Abstract
Bioemulsifier production by Aureobasidium pullulans LB83 from pretreated sugarcane bagasse was evaluated in solid-state fermentation. Alkaline (0.2 mol.L−1 NaOH; 12 min) and hydrodynamic cavitation (0.5 mol.L−1 NaOH, 25 min) pretreatments showed a maximum lignin removal of 55.1 % and 44.7 %, respectively. Pretreatment biomass effectivity was assessed by X-ray diffraction, FT-MIR, FTIR-NIR, and RAMAN techniques. Maximum kerosene emulsification indexes of 65.8 % and 41.3 % were obtained in solid-state fermentation, respectively, for alkaline and hydrodynamic cavitation pretreated sugarcane bagasse. Synthesis of cellulases was observed in fermentation, showing maximum values of endoglucanase and exoglucanase, respectively, of 2.25 U.g−1 and 1.39 U.g−1, for alkaline pretreated sugarcane bagasse, and 2.43 U.g−1 and 1.45 U.g−1, for hydrodynamic cavitation-pretreated sugarcane bagasse. The biomolecule was characterized as a mixture of mannitol and arabitol-type liamocin. Microorganism was able to produce bioemulsifier using sugarcane bagasse as support and carbon source in solid-state fermentation, thus showing the potential of this system to obtain value-added products from this biomass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The worldwide demand for fuels is projected to reach 5.7 million barrels a day–1 over 2019–2025 at an average annual rate of 950 kb day–1. However, fuels from petroleum will be less abundant in the near future and their use results in environmental concerns, including the emission of greenhouse gasses. Some factors such as oil prices, volatility, and geopolitical factors have also been decisive in the search for bio-based alternatives [1].
Some fuels such as ethanol and biodiesel are produced from biomass. However, the biorefinery concept implies not only in the conversion of biomass into biofuels, but also in biochemical products such as amino acids, organic acids, enzymes, biopolymers, and bioemulsifiers. Biomass are sustainable and renewable sources of energy and carbon, presenting environmental and economic advantages [2]. Among the different plant-based materials, lignocellulosic biomass is the most abundantly available, renewable, and low-cost alternative for the production of biofuels and other bioproducts. Another fact is that lignocellulosic sources do not compete with the food supply, coming from forestry, agro-industrial, and agricultural wastes and byproducts [3].
Brazil is the main sugarcane producer, with an estimated forecast of 592 million tons projected for the 2021/22 season [4]. Some of the byproducts obtained in sugarcane biorefineries are vinasse, molasses, straw, and bagasse [5]. Sugarcane bagasse, which is produced in large amounts, is mainly composed of cellulose (45 %), hemicellulose (27 %), and lignin (21.1 %). It is used for purposes such as the generation of electricity, paper, and microbial products [6]. However, one of the main drawbacks for the utilization of sugarcane bagasse as a carbon source in bioprocesses is the generation of monomers from polysaccharides by hydrolysis, due to the complex recalcitrant structure, which hinders the access of microbial enzymes. Thus, alkaline pretreatment is one of the traditional methods that play an important role in the deconstruction and fractionation of lignocellulose, mainly removing lignin from its structure [7]. Besides traditional pretreatment methods, alternatives have also been evaluated, as hydrodynamic cavitation-assisted methods [5, 8].
Among the interesting bioproducts to be obtained in biorefineries, bioemulsifiers can be highlighted. They are secondary metabolites characterized by their high molecular weight and amphiphilic chemical structure, and can be classified as heteropolysaccharides, lipopolysaccharides, lipoproteins, glycoproteins, and proteins [9]. These molecules form and stabilize emulsions and foams between immiscible liquids or the interface between air and water. The attractiveness of bioemulsifiers come from their ability to act in broad ranges of pH, temperature, and salinity, low toxicity, biodegradability, and the production from sustainable sources [10]. Currently, bioemulsifiers are considered valuable for different industrial applications such as cosmetics, food industry, and bioremediation among others [11]. The bioemulsifier market is expected to reach USD 17.53 billion by 2027, with an increase of rate of 6.90 % for the forecast period of 2020 to 2027 [12].
However, producing these compounds on an industrial scale is still a challenge due to their low yield and the cost of production. To overcome these drawbacks, some alternatives have been evaluated, including the use of agroindustrial residues as a source of carbon and nitrogen for different microbial strains, and the selection of the fermentative process system [13].
Several yeasts that are generally recognized as safe (GRAS) produce bioemulsifiers, which allow the wide use of these metabolites in industrial processes [14]. Among the yeasts with GRAS status, Aureobasidium pullulans has stood out also for its ability to produce cellulases and xylanases, which enables the degradation of the carbohydrate fraction contained in lignocellulosic materials. In addition, the synthesis of different surface-active compounds by A. pullulans has been confirmed by several authors [15,16,17]. A. pullulans LB83 was previously evaluated to produce a biomolecule with surface activity from sugarcane bagasse in submerged fermentation in the work of Brumano [18]. In that work, the authors evaluated for the scale-up of the process in a stirred tank reactor. They observed that the maximum biomolecule tensoactivity was 8.05 cm with a productivity of 0.0838 cm.h–1 when aeration rate (Ar) and carbon source (SC) concentrations were 1.1 min–1 and 80 g.L–1, respectively.
Regarding the fermentative system, solid-state fermentation could contribute to the viability of the industrial production of bioemulsifiers. The solid-state fermentation process allows the microbial growth on wet solid materials with minimal quantities of free water in the system. In addition, solid-state fermentation offers some advantages such as lower sterilization costs, the reduced downstream processing, the low risk of bacterial contamination, and the consumption of less energy [19, 20].
The use of lignocellulosic wastes or organic by-products can perform better in solid-state fermentation than inert materials (perlite and polyurethane foams). However, in the case of cellulosic biomass, some studies show that pretreatment contributes to obtaining value-added products of industrial interest. In this context, the aim of this work was to evaluate the production of bioemulsifier by A. pullulans LB83 in solid-state fermentation using sugarcane bagasse as support and carbon source. Alkaline and hydrodynamic cavitation pretreatments were evaluated as alternatives to enhance the performance of the process. To our knowledge, the present study shows for the first time the synthesis of a bioemulsifier produced by A. pullulans LB83 from pretreated sugarcane bagasse used as a support and carbon source.
