mass of FVW to organic acids.
The first phase of AD, hydrolysis, and acidification of FVW was assumed to follow the first-order rate of kinetics which is given by the below equations as
where k is the first-order specific rate constant (day−1), C is the COD concentration of FVW (mg/L) at time t, and Co is the initial COD concentration of FVW (mg/L) at zero time.
Using the above equations, solubilization rate data has been plotted to determine the rate constant for the first-order kinetics as presented in Fig. 8. The value of the specific rate constant for the FVW was determined as 0.1785 d−1. Similar results were obtained by [29].
3.4 Hydrolysis yield
The hydrolysis yield of the FVW is calculated as the difference between the input COD and the remaining COD in the digester over the input COD × 100 [29].
The hydrolysis yield for FVW is as shown in the Fig. 9. The hydrolysis yield is found to increase with the increase in the retention time. Hydrolysis yield varies from 44 to 83% for the FVW.
4 Conclusion
Anaerobic digester treating organic fraction of municipal solid waste (OFMSW) like FVW and FW easily undergo hydrolysis in the initial stage; the problem comes later on the hydrolysis of the complex organic matter which is present in the skin and seed. This requires application of the WAS to carry out enzymatic hydrolysis which enhances the degree of hydrolysis and results in the high concentration of the VFA and COD in the leachate. Hence, for the practical applicability of the pilot-scale anaerobic digester in the field, the entire process yield depends on the hydrolysis yield, whereas the absence of the WAS prolongs the duration of AD and, thus, results in an economic failure. The process parameters also play a crucial role for enhancing the hydrolysis and acidogenesis. According to our finding, pH 6 and the solid-to-liquid ratio 7% was found to be optimum. Hydrolysis data fits well with the first-order kinetics. The first-order rate constant of the FVW was found to be 0.1785 d−1. It is envisaged that leach bed reactor coupled with upflow anaerobic sludge blanket (offers a great opportunity or the treatment of the waste such as FVW and FW). This holds a strong potential for the application in real-life situation for treatment of OFMSW and thus leads to better utilization of the anaerobic digestion technology.
Data availability
Not applicable
References
Doll CNH, Pachauri S (2010) Estimating rural populations without access to electricity in developing countries through night-time light satellite imagery. Energy Policy 38:5661–5670. https://doi.org/10.1016/j.enpol.2010.05.014
Berg P, Boland A (2014) Analysis of ultimate fossil fuel reserves and associated CO2 emissions in IPCC scenarios. Nat Resour Res 23:141–158. https://doi.org/10.1007/s11053-013-9207-7
Agrawal A, Chaudhari PK, Prabir G (2022) Anaerobic digestion of fruit and vegetable waste : a critical review of associated challenges. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-21643-7
Grimberg SJ, Hilderbrandt D, Kinnunen M, Rogers S (2015) Anaerobic digestion of food waste through the operation of a mesophilic two-phase pilot scale digester - assessment of variable loadings on system performance. Bioresour Technol 178:226–229. https://doi.org/10.1016/j.biortech.2014.09.001
Kobayashi T, Xu KQ, Li YY, Inamori Y (2012) Evaluation of hydrogen and methane production from municipal solid wastes with different compositions of fat, protein, cellulosic materials and the other carbohydrates. Int J Hydrogen Energy 37:15711–15718. https://doi.org/10.1016/j.ijhydene.2012.05.044
