Abstract
Gold nanoparticles (AuNPs) have a numerous biomedical applications including their antioxidant, antimicrobial, and anticancer applications. We have synthesized AuNPs laced with the extracts of red algae Halymenia pseudofloresii using the green method (Hp-AuNPs). The synthesized nanoparticles were characterized by UV spectroscopy, XRD, FTIR, and SEM. In UV spectroscopy, Hp-AuNP’s surface plasmon resonance (SPR) peak was discovered to be at 545 nm, which initially confirmed the formation of nanoparticles. The X-ray diffraction analysis confirmed the biosynthesized Hp-AuNP structures to be crystal in nature. Bioactive molecules, such as phenolic compounds and carboxylic groups, were identified by FTIR analysis as contributing to the reduction of Hp-AuNPs. The scanning electron microscope (SEM) analysis was used to identify the Hp-AuNP morphology as cubic and rectangular structures with 27 nm size. The antioxidant activity demonstrated that H. pseudofloresii extract (61.3%) and synthesized Hp-AuNPs (71.87%) effectively inhibited the DPPH radicals at 50 µg/mL concentration. The Hp-AuNPs exhibited efficient antibacterial effects against Staphylococcus aureus (24 mm), Lactobacillus (23 mm), and Pseudomonas aeruginosa (22 mm). The biosynthesized Hp-AuNPs showed potential cytotoxic activity against A549 lung cancer (IC50 = 19.02 µg/mL) than LN-18 glioblastoma cancer cells (IC50 = 32.46 µg/mL). Hence, further anticancer screening was tested against lung cancer cells. In the clonogenic assay, Hp-AuNPs effectively control the lung cancer cell colony formation at the concentration of 30 µg/mL, whereas the Hp-AuNPs induce apoptotic activity in lung cancer cells was confirmed through reactive oxygen species (ROS) generation assay. Therefore, the unique biologically synthesized Hp-AuNPs have the ability to function as an anticancer agent, and they may further be used for a variety of biomedical purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology is a recently using novel approach that offers vast applications and possibilities for biomedical research. As a tool for biological research, the synthesis of nanomaterials and nanoparticles has grown exponentially in recent years [1]. The nanomaterials have more biological features including anti-tumor, drug-microbial, anti-parasitic, immunosuppressive, and bio-catalytic activities [2]. Nanoparticles that are produced using environmentally friendly methods (green synthesis) have become more popular for drug delivery systems, than chemical and physical approaches due to their advantages in rapid development, economy, and biodegradable, which negate the need for harmful chemicals, high temperatures, and pressure [3]. The unique properties of nanoparticles have a great deal of potential to increase the specificity for disease detection and treatment and it could aid in overcoming the restrictions [4]. There are various classes of nanoparticles, including lipid-based nanoparticles and inorganic nanoparticles. There are several different nanoparticles being synthesized at the moment, including gold, titanium, silver, and palladium. Recent data indicates that gold nanoparticles are highly effective in delivering cancer drugs [5]. Thus, they have displayed exceptional properties including enzyme-mimicking abilities that are similar to those of peroxidase, oxidase, catalase, superoxide dismutase, or reductase. Due to the availability of numerous bioactive components, the production of gold nanoparticles using plant and seaweed extract drew increased attention and also said to be non-toxic nanoparticles [6]. At a point when administered to cells and tissues, functionalized gold nanoparticles display good biocompatibility and controlled distribution patterns, making them particularly excellent candidates for the foundation of novel therapeutics drugs [7].
The term “cancer” refers to a group of genetic functions that are developed by human cells when they go from normal states to neoplastic growth stages. More specifically, these functions are essential for the formation of malignant tumors [8]. As one of the most lethal forms of cancer, lung cancer occurrences and deaths are on the rise globally, with an estimated 2.09 million additional cases and 1.76 million more fatalities [9]. Lung and glioblastoma are the most dangerous cancer worldwide. Based on data from the National Centre for Health Statistics’ death-certificate mortality records, the predicted rate of cancer is higher than other diseases [10]. Recently, cancer research has made significant advancements. Despite great improvements in therapy, it is still a major concern for global health today. Preventive measures and early detection may reduce the number of cancer fatalities [11], but treatment options for advanced stages of cancer include some therapies such as chemotherapies, immunotherapies, and nanoparticle drug delivery. Numerous applications that are continually being developed for use in the diagnosis, detection, imaging, and therapy of lung cancer demonstrate the enormous potential of nanoparticle-based medicine [12]. Understanding the biology of the tumor, the microenvironment, and the interaction between cancerous cells and nanoparticles is essential for developing effective ways for selective drug delivery to lung metastases and tumours [13].