2 Material and methods
2.1 Sugarcane bagasse
Sugarcane bagasse used for alkaline pretreatment experiments was provided by Usina Costa Pinto (COSAN, Piracicaba, São Paulo, SP, Brazil) and the sugarcane bagasse used for hydrodynamic cavitation experiments was supplied by the company Ipiranga Agroindustrial SA, (Descalvado, SP. Brazil). The biomasses were exposed to the sun for drying until reaching a moisture content of 10 % (mass per dry mass) determined in an infrared moisture balance (Marte ID-50) and screened to collect the average size fraction of 14 mesh (2.36–0.85 mm).
2.2 Optimization of the alkaline pretreatment
In order to determine the most favorable conditions for lignin removal, a 22 Central composite rotatable design (CCRD) with triplicate at center point, totaling 11 experiments, was carried out. The two independent variables studied were NaOH concentration (A): 0.083–0.416 mol.L–1, and time at 121 °C (B): 20–60 min. The alkaline pretreatment was carried out in 1-L Erlenmeyer flasks containing 30 g of biomass and 450 mL of NaOH solution. The flasks were autoclaved at 1 atm and 121 °C. After the pretreatment, the biomasses were separated from the liquid fraction using a cloth strainer. The solid fractions were washed with water until neutral pH and dried at 65 °C for 24 h. The obtained solids were characterized regarding their content of cellulose, hemicellulose, and lignin. Also, fermentation was performed, according to section 2.8, using the sugarcane bagasse pretreated in each condition evaluated in the statistical design, aiming to obtain the emulsification index as an indicative of bioemulsifier production.
2.3 Optimization of the hydrodynamic cavitation pretreatment
The pretreatment of the sugarcane bagasse was carried out in a hydrodynamic cavitation system based on a reservoir and a stainless-steel cylindrical cavitation reactor that is interconnected to two centrifugal pumps, as described by Terán Hilares [5]. An orifice plate with 27 holes of 1 mm in diameter was used as a cavitation generator in all experiments [16]. The sugarcane bagasse was kept in a cylindrical cloth (40 mesh) kept within the cavitation zone in the cavitation reactor. The loading of solids (%) was calculated by dividing the bagasse mass (in grams) by the volume of the cavitation reactor cylinder (1425 mL) and different concentrations of NaOH aqueous solution were used for each experiment. After pretreatment, the solid fraction was washed to pH 7.0 and dried. A factorial Box-Behnken design with 3 replicates at the central point, totaling 15 experiments, was carried out in order to optimize the hydrodynamic cavitation associated with the alkaline treatment of the sugarcane bagasse. The three independent variables studied were as follows: NaOH concentration (X1), 0.1–0.5 mol.L–1; cavitation time (X2), 5–25 min; and loading of solids (X3), 1–2 %. The pretreated biomass was characterized regarding its content of cellulose, hemicellulose, and lignin.
2.4 Compositional analysis of biomass
The raw sugarcane bagasse and the pretreated sugarcane bagasse were characterized in terms of cellulose, hemicellulose, lignin and ash content, according to the methodology as described [21].
2.5 Characterization techniques
Considering the similar composition of different samples of sugarcane bagasse in natura used in this work, only the sample supplied by the company Ipiranga Agroindustrial SA was selected as raw sugarcane bagasse for comparison with pretreated sugarcane bagasse in the following analysis.
2.5.1 Infrared spectroscopy
The samples were prepared in the form of pellets, a process carried out in a hydraulic press (SPECAC 25T, ATLAS TM), submitting 200 mg of the material to a pressure of 10 tons for 3 min. The signal analyzed in the mid-infrared (MIR) was performed by a VERTEX 70 spectrometer and in the near-infrared spectroscopy (NIR) by the MPA (Multi Purpose Analyser), both from Bruker Optics. The spectra were acquired by the OPUS 6.5 software after 64 scans. In the MIR it covers the range from 4000 to 400 cm–1 and in the NIR from 12,000 to 4000 cm–1 wavenumber.
2.5.2 Raman spectroscopy
The Raman analysis was performed using a Renishaw micro-Raman in Via spectrometer equipped with a 785 nm diode laser to avoid fluorescence. The spectra were obtained through a 50 × objective with NA = 0.75. The measurements were performed in the sample pellets, with a laser spot diameter of approximately 1μm.
2.5.3 X-ray diffraction (XDR)
The crystallinity of the samples was evaluated by X-ray diffraction using a Rigaku MiniFlex diffractometer with copper Kα radiation (λ = 0.1542 nm). The monochromator operated at 30 KV and 15 mA with a speed of approximately 2°/min and scanning at an angle (2θ) in the range of 5–40°. The intensity of the 002 peak (I002, 2θ = 22.5°) and the minimum dip (Iam, 2θ = 19°) were considered to estimate the crystallinity index (CrI), which was calculated using the method described by [22] in a following equation:
2.6 Determination of the absorptive capacity of sugarcane bagasse
The absorptive capacity test was carried out in order to determine the maximum volume of medium that raw sugarcane bagasse and pretreated sugarcane bagasse can retain as a solid matrix. In a 125-mL beaker, 2 g of bagasse with a particle size greater than 14 mesh were weighed. Subsequently, a 1 mL aliquot of modified Kitamoto (without sucrose) medium was added. The process was repeated until the appearance of free liquid medium in the system [18].
2.7 Microorganism and inoculum preparation
A. pullulans LB83 was isolated and kindly donated by the Center for Study of Social Insects (CEIS/São Paulo State University - Rio Claro - SP), Brazil. The microorganism was kept at 4 °C in slants containing yeast malt extract solid medium (YMA) (glycose 10 g.L–1, yeast extract 3 g.L–1, peptone 5 g.L–1, malt extract 3 g.L–1, and 20 g.L–1 agar).
For the inoculum preparation, a loop of microorganisms was transferred from the test tube with YMA medium to 125 mL Erlenmeyer flasks containing 40 mL of culture medium proposed by [23] (peptone 0.6 g.L–1, yeast extract 0.4 g.L–1, NaCl 1 g.L–1, K2HPO4 5 g.L–1, and MgSO4 0.4 g.L–1, with 50 g.L–1 of sucrose. The flasks were incubated on a rotary shaker (New Brunswick Scientific - Excella E24, Hamburg, Germany) at 200 rpm, 28 °C for 48 h. The cells were aseptically separated by centrifugation at 2930 × g for 15 min, suspended in 0.9 % saline solution (g.L–1), and counted in an Agasse-Lafont-R chamber containing reticles of 0.0025 mm2 and depth of 0.100 mm, in order to adjust the initial cell concentration in the inoculum suspension for 108 cells.mL–1 [18].