Micolucci F, Gottardo M, Pavan P et al (2018) Pilot scale comparison of single and double-stage thermophilic anaerobic digestion of food waste. J Clean Prod 171:1376–1385. https://doi.org/10.1016/j.jclepro.2017.10.080
Agrawal A, Chaudhari PK, Prabir G (2023) Effect of mixing ratio on biomethane potential of anaerobic co - digestion of fruit and vegetable waste and food waste. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-023-03737-5
Edwiges T, Frare L, Mayer B et al (2018) Influence of chemical composition on biochemical methane potential of fruit and vegetable waste. Waste Manag 71:618–625. https://doi.org/10.1016/j.wasman.2017.05.030
Arhoun B, Villen-Guzman M, Gomez-Lahoz C et al (2019) Anaerobic co-digestion of mixed sewage sludge and fruits and vegetable wholesale market waste: composition and seasonality effect. J Water Process Eng 31. https://doi.org/10.1016/j.jwpe.2019.100848
Ji C, Kong CX, Mei ZL, Li J (2017) A review of the anaerobic digestion of fruit and vegetable waste. Appl Biochem Biotechnol 183:906–922. https://doi.org/10.1007/s12010-017-2472-x
Lytras G, Lytras C, Mathioudakis D et al (2021) Food waste valorization based on anaerobic digestion. Waste Biomass Valorization 12:1677–1697. https://doi.org/10.1007/s12649-020-01108-z
Zia M, Ahmed S, Kumar A (2020) Anaerobic digestion (AD) of fruit and vegetable market waste (FVMW): potential of FVMW, bioreactor performance, co-substrates, and pre-treatment techniques. Biomass Convers Biorefinery 12:3573–3592. https://doi.org/10.1007/s13399-020-00979-5
Surendra KC, Takara D, Hashimoto AG, Khanal SK (2014) Biogas as a sustainable energy source for developing countries: opportunities and challenges. Renew Sustain Energy Rev 31:846–859. https://doi.org/10.1016/j.rser.2013.12.015
Chatterjee B, Mazumder D (2019) Role of stage-separation in the ubiquitous development of anaerobic digestion of organic fraction of municipal solid waste: a critical review. Renew Sustain Energy Rev 104:439–469
Chatterjee B, Mazumder D (2018) Performance evaluation of three-stage anaerobic digestion (AD) for stabilization of fruit and vegetable waste (FVW). J Indian Chem Soc 95:65–80
Chakraborty D, Venkata Mohan S (2018) Effect of food to vegetable waste ratio on acidogenesis and methanogenesis during two-stage integration. Bioresour Technol 254:256–263. https://doi.org/10.1016/j.biortech.2018.01.051
Xiao B, Qin Y, Zhang W et al (2018) Temperature-phased anaerobic digestion of food waste: a comparison with single-stage digestions based on performance and energy balance. Bioresour Technol 249:826–834. https://doi.org/10.1016/j.biortech.2017.10.084
Chatterjee B, Mazumder D (2018) A study on enzymatic hydrolysis of fruit and vegetable waste using waste activated sludge. J Indian Chem Soc 95:285–293
Chatterjee B, Mazumder D (2018) Enzyme mediated hydrolysis of organic fraction of municipal solid waste (OFMSW): A short review. J Indian Chem Soc 95:295–312
Menzel T, Neubauer P, Junne S (2020) Role of microbial hydrolysis in anaerobic digestion. Energies 13. https://doi.org/10.3390/en13215555
Kim M, Gomec CY, Ahn Y, Speece RE (2003) Hydrolysis and acidogenesis of particulate organic material in mesophilic and thermophilic anaerobic digestion. Environ Technol (United Kingdom) 24:1183–1190. https://doi.org/10.1080/09593330309385659
Silva FMS, Mahler CF, Oliveira LB, Bassin JP (2018) Hydrogen and methane production in a two-stage anaerobic digestion system by co-digestion of food waste, sewage sludge and glycerol. Waste Manag 76:339–349. https://doi.org/10.1016/j.wasman.2018.02.039
Edwiges T, Frare LM, Lima Alino JH et al (2020) Methane potential of fruit and vegetable waste: an evaluation of the semi-continuous anaerobic mono-digestion. Environ Technol (United Kingdom) 41:921–930. https://doi.org/10.1080/09593330.2018.1515262