The rise of multidrug-resistant bacteria is a major public health problem since it has resulted from improper antibiotic usage, which has led to longer hospital stays, higher medical costs, and even death in certain cases. Thus, a cost-effective, comprehensive antibiotic is needed, and using nanoparticles to treat drug-resistant bacteria is especially appealing [14]. In nanotherapy, the nanoparticles are developed from various bioactive compounds that combine with a drug at the point of delivery. To increase drug effectiveness and lessen negative effects, they alter the pharmacokinetic parameters of the drug [15]. The features of 10–100 nm nanoparticles have attracted a lot of interest in drug delivery. Its decreased availability in blood flow and tissues is due to its extraordinarily high surface-area-to-volume ratios and the fact that immune cells can digest particles larger than 100 nm [16]. In the past few years, microbes can produce nanoparticles either intracellularly or extracellularly through a variety of mechanisms [17]. But at present, seaweeds are widely used for nanoparticle synthesis because they have numerous bioactive molecules with an excellent antioxidant capacity [18].
Seaweeds are an abundant source of several different types of bioactive chemicals, including polyphenols, proteins, carotenoids, minerals, vitamins, and polysaccharides. The functional groups such as carboxylic, amino, and hydroxyl molecules in the seaweed phytonutrients act as an efficient metal-reducing agent as well as a capping agent in order to create a strong coating on the metal nanoparticles in a single stage of production [19]. Seaweed has been found to have a variety of biological properties, including antibacterial, antifungal, and anticancer actions, among others due to the bioactive chemical compounds [20]. Although there are several reports of metal nanoparticle synthesis using terrestrial plants, there is less evidence of AuNP fabrication utilizing sustainable marine habitats such as seaweeds. Red marine algae found in seaweed are used in the production of nanoparticles because they are thought to be extremely effective living marine resources that are renewable [21]. Halymenia, a genus of red marine algae, has a wide number of species that have been found in Indo-Pacific coastal region. Previous reports described that metal nanoparticles were developed from Halymenia dilatata [22] and H. poryphyroides [23] for biomedical applications. To the best of our knowledge, a paucity of data on the development of nanoparticles use H. psudofloresii and its pharmacological properties. Thus, in the present study, we investigated the possibility of using an aqueous extract of H. psudofloresii in the production of AuNPs and their antioxidant, antibacterial, and anticancer activities.
2 Materials and methods
2.1 Collection of seaweed
The coastal region of Mandapam, Tamil Nadu, India (916′51″ N, 7910′37″ E) is where the fresh red marine algae Halymenia pseudofloresii was collected. After being cleaned with both tap water and distilled water, the sample was subsequently dried off. An electric blender was used to grind the dried seaweed, and the powder was kept in a sterile container for later usage.
2.2 Chemicals used
Synthesis of gold nanoparticles was carried out using gold III chloride solution (HAuCl4). For cancer cell culture, Dubecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS)-PH-7.4, trypsin–EDTA, antimycotic solution, antibiotic solution, and Tryptan Blue were used. In vitro, anticancer activity was tested using dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), methanol, glacial acetic acid, crystal violet, acridine orange, and ethidium bromide. These chemicals were bought from Gibco, Thermo Fisher Scientific, and HiMedia in Mumbai, India.
2.3 Preparation of Halymenia pseudofloresii aqueous extract
At the time of the preparation process, 1 g of algae powder was dissolved in 100 mL of distilled water to create an aqueous extract. After 20 min of heating the mixture at 60 °C, the Whatmann No. 1 filter paper was used to separate the extract. The synthesis of Hp-AuNPs was carried out using the filtrate.
2.4 Biosynthesis of gold nanoparticles
The gold nanoparticles were synthesized by reducing 70 mL of 1 mM HAuCl4 with 30 mL of H. pseudofloresii extract in a beaker at 60 °C for 20 min with continuous stirring using a magnetic stirrer. After 20 min, the reaction mixture changed its color from pale yellow to dark purple color. The gold colloidal solution was monitored UV spectroscopically at a 1-h time interval. After the complete reduction reaction, a colloidal solution containing gold nanoparticles was obtained by centrifuging the mixture at 12,000 rpm [24]. The obtained pellet form of Hp-AuNPs was dried for characterization analysis.