2.8 Solid-state fermentation in Erlenmeyer flasks
In order to evaluate the bioemulsifier production, the solid-state fermentation was carried out in Erlenmeyer flasks of 125 mL, containing 2 g of raw sugarcane bagasse or pretreated sugarcane bagasse (alkaline pretreatment or hydrodynamic cavitation) with neutral pH. Erlenmeyer flasks were sterilized at 121 °C for 15 min, cooled, and inoculated with the moistening solution (sterile modified Kitamoto medium containing a cell concentration of 108 cells.mL–1), according to the absorptive capacity test.
The flasks were incubated in a microbiological oven (Quimis - Q316M4, Diadema, Brazil) maintained at 28 ± 2 °C, under 80 % relative humidity (maintained with the aid of textile fibres) for a period of 14 days (336 h). Samples were removed at 0, 3, 7, 9, 11, and 14 days for analysis. The samples were analyzed for the emulsifying properties of the produced bioemulsifier as described by [24] and the measure of cellulolytic enzymes.
Due to the difficulty of removing a representative and homogeneous sample in this type of culture, replicates were prepared for each point to be analyzed, with each flask being considered a sample. For each sampling time, six flasks were prepared, corresponding to three flasks to be used for enzymatic tests, and three flasks for emulsification index tests.
2.9 Extraction of the raw extract
For the enzymatic tests, the crude extract from the fermentation was extracted with the addition of 5 mL of 0.05 M acetate buffer, pH 4.8 per gram of bagasse. For the emulsification index tests, 5 mL of distilled water were added per gram of bagasse and the flasks were left under incubation in a rotary shaker (New Brunswick Scientific – Excella E24, Hamburg, Germany) at 200 rpm and 28 °C for 1 h. The liquid fraction was separated from the flask by pressing and centrifuging (Novatécnica – NT 810, Piracicaba, Brazil) for 15 min at 2198 × g, separating the cell-free liquid adapted by [18].
2.10 Bioemulsifier recovery
Bioemulsifier recovery was adapted by [18]. Butanone was added in a 1:1 ratio to the raw extract. The mixture was shaken in a rotary shaker (New Brunswick Scientific – Excella E24, Hamburg, Germany) at 200 rpm for 1 h and then centrifuged (Novatécnica – NT 810, Piracicaba, Brazil) for 10 min at 1610 × g, and the bioemulsifier was extracted to the non-polar phase. Then, the excess solvent was evaporated and the bioemulsifier concentration was determined by gravimetry.
2.11 Characterization of the bioemulsifier
The bioemulsifier sample was retrieved as per section 2.10The sample was dissolved in butanone and analyzed by HPLC UltiMate3000 series, following the methodology developed by [25].
2.12 Determination of sugars by high performance liquid chromatography (HPLC)
To determine the concentrations of sugars and organic acids present in the samples, HPLC analysis was performed using an Agilent Technology series A1100 chromatograph (Palo Alto, California). The samples were previously filtered through a Sep-Pak C18 filter (Millipore) and analyzed using the following conditions: Bio-Rad Aminex HPX-87H column (300 × 7.8 mm) maintained at a temperature of 45 °C; injection volume of 20 μL; Agilent refractive index detector; mobile phase H2SO4 0.01 eq.L–1 and flow of 0.6 mL.min–1.
2.13 Emulsification index
The raw extract of the fermentation was mixed in test tubes with a screw cap with kerosene in the proportion of 1:1 and homogenized in a tube shaker (Scientific Industries - Vortex Genie 2, Bohemia, United States), at a maximum speed of 1.5 min. The tubes were left to stand at 25 °C for 24 h. Measurements were performed in the test tubes after 24 h, and the emulsification index (EI) was expressed through the equation below [26]:
where
EL: emulsified layer
TH: total height
2.14 Enzymatic activities
2.14.1 Analysis of endoglucanase activity
The endo-1,4-β-glucanase activity was determined through the protocol described by [27]. 0.9 mL of 0.44 % carboxymethylcellulose (SIGMA®) and 0.1 mL of enzyme extract were added to the test tubes, where the reaction was maintained for 10 min at 50 °C. After that, 1.5 mL of 3,5-Dinitrosalicylic acid (DNS) was added, and the mixture was boiled at 100 °C for 5 min. The absorbance reading was carried out at 540 nm. Absorbance was converted to glucose concentration according to a standard curve prepared (2.7; 2.2; 1.6; 1.1; 0.5 μmol.mL–1).
2.14.2 Analysis of exoglucanase activity
The exo-1,4-β-glucanase activity was determined using the protocol of Wood and Bhat [28]. 0.9 mL of Avicel 1 % (SIGMA®) and 0.1 mL of enzymatic extract were added to the test tubes, where the reaction was maintained for 60 min at 50 °C. After that, 1.5 mL of DNS was added, and the mixture was boiled at 100 °C for 5 min. The test tubes were centrifuged at 2564 × g for 20 min, and the insoluble fraction was separated from the supernatant. The supernatant was read at 540 nm. Absorbance was converted to glucose concentration according to a standard curve prepared (2.7; 2.2; 1.6; 1.1; 0.5 μmol.m–1).
2.15 Statistical analysis
The statistical analysis of the results obtained in the factorial design experiments was performed using the Design-Expert 6.0.8 Portable software (Stat-Ease, Inc.) for the generation of a mathematical model, Statistica 7.0 (StatSoft) for the generation of the response surface and the figures showing enzymatic behavior were created using GraphPad Prism version 8.0.
3 Results and discussion
3.1 Optimization of alkaline and hydrodynamic cavitation pretreatments
The alkaline pretreatment of the sugarcane bagasse was carried out according to CCRD with 3 replicates at the center point. The input variables were alkali concentration and pretreatment time. Table 1 shows the composition of the sugarcane bagasse and the pretreated sugarcane bagasse in each condition. The results indicated that condition 6, with the highest NaOH concentration, resulted in the maximum lignin and hemicellulose removal, with 80.5 % and 41.3 %, respectively. On the contrary, pretreatment conducted with the lower alkali concentration led to the lower lignin removal of 9.6 %, in condition 5. The effectiveness of NaOH pretreatment under different conditions was evaluated based on lignin removal [29]. However, care must be taken with the reaction conditions (time, reagent concentration, and temperature) because, if they are severe, in addition to lignin and hemicellulose, sugars and other soluble components can also be decomposed [17].