Shanthi M, Rajesh Banu J, Sivashanmugam P (2018) Effect of surfactant assisted sonic pretreatment on liquefaction of fruits and vegetable residue: characterization, acidogenesis, biomethane yield and energy ratio. Bioresour Technol 264:35–41. https://doi.org/10.1016/j.biortech.2018.05.054
Chatterjee B, Mazumder D (2020) New approach of characterizing fruit and vegetable waste (FVW) to ascertain its biological stabilization via two-stage anaerobic digestion (AD). Biomass and Bioenergy 139:105594. https://doi.org/10.1016/j.biombioe.2020.105594
Esparza I, Jiménez-Moreno N, Bimbela F et al (2020) Fruit and vegetable waste management: conventional and emerging approaches. J Environ Manage 265:110510. https://doi.org/10.1016/j.jenvman.2020.110510
Zhang B, Zhang L, Zhang S et al (2010) The influence of pH on hydrolysis and acidogenesis of kitchen wastes in two-phase anaerobic digestion. Environ Technol 26:329–340. https://doi.org/10.1080/09593332608618563
Di Maria F, Barratta M (2015) Boosting methane generation by co-digestion of sludge with fruit and vegetable waste: internal environment of digester and methanogenic pathway. Waste Manag 43:130–136. https://doi.org/10.1016/j.wasman.2015.06.007
Tembhurkar AR, Mhaisalkar VA (2007) Studies on hydrolysis and acidogenesis of kitchen waste in two phase anaerobic digestion. Inst Public Heal Eng 8:10–18
Zheng M, Zheng M, Wu Y et al (2015) Effect of pH on types of acidogenic fermentation of fruit and vegetable wastes. Biotechnol Bioprocess Eng 20:298–303. https://doi.org/10.1007/s12257-014-0651-y
Browne JD, Allen E, Murphy JD (2013) Improving hydrolysis of food waste in a leach bed reactor. Waste Manag 33:2470–2477. https://doi.org/10.1016/j.wasman.2013.06.025
Khadka A, Parajuli A, Dangol S et al (2022) Effect of the substrate to inoculum ratios on the kinetics of biogas production during the mesophilic anaerobic digestion of food waste. Energies 15. https://doi.org/10.3390/en15030834
Zhang L, Loh KC, Dai Y, Tong YW (2020) Acidogenic fermentation of food waste for production of volatile fatty acids: bacterial community analysis and semi-continuous operation. Waste Manag 109:75–84. https://doi.org/10.1016/j.wasman.2020.04.052
Di Maria F, Sordi A, Cirulli G et al (2014) Co-treatment of fruit and vegetable waste in sludge digesters. An analysis of the relationship among bio-methane generation, process stability and digestate phytotoxicity. Waste Manag 34:1603–1608. https://doi.org/10.1016/j.wasman.2014.05.017
Mata-Alvarez J, Llabrés P, Cecchi F, Pavan P (1992) Anaerobic digestion of the Barcelona central food market organic wastes: experimental study. Bioresour Technol 39:39–48. https://doi.org/10.1016/0960-8524(92)90054-2
Naik GP, Poonia AK, Chaudhari PK (2022) Alkaline electro-hydrolysis pretreatment of rice straw for enhanced biogas production under ambient temperature. Int J Chem React Eng 20:549–559. https://doi.org/10.1515/ijcre-2021-0099
Lin J, Zuo J, Gan L et al (2011) Effects of mixture ratio on anaerobic co-digestion with fruit and vegetable waste and food waste of China. J Environ Sci 23:1403–1408. https://doi.org/10.1016/S1001-0742(10)60572-4
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and experimental analysis were performed by Akanksha Agrawal. The first draft of the manuscript was written by Akanksha Agrawal. Data validation was done by Dr. P.K. Chaudhari band Dr. Prabir Ghosh. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agrawal, A., Chaudhari, P.K. & Ghosh, P. Hydrolysis and acidogenesis study of fruit and vegetable waste using activated sludge. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03937-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03937-z