2.5 Characterization of synthesized gold nanoparticles
The goal of gold nanoparticle synthesis is to investigate the properties, which was done using various analytical techniques. That they were initially assessed using double-beam UV–Vis spectroscopy, which is the most important method to detect nanoparticle existence, at various wavelengths between 400 and 700 nm (double beam UV-spectrophotometer, Perkin-Elmer MA, USA). Then, the structural characterization of Hp-AuNPs was determined using the SEM (CM200, PHILIPS, Netherland). Every existing particle has a type of face that mean diffraction pattern of crystalline structure. So, we used XRD method to determine the crystalline composition of the biosynthesized Hp-AuNPs (Bruker AXS, Inc. Madison, USA). Knowing the functional group of the particle in question is crucial for every practical aspect of the experiment. In this case, Fourier transform infrared spectroscopy was used to make the determination of functional groups capped on the Hp-AuNPs (FTIR Perkin-Elmer, MA, USA).
2.6 Antioxidant activity
2.6.1 DPPH radical scavenging assay
The DPPH free radicals quenching ability of Hp-AuNPs were analyzed to measure the antioxidant properties. The various concentration of biosynthesized Hp-AuNPs (10, 20, 30, 40, and 50 µg/mL) were added to the 0.1-mM DPPH solution in ethanol. The purple-colored solution was transformed into a colorless solution after 30 min of incubation. The absorbance at 517 nm was then measured using UV spectrophotometry. The following equation was used to get the percentage of inhibition [25].
2.7 Antimicrobial activity
The microbial activity of synthesized Hp-AuNPs were determined by agar well diffusion method against clinical pathogens. The test culture of Lactobacillus, Staphylococcus aureus, Pseudomonas aeruginosa, and Vibrio cholerae were maintained in Luria Bertani broth. The cultured bacterial species was swabbed on Muller Hinton agar in the petri plates separately. Then, gel puncture was used to create the four wells, each of which has a diameter of 6 mm. Various concentration of synthesized Hp-AuNPs (25, 50, and 100 μg/mL) and standard antibiotic as a control was poured into the well. The drug-loaded petri plates were incubated at 37 °C for 24 h. The inhibitory effect was recorded based on the zones formed in the culture plates, and the diameter of the zone of inhibition was measured in millimeters [25].
2.8 Anticancer activity
2.8.1 Cancer cell culture
The human lung cancer cells (A549) and glioblastoma cells (LN-18), which were used for anticancer studies, were bought from National Centre for Cell Science (NCCS), Pune, India. Each cell line was cultured on DMEM (Dulbecco’s modified Eagle’s media) as per the general protocol, with the addition of necessary components including 10% FBS, 1% antibiotic, and antimitotic solution and maintained at 37 °C in a CO2 incubator. The T-25 tissue culture flasks in which these cell lines were placed, underwent a subculture process every 3 days.
2.8.2 MTT assay
The primary goal of the MTT analysis was to assess the cytotoxicity of produced Hp-AuNPs against lung and glioblastoma cancer cells. In this analysis, the 90% confluent cell lines were trypsinized and loaded into the 96-well plate in the density of 2 × 104 cells/well. After 24 h of incubation, spent media was discarded and cancer cells were treated with the Hp-AuNPs as an anticancer drug at the concentration range of 3 to 50 µg/mL; one was kept as a control well that is not treated with Hp-AuNP. Next day, drug-treated cells were rinsed with PBS buffer and stained with 5 mg/mL concentration of MTT reagent (100 µL) and then incubated for 4 h in a CO2 incubator to obtain the desired results. After some time, crystals started to form; these crystals were then completely solubilized by adding 50 µL of DMSO solution. Finally, the cell viability was measured at 450 nm using an ELISA microplate reader and the percentage inhibition and IC50 concertation were calculated [26].
2.8.3 Clonogenic assay
Clonogenic tests are an extremely helpful tool for determining the anticancer agents that inhibit or destroy cancer cell colonies [27]. For this activity, the fully confluent A549 cells were loaded to a 6-well plate (7.5 × 104 cells/well). Then, the objective of forming colonies, the cells were cultured for 72 h at 37 °C in a CO2 incubator. Then, at different doses (10, 20, and 30 µg/mL), the prepared or biosynthesized gold nanoparticles are added to the cultivated cell line present in the well plate, with the untreated cells in the wells serving as the control. Cells were rinsed with PBS and given a 1-mL fixing solution for 5 min after being exposed to the drug treatment for 24 h. After removing the fixing solution, 0.5% crystal violet dye was added and incubate the mixture for 2 h at room temperature. After that stanning procedure, that crystal violet was washed off with running water, the plates were air-dried, and colonies were counted using an inverted microscope.