A quadratic model was proposed for lignin removal in the alkaline pretreatment and the correspondent analysis of variance (ANOVA) is shown in Table 2. The adequate fit of the model was confirmed by the p-value < 0.05 (95 % confidence level) and the non-significance of the lack of fit test with p-value > 0.05. Additionally, the adjusted R2 was 0.966, indicating that the model explains 96.6 % of the variability of the experimental data. In addition, it was observed that factor A (NaOH concentration) had a great influence on the removal of lignin content, while factor B (pretreatment time) was significant only at 90 % of confidence level. The quadratic mathematical model which relates the NaOH concentration (A) and the pretreatment time (B) to lignin removal (LR) was described in Eq. 3:
where
LR = lignin removal (%), (A) = NaOH concentration (mol.L–1), (B) = time (min)
The model also allowed the generation of a response surface (Fig. 1a), showing that higher concentrations of NaOH and prolonged process times resulted in high removal of lignin. In the same figure, it can be seen that the concentration of NaOH between 0.2 and 0.28 mol.L–1 removed between 60 and 70 % of the bagasse’s lignin.
Aided by the desirability function of Design-Expert 6.0.8 software, it was specified an EI96h between 50 and 100 %, reducing the NaOH concentration and the pretreatment time in the autoclave. From these requirements, the software returned optimized conditions with an EI96h of 57.4 % obtained by using 0.2 mol.L–1 of NaOH and 12 min in the autoclave. This software prevision was experimentally confirmed, with a result of 54.0 ± 2.7 % of lignin removal. Thus, these conditions were selected for further solid-state fermentations.
A similar trend was observed in the optimization of biomass pretreated by the hydrodynamic cavitation-assisted process. As observed in Table 3, the maximum lignin removal was observed in condition 8 (40.2 %), which coincided with the highest levels of NaOH concentration and process time. On the other hand, the minimum removal was observed in condition 5 (2.6 %), using the lowest levels of the studied variables. By comparing the results of Table 3 with the reported in Table 1, higher results of removal of lignin were observed when alkaline pretreatment was used. Actually, the best emulsification index values were obtained, for alkaline pretreatment, with lignin removal of about 60 %, and the higher removal obtained by hydrodynamic cavitation pretreatment was lower than 50 %. Thus, the optimization of hydrodynamic cavitation-assisted pretreatment was performed considering only the answer lignin removal.
An analysis of variance was performed for a 2FI model composed for the answer removal of lignin. As shown in Table 4, the model was significant (p < 0.05), with significant coefficients and non-significant lack of fit. Also, the coefficient of determination (R2) was 0.9619. In addition, the solids loading had no significance and the corresponding terms were excluded from the model. The composed model is shown in Eq. 4:
where
Y 1 = Lignin removal (%), X1 = NaOH concentration (mol.L–1), X2 = time (min)
In addition, the model also allowed the generation of a response surface graph (Fig. 1c), taking into account solid loading of 1 %. As shown, higher concentrations of NaOH and prolonged process times increased the removal of lignin. The numerical optimization tool of the Design-Expert program was used to optimize the lignin removal, adopting as a criterion to maximize this answer. The established conditions were: NaOH solution of 0.5 mol.L–1, time of 25 min and lower solids loading of 1 %, which led to a predicted value of 41.99 % (mean ± confidence interval of 95 %). Thus, three experiments were performed using these parameters to calculate the experimental result and the mean lignin removal was 44.7 %. Hence, these conditions of hydrodynamic cavitation were selected for the pretreatment of biomass, which was used for further experiments.
Even after optimization, it was observed that the alkaline pretreatment had a comparatively higher delignification rate, although requiring a higher temperature (121 °C). However, despite the hydrodynamic cavitation showing less lignin removal, the process took place at a lower temperature (30–60 °C) and fermentation was carried out at the selected conditions for pretreatments.
3.2 Chemical characterization of biomass
3.2.1 X-ray diffraction (XRD)
The relative amount of crystalline cellulose in the lignocellulosic biomass varies according to its nature and composition [30]. Diffractograms of raw sugarcane bagasse and pretreated sugarcane bagasse by hydrodynamic cavitation and alkaline process are shown in Fig. 2.
All samples analyzed exhibited typical cellulose diffraction peaks (2θ = 16.0° and 2θ = 22.5°). The highest peak corresponded to the crystallographic planes of 002. The raw sugarcane bagasse presented the lowest value of CrI (48.4 %). On the contrary, the CrI values corresponding to biomass pretreated by hydrodynamic cavitation and alkaline pretreatment were 57.4 % and 63.8 %, respectively. These results are consistent with several studies that reported that an increase in the value of this index can be observed when the biomass undergoes pretreatment. The phenomenon is due to the fact that some pretreatments remove certain amounts of lignin and hemicellulose, which represent the amorphous fractions, and not necessarily due to transformations in the crystal structure of lignocellulosic biomass [31].
3.2.2 Sugarcane bagasse samples analyzed through mid-infrared spectroscopy (MIR)
Mid-infrared analysis was used to detect the presence of the main organic groups that constitute the lignocellulosic structure. The absorption bands detected covered the mid-infrared region between 400 and 4000 cm–1 (Online Resource 1). All treated samples had well-defined bands at regions similar to those of the raw sugarcane bagasse. Nevertheless, there were some bands that can be used as signatures of the pretreatment processes, such as 2850 cm–1 and 1728 cm–1, where the raw bagasse spectrum presents absorption intensities which were not observed in the other samples. The bands around 1631 cm–1 and 1604 cm–1 are slightly shifted to smaller wavelengths when comparing pretreated samples with the raw biomass. On the other hand, the samples showed wide absorptions in the range from 1190 to 862 cm–1, with peaks at 1161 cm–1, 1029 cm–1, and 896 cm–1. A second absorption region can be seen in the range of 744 to 405 cm–1, with peaks at 663, 476, 559, 522, and 432 cm–1. The 3334 cm–1 band is related to the O–H stretching band, due to the presence of water in the samples. The 2916 cm–1 band is related to the CH2 and CH3 groups that are present in cellulose, lignin, and hemicellulose. The presence of lignin also appears at 2850 cm–1, due to vibration of OCH groups [32].
The band at 1728 cm–1 can be attributed to lignin ester groups or acetyl groups present in hemicellulose. The band at 1631 cm–1 and 1604 cm–1 are attributed to the aromatics C-Phenyl and C≡C, respectively, normally found in lignin. The band at 1514 cm–1 is related to the C≡C stretch of the aromatic ring of lignin [33]. The region from 1487 to 1299 cm–1 was related to crystalline cellulose while the 1242 cm–1 band is related to the elongation of the C–O bond of hemicellulose and lignin. The 1161 cm–1 band was attributed to the stretching of the C–O–C bonds, while the 1029 cm–1 band was detected from the C–O stretch and strain bonds in lignin [33]. The band at 896 cm–1 has a higher intensity for the alkaline pretreatment and lower intensity for the hydrodynamic cavitation process. This is because this band corresponds to the amorphous portion of cellulose [34].
3.2.3 Sugarcane bagasse analysis via near-infrared spectroscopy (NIR)
Near-infrared spectroscopy provides information on numerous combinations of vibrational bands and it is considered to be in the range of electromagnetic spectrum from 12,000 to 4000 cm–1 [35]. This is a very convenient method to be used for the structural characterization of materials. The simplicity, speed, and relative abundance of near-infrared light sources are the strength of the technique. The NIR bands are harmonics originated, for example, from CH, OH, and NH groups (Online Resource 2). Bands in the region of 10,000 to 8000 cm–1 are characterized by normal modes that are polymeric combinations of OH [6]. The lignin band is observed at 8240 cm–1. This characteristic is confirmed with the analysis of the second derivative (not shown). The band at 5806 cm–1 is attributed to the first harmonic of the CH stretch band. The region from 6111 to 5947 cm–1 corresponds to the first harmonic of the aromatic CH stretch vibration, which is responsible for the amount of lignin. Amplitude changes and displacement in this region may indicate degradation in lignin macromolecules [34].
3.2.4 Raman spectroscopy analysis of sugarcane bagasse samples
Raman spectra showed that the expected intensities in the raw bagasse sample were related to cellulose, hemicellulose, and lignin. In the spectra of the raw sample, it was possible to observe a band at 1091 cm–1 which corresponded to cellulose and a band at 2904 cm–1 attributed to hemicellulose. The bands between 1140 and 1230 cm–1, with peaks at 1171 and 1202 cm–1 are due to the cellulose. These bands varied in function to the incident and scattered polarization of the laser light. Finally, it was possible to observe another band between 1555 and 1650 cm–1 which was associated with the phenyl group of lignin [36] (online Resource 3). In the case of the treated samples, it was possible to observe a significant or total decrease in the mentioned bands. In the alkaline pretreated sample, the region of 2904 (not shown), 1650 to 1550 cm–1, 1230 to 1140, and 1091 cm–1 do not show any significant signal. Comparison between the samples that was submitted to hydrodynamic cavitation pretreatment still showed a weak signal of cellulose, but the band from 1555 to 1650 cm–1 associated with lignin disappeared which indicated the effectiveness of the method (Online resource 3). This reinforces the information that this pretreatment was not as efficient for removing the lignin content as the alkaline pretreatment.
3.3 Solid-state fermentation of raw sugarcane bagasse
Table 5 shows the results of 9 days of solid-state fermentation of raw sugarcane bagasse used as a control and the biomass pretreated. As can be seen, raw sugarcane bagasse had lower EI24h when compared to alkaline and hydrodynamic cavitation pretreated material. Likewise, no enzymatic activities were detected in the raw extract of raw sugarcane bagasse. However, cellulolytic enzymes were detected in the cell-free liquid of biomass submitted to alkaline pretreatment and hydrodynamic cavitation. Thus, the solid-state fermentation was extended to 14 days to analyze the performance of the bioemulsifier and the hydrolytic enzymes produced by the microorganism from pretreated biomass.
In addition, Fig. 3 shows the variation of the emulsification index (EI24h) of cell-free liquids from solid-state fermentation, measured at different intervals during 14 days. This parameter was used as an indicator of the bioemulsifier production from the optimized conditions selected for alkaline pretreatment (NaOH: 0.2 mol.L−1, time: 12 min) and hydrodynamic cavitation (NaOH solution: 0.5 mol.L−1, time: 25 min, and lower solids loading 1 %).
In general, it was observed that the bioemulsifier produced by A. pullulans LB83 can emulsify kerosene, which was used as a hydrophobic substrate. The results showed an increase in bioemulsifiers production in both alkaline pretreatment and hydrodynamic cavitation. This is likely due to reduced water activity, which led to a longer adaptation phase before growth began [37]. In the case of hydrodynamic cavitation, the bioemulsifier production was observed from 3rd day. The maximum values of the emulsification index (EI24h) were observed for the biomass submitted to alkaline pretreatment. The comparison of the emulsification index on the 3rd and 14th day showed an increase from 36.8 to 65.8 % for the alkaline pretreated biomass, whereas, for hydrodynamic cavitation, the EI24h ranged from 0 to 41.3 %. This difference can be explained by the lignin removal rate from the biomass, which was 55.1 % and 44.7 % for alkaline and hydrodynamic cavitation pretreatments, respectively. In this sense, lignin is often considered as a barrier that prevents the access of microbial enzymes to lignocellulosic biomass. The synthesis of some bioproducts, such as second-generation bioethanol [38], enzymes [39], the single cell protein [40], among others, have been improved after biomass delignification processes.
In the literature, several studies report the use of lignocellulosic biomass as support for the production of surface-active compounds [41]. For example, Zhu et al. [42] analyzed six agro-industrial by-products for the production of biosurfactants in solid-state fermentation from Bacillus amyloliquefaciens XZ-173. The authors selected rice straw and soy flour as the main substrates for the production of surfactin. After optimizing the variables that influenced the synthesis of the biosurfactant, a yield of 15.03 mg.gds–1 was achieved in a 50 L fermentor. However, to our knowledge, the present studied reports, for the first time, the use of pretreated sugarcane bagasse as both, support and culture medium for bioemulsifier production in solid-state fermentation.
3.4 Enzymatic activity
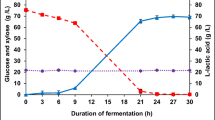
Sugarcane bagasse contains fermentable sugars that stimulate the production of bioemulsifiers. At the same time, this type of biomass stimulates the production of cellulolytic enzymes, required by the microorganism to use the bagasse as a substrate source. In the present study, cellulolytic enzymes such as endoglucanase and exoglucanase were measured for 14 days in the solid-state fermentation for the production of bioemulsifiers of A. pullulans LB83 (Fig. 4).
In general, it was observed that the enzyme production of the raw extract obtained from the solid-state fermentation of both alkaline and hydrodynamic cavitation pretreated biomasses varied during the fermentation period. However, among the two enzymes determined, the highest values corresponded to endoglucanase (Fig. 4). In this sense, the maximum values of endoglucanase were observed on the 9th day, with 2.25 U.g–1 and 2.43 U.g–1 for alkaline and hydrodynamic cavitation pretreated materials, respectively.
For exoglucanase, the maximum values were detected on the 7th and 14th days for alkaline and hydrodynamic cavitation pretreated biomass, with values of 1.39 U.g–1 and 1.45 U.g–1, respectively. In this sense, Marques et al. [43] reported that Colletotrichum crassipes CSY 02 and Paecilomyces sp. SF 021 produced 5.32 U.g– and 1.43 U.g– of endoglucanases after 7 days in a mixture 1:1 of sugarcane bagasse and wheat bran. In the current study, the enzymatic results obtained allowed to corroborate that A. pullulans LB83 produced hydrolytic enzymes having only pretreated sugarcane bagasse as a carbon source. At the same time, the sugars released by these enzymes were used by the yeast for cell growth and bioemulsifier production. On the other hand, the reports of exoglucanase detection by yeast in solid-state fermentation are scarce when compared with filamentous fungi. In the study of da Silva et al. [44] showed an exoglucanase production of 25.4 IU.g–1 by Penicillium roqueforti ATCC 10110 using sugarcane bagasse. However, the authors detected an increase (62.5 IU.g–1) in the synthesis of the enzyme when it was used a blend of green coconut shell, corn cob, and sugarcane bagasse. Another study of Zia et al. [45] also demonstrated the suitability of sugarcane bagasse for exoglucanase production and characterization from Phaeolus spadiceus. Maximum activity of exoglucanase reported was 57.64 IU.mL.min–1 after day 4 when using this biomass as support for solid-state fermentation. Maximum activity of exoglucanase was observed after day 4 (57.64 IU.mL–1.min–1), at 25–30 °C and 50 % moisture level (31.5 IU.mL–1.min–1), at pH 4.5 (47.03 IU.mL–1.min–1) and also with carbon source (5 % sucrose) and nitrogen source (2.5 % urea).
The results obtained for the enzymatic profile allowed to corroborate that A. pullulans LB83 produced hydrolytic enzymes, using the pretreated sugarcane bagasse as a carbon source. At the same time, the sugars released by these enzymes were used by the yeast for cell growth and bioemulsifier production.
3.5 Characterization of the bioemulsifier produced by A. Pullulans
The composition of bioemulsifier produced by A. pullulans LB83 in solid-state fermentation using pretreated sugarcane bagasse is shown in Table 6. The literature shows that A. pullulans strains produce heavy extracellular oils called liamocins, which have surface activity. The unique chemical structure of these molecules consists of a single sugar alcohol as a main group (e.g., mannitol or arabitol), which is partially O-acylated with a polyester tail with up to six 3,5-dihydroxydecanoic ester groups that can be acetylated [46].
Liamocines A1, A2, B1, and B2 are the most common variants produced among the different strains of A. pullulans. According to [47] A. pullulans strain RSU 12 (NRRL 50381) produced a mixture of mannitol and arabitol-type liamocin when sucrose was used as a carbon source in the medium. Likewise, in the present study, the compound produced by A. pullulans LB83 was identified as a mixture of mannitol and arabitol-type liamocin, but the molecules were synthesized from sugars contained in the pretreated sugarcane bagasse when used as a carbon source.
In this sense, this result reinforces the potential of lignocellulosic biomass as an economic source for the synthesis of microbial metabolites in the context of biorefineries. Bischoff et al. [47] also observed the production of mannitol A1, A2, B1, B2, and to a lesser extent C1 and C2 mannitol from A. pullulans CU 43 (NRRL 50380) cultivated on sucrose as a carbon source.
In addition, other structurally related compounds that are synthesized together with liamocines are the exophilins. The main characteristic of these molecules is the presence of the polyester tail without a head group and its synthesis takes place in a smaller amount. According to Price et al. [46], exophilin A was the minor component in the antibiotic activity of the mixture produced by A. pullulans NRRL 50380. However, in the bioemulsifier produced by A. pullulans LB83 in the present study, the highest percentage corresponded to exophilin, mainly type B2.
4 Conclusions
The results indicate that, under the evaluated conditions, alkaline pretreatment showed to be more efficient when compared with hydrodynamic cavitation for the sugarcane bagasse delignification. The detection of cellulolytic enzymes confirmed the use of the pretreated sugarcane bagasse as a substrate for microorganism growth and the synthesis of the bioemulsifier. The biomolecule produced was identified as a mixture of mannitol and arabitol-type liamocin. To the best of our knowledge, this is the first study of the bioemulsifier production by A. pullulans LB 83 using pretreated sugarcane bagasse as the sole carbon source in solid-state fermentation. Considering the need for sustainable alternatives to synthetic emulsifiers, it is mandatory to develop future studies for scale-up in bioreactors aiming at the availability of bioemulsifiers in the market.
References
Botshekan M, Moheb A, Vatankhah F, Karimi K, Shafiei M (2021) Energy saving alternatives for renewable ethanol production with the focus on separation/purification units: a techno-economic analysis. Energy 122363. https://doi.org/10.1016/j.energy.2021.122363
Sanford K, Chotani G, Danielson N, Zahn JA (2016) Scaling up of renewable chemicals. Curr Opin Biotechnol 38:112–122. https://doi.org/10.1016/j.copbio.2016.01.008
Capolupo L, Faraco V (2016) Green methods of lignocellulose pretreatment for biorefinery development. Appl Microbiol Biotechnol 100(22):9451–9467. https://doi.org/10.1007/s00253-016-7884-y
Companhia Nacional de Abastecimento - (CONAB) (2021) Acompanhamento da Safra Brasileira. Observatório Agrícola 2(4):1–60
Terán Hilares R, de Almeida GF, Ahmed MA, Antunes FAF, da Silva SS, Han JI, dos Santos JC (2017) Hydrodynamic cavitation as an efficient pretreatment method for lignocellulosic biomass. a parametric study. Bioresour Technol 235:301–308. https://doi.org/10.1016/j.biortech.2017.03.125
Workman JJ, Weyer L (2007) Practical guide to interpretive near-infrared spectroscopy. CRC Press
De Bhowmick G, Sarmah AK, Sen R (2018) Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour Technol 247:1144–1154. https://doi.org/10.1016/j.biortech.2017.09.163
Prado CA, Antunes FAF, Rocha TM, Sánchez-Muñoz S, Barbosa FG, Terán-Hilares R, Santos JC (2021) A review on recent developments in hydrodynamic cavitation and advanced oxidative processes for pretreatment of lignocellulosic materials. Bioresour Technol 345:126458
Sałek K, Euston SR (2019) Sustainable microbial biosurfactants and bioemulsifiers for commercial exploitation. Process Biochem 85(July):143–155. https://doi.org/10.1016/j.procbio.2019.06.027
Al-Sakkaf MK, Onaizi SA (2022) Rheology, characteristics, stability, and pH-responsiveness of biosurfactant-stabilized crude oil/water nanoemulsions. Fuel 307:121845. https://doi.org/10.1016/j.fuel.2021.121845
Song B, Zhu W, Song R, Yan F, Wang Y (2019) Exopolysaccharide from Bacillus vallismortis WF4 as an emulsifier for antifungal and antipruritic peppermint oil emulsion. Int J Biol Macromole 125:436–444. https://doi.org/10.1016/j.ijbiomac.2018.12.080
Emulsifiers Market Future on Recent Innovation 2026 Key Players (2020) Corbion, BASF SE, Lonza., Stepan Company, Akzo Nobel N.V., Estelle Chemicals Pvt. Ltd. https://www.openpr.com/news/2063526/emulsifiers-market-future-on-recent-innovation-2026-key-players. Acessed 23 January 2023.
Dhagat S, Jujjavarapu SE (2021) Simulated annealing and artificial neural network as optimization tools to enhance yields of bioemulsifier and exopolysaccharides by thermophilic Brevibacillus borstelensis. J Environ Chem Engineer 9(4):105499. https://doi.org/10.1016/j.jece.2021.105499
Bourdichon F, Casaregola S, Farrokh C, Frisvad JC, Gerds ML, Hammes WP, Harnett J, Huys G, Laulund S, Ouwehand A, Powell IB, Prajapati JB, Seto Y, Ter Schure E, Van Boven A, Vankerckhoven V, Zgoda A, Tuijtelaars S, Hansen EB (2012) Food fermentations: microorganisms with technological beneficial use. Int J Food Microbiol 154(3):87–97. https://doi.org/10.1016/j.ijfoodmicro.2011.12.030
Brumano LP, Antunes FAF, Souto SG, Silva G, dos Santos JC, da Silva SS (2017) Biosurfactant production by sugarcane bagasse as a renewable alternative for bioremediation process. Explor Microorg Recent Adv Appl Microbiol 15:50
Kim I, Lee I, Jeon SH, Hwang T, Han JI (2015) Hydrodynamic cavitation as a novel pretreatment approach for bioethanol production from reed. Bioresour Technol 192:335–339. https://doi.org/10.1016/j.biortech.2015.05.038
Kim JS, Lee IK, Kim DW, Yun BS (2016) Aureosurfactin and 3-deoxyaureosurfactin, novel biosurfactants produced by Aureobasidium pullulans L3-GPY. J Antibio 69(10):759–761. https://doi.org/10.1038/ja.2015.141
Brumano LP, Antunes FAF, Souto SG, dos Santos JC, Venus J, Schneider R, da Silva SS (2017) Biosurfactant production by Aureobasidium pullulans in stirred tank bioreactor: new approach to understand the influence of important variables in the process. Bioresour Technol 243:264–272. https://doi.org/10.1016/j.biortech.2017.06.088
Velioglu Z, Ozturk UR (2014) Concurrent biosurfactant and ligninolytic enzyme production by Pleurotus spp. in solid-state fermentation. Appl Biochem Biotechnol 174(4):1354–1364. https://doi.org/10.1007/s12010-014-1136-3
Rodríguez A, Gea T, Sánchez A, Font X (2021) Agro-wastes and inert materials as supports for the production of biosurfactants by solid-state fermentation. Waste and Biomass Valoriz 12(4):1963–1976. https://doi.org/10.1007/s12649-020-01148-5
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2011) Determination of structural carbohydrates and lignin in biomass. Biomass Anal Technol Team Lab Anal Proced 2008:1–14
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Textile Res J 29(10):786–794. https://doi.org/10.1177/004051755902901003
Manitchotpisit P, Price NPJ, Leathers TD, Punnapayak H (2011) Heavy oils produced by Aureobasidium pullulans. Biotechnol| Lett 33(6):1151–1157. https://doi.org/10.1007/s10529-011-0548-1
Camilios-Neto D, Bugay C, de Santana-Filho AP, Joslin T, de Souza LM, Sassaki GL, Mitchell DA, Krieger N (2011) Production of rhamnolipids in solid-state cultivation using a mixture of sugarcane bagasse and corn bran supplemented with glycerol and soybean oil. Appl Microbiol Biotechnol 89(5):1395–1403. https://doi.org/10.1007/s00253-010-2987-3
Saur KM, Brumhard O, Scholz K, Hayen H, Tiso T (2019) A pH shift induces high-titer liamocin production in Aureobasidium pullulans. Biotechnol Prod Pro Engineering 103:4741–4752. https://doi.org/10.1007/s00253-019-09677-3
Barros FFC, Quadros CP de, Pastore GM (2008) Propriedades emulsificantes e estabilidade do biossurfactante produzido por Bacillus subtilis em manipueira. Food Sci Technol, 28(4), 979–985. https://doi.org/10.1590/s0101-20612008000400034
Tanaka M, Taniguchi M, Matsuno R, Kamikubo T (1981) Purification and properties of cellulases from Eupenicillium javanicum. J Ferment Technol 59:177–183
Wood TM, Bhat KM (1988) Methods for measuring cellulase activities. Methods in Enzymol 160(C):87–112. https://doi.org/10.1016/0076-6879(88)60109-1
Wang C, Qi W, Liang C, Wang Q, Wang W, Wang Z, Yuan Z (2021) Impact of alkaline pretreatment condition on enzymatic hydrolysis of sugarcane bagasse and pretreatment cost. Appl Biochem Biotechnol 193(7):2087–2097. https://doi.org/10.1007/s12010-021-03530-y
Marakana PG, Dey A, Saini B (2021) Isolation of nanocellulose from lignocellulosic biomass: synthesis, characterization, modification, and potential applications. J Environ Chem Engineering 9(6):106606. https://doi.org/10.1016/j.jece.2021.106606
Vanitjinda G, Nimchua T, Sukyai P (2019) Effect of xylanase-assisted pretreatment on the properties of cellulose and regenerated cellulose films from sugarcane bagasse. Int J Biol Macromole 122:503–516. https://doi.org/10.1016/j.ijbiomac.2018.10.191
Poletto M, Ornaghi Júnior HL, Zattera AJ (2014) Native cellulose: structure, characterization and thermal properties. Materials 7(9):6105–6119. https://doi.org/10.3390/ma7096105
Jiménez IM, Chandel AK, Marcelino PRF, Anjos V, Batesttin Costa C, Jose V, Bell M, Pereira B, da Silva SS (2020) Comparative data on effects of alkaline pretreatments and enzymatic hydrolysis on bioemulsifier production from sugarcane straw by Cutaneotrichosporon mucoides. Bioresour Technol 301:122706. https://doi.org/10.1016/j.biortech.2019.122706
Chandel AK, Antunes FAF, Anjos V, Bell MJV, Rodrigues LN, Polikarpov I, De Azevedo ER, Bernardinelli OD, Rosa CA, Pagnocca FC, da Silva SS (2014) Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid-base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol Biofuels 7(1):1–17. https://doi.org/10.1186/1754-6834-7-63
Smith-Moritz AM, Chern M, Lao J, Sze-To WH, Heazlewood JL, Ronald PC, Vega-Sánchez ME (2011) Combining multivariate analysis and monosaccharide composition modeling to identify plant cell wall variations by Fourier transform near infrared spectroscopy. Plant Methods 7(1):10–16. https://doi.org/10.1186/1746-4811-7-26
Antunes FAF, Thomé LC, Santos JC, Ingle AP, Costa CB, Anjos VD, Bell MJV, Rosa CA, Silva SS (2021) Multi-scale study of the integrated use of the carbohydrate fractions of sugarcane bagasse for ethanol and xylitol production. Renew Energy 163:1343–1355. https://doi.org/10.1016/j.renene.2020.08.020
Hamill PG, Stevenson A, McMullan PE, Williams JP, Lewis ADR, Sudharsan S, Stevenson KE, Farnsworth KD, Khroustalyova G, Takemoto JY, Quinn JP, Rapoport A, Hallsworth JE (2020) Microbial lag phase can be indicative of, or independent from, cellular stress. Sci Rep 10(1):1–20. https://doi.org/10.1038/s41598-020-62552-4
Bera S, Banerjee T, Samanta A (2021) Evaluation of enzymatic delignification of rice straw residues for bioethanol production. Int J Renew Energy Technol 12(2):99. https://doi.org/10.1504/ijret.2021.115276
Karp SG, Faraco V, Amore A, Letti LAJ, Thomaz Soccol V, Soccol CR (2015) Statistical optimization of laccase production and delignification of sugarcane bagasse by Pleurotus ostreatus in solid-state fermentation. BioMed Res Int 2015. https://doi.org/10.1155/2015/181204
Said SD, Zaki M, Asnawi TM, Novita E (2019) Single cell protein production by a local Aspergillus niger in solid state fermentation using rice straw pulp as carbon source: effects of fermentation variables. IOP Conf Series: Mat Sci Engineer 543(1). https://doi.org/10.1088/1757-899X/543/1/012002
Pele MA, Ribeaux DR, Vieira ER, Souza AF, Luna MAC, Rodríguez DM, Andrade RFS, Alviano DS, Alviano CS, Barreto-Bergter E, Santiago ALCMA, Campos-Takaki GM (2019) Conversion of renewable substrates for biosurfactant production by Rhizopus arrhizus UCP 1607 and enhancing the removal of diesel oil from marine soil. Elec J Biotechnol 38:40–48. https://doi.org/10.1016/j.ejbt.2018.12.003
Zhu Z, Zhang F, Wei Z, Ran W, Shen Q (2013) The usage of rice straw as a major substrate for the production of surfactin by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation. J Environ Manage 127:96–102. https://doi.org/10.1016/j.jenvman.2013.04.017
Marques NP, de Cassia PJ, Gomes E, da Silva R, Araújo AR, Ferreira H, Rodrigues A, Dussán KJ, Bocchini DA (2018) Cellulases and xylanases production by endophytic fungi by solid-state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Industrial Crops and Products 122:66–75. https://doi.org/10.1016/j.indcrop.2018.05.022
da Silva Nunes N, Carneiro LL, de Menezes LH, de Carvalho MS, Pimentel AB, Silva TP, Pacheco CS, de Carvalho Tavares IM, Santos PH, das Chagas TP, da Silva EG (2020) Simplex-centroid design and artificial neural network-genetic algorithm for the optimization of exoglucanase production by Penicillium Roqueforti ATCC 10110 through solid-state fermentation using a blend of agroindustrial wastes. Bioenergy Res 13(4):1130–1143. https://doi.org/10.1007/s12155-020-10157-0
Zia N, Mahmood RT, Asad MJ, Hadri SH, Javaid B, Nasreen S, Safder A (2021) Fermentation of sugarcane bagasse for the production and characterization of exoglucanase by Phaeolus spadiceus. Pak J Biochem Biotechnol 2:38–48. https://doi.org/10.52700/pjbb.v2i1.33
Price NPJ, Manitchotpisit P, Vermillion KE, Bowman MJ, Leathers TD (2013) Structural characterization of novel extracellular liamocins (mannitol oils) produced by Aureobasidium pullulans strain NRRL 50380. Carbohy Res 370:24–32. https://doi.org/10.1016/j.carres.2013.01.014
Bischoff KM, Leathers TD, Price NP, Manitchotpisit P (2015) Liamocin oil from Aureobasidium pullulans has antibacterial activity with specificity for species of Streptococcus. J Antibio 68(10):642–645. https://doi.org/10.1038/ja.2015.39
Acknowledgements
The authors gratefully acknowledge the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo – Process number 2020/06323-0 and 2016/10636-8) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil) (DS 88882.379239/2019-01) for financial support.
Data availability
Not applicable
Funding
This work was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo – Process number 2020/06323-0 and 2016/10636-8) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil) (DS 88882.379239/2019-01) for financial support.
Author information
Authors and Affiliations
Contributions
Rogger Alessandro Mata da Costa: conceptualization, software, formal analysis, investigation, data curation, and writing—original draft preparation. Bruna Carneiro: methodology and validation. Daylin Rubio-Ribeaux: conceptualization and writing—original draft preparation. Paulo Marcelino Franco: conceptualization. Geissy de Azevedo Mendes: formal analysis. Isis Lee da Silva: formal analysis. Virgílio de Carvalho dos Anjos: software and formal analysis. Júlio César dos Santos: supervision and data curation. Till Tiso: supervision. Silvio Silvério da Silva: supervision, resource and funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
- Alkaline pretreatment was effective for lignin removal
- Biomass pretreated was used as carbon source for the bioemulsifier production
- Aureobasidium pullulans produced bioemulsifier through solid-state fermentation
- Detection of cellulolytic enzymes confirms the use of sugarcane bagasse as carbon source
- Bioemulsifiers were identified as mannitol and arabitol-type liamocin
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Costa, R.A.M., Rubio-Ribeaux, D., Carneiro, B.C. et al. Sugarcane bagasse pretreated by different technologies used as support and carbon source in solid-state fermentation by Aureobasidium pullulans LB83 to produce bioemulsifier. Biomass Conv. Bioref. 14, 18579–18592 (2024). https://doi.org/10.1007/s13399-023-03896-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03896-5