2.8.4 Apoptotic assay
Apoptosis activity was analyzed by reactive oxygen species generation assay. First, the required concentration of A549 cells was added to 6-well plates and allowed to grow for 24 h. The cells were treated with IC50 concentration of Hp-AuNPs, untreated cells acted as a control and were incubated for 24 h. After drug treatment, 1 mg/mL of 2,7-dichlorodihydrofluorescein diacetate (DCF-DA) stain was used to determine the generation of ROS level in the Hp-AuNPs treated cells. The DCF-DA dye-treated cells were examined using fluorescence microscopy in order to identify the apoptosis-induced cells [28].
3 Results
3.1 Synthesis of gold nanoparticles using H. pseudofloresii
The synthesis of gold nanoparticles was performed by the reduction process with H. pseudofloresii extract. Respectively, at the initial stage of the reaction, solution remains colorless, after 30 min of heating condition the solution started to change its color rapidly. The reduction of gold ions into gold nanoparticles was visually confirmed by the color change of the colorless solution to deep purple color after 1 h of reaction (Fig. 1A).
3.2 UV Spectroscopy characterization of Hp-AuNPs
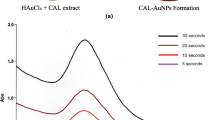
Monitoring the reduction reaction between H. pseudofloresii and chloroauric acid was accomplished by the use of UV spectroscopy. At the initial stage of the reduction reaction, there is no progression in the surface plasmon resonance peak. The SPR peak rapidly increased and was clearly observed at 545 nm, after 1 h of incubation which confirmed the formation of Hp-AuNPs (Fig. 1B). In this observation, there was no variation in SPR peak value after the complete reduction of gold nanoparticles proved the stability of nanoparticles.
3.3 XRD analysis of Hp-AuNPs
The crystalline structure of the gold nanoparticles produced by H. pseudofloresii is confirmed using the X-ray diffraction (XRD) technique. A typical XRD pattern of biosynthesized Hp-AuNPs were displayed in the intensity between 20 and 80°. The XRD spectrum showed 3 major peaks at 2Ɵ value (Fig. 1C). The diffraction peaks of Hp-AuNPs were detected at 2Ɵ values of 38.30, 44.53, 64.73, and 77.70, which corresponded to the crystal plans of (111), (200), and (222), respectively. All of the peaks in the diffraction pattern could be indexed to the face-centered cubic structure of the metal nanoparticles. The crystalline structure of AuNPs developed by red seaweed is quite similar to that previously described standard JCPDS No-03–065-2870.
3.4 FTIR analysis of Hp-AuNPs
The FTIR technique was utilized for the identification of biomolecules present in the aqueous red seaweed extract-capped gold nanoparticles. The FTIR spectra of synthesized Hp-AuNP displayed bands at 1637, 2110, and 3302 cm−1 (Fig. 1D). These absorption peaks denote compounds involved in the nanoparticle reduction mechanism. The vibrations of the conjugated alkene compounds (C = C stretching) were identified from the medium peak of 1637 cm−1. The (C = C stretching) of alkyne groups (protein molecules) was shown by the relatively faint signal at 2110 cm−1. The carboxylic acid presence is shown by the prominent peak that was absorbed at 3302 cm−1 (O–H stretching of phenolic compounds). This peak confirms the presence of proteins and phenolic compounds in the biosynthesized Hp-AuNPs.
3.5 SEM analysis of Hp-AuNPs
The morphological characteristic of synthesized Hp-AuNP particles were observed to be well dispersed as seen in the scanning electron microscope. The size range of the particles was observed in various magnifications and the Hp-AuNP size range from 22 to 38 nm, with an average size of 27 nm. The analysis showed that the biosynthesized Hp-AuNPs were mostly cubic and rectangular, and some particles were irregular-shaped (Fig. 2).
3.6 Antioxidant activity
The DPPH free radical scavenging experiment was used to evaluate or examine the antioxidant activity of H. pseudofloresii and Hp-AuNPs. Various concentration range of (10–50 µg/mL) test samples and standard sample (ascorbic acid) was investigated for DPPH scavenging assay. The increased free radical scavenging ability was observed based on the test samples concentration ranges from low to high. In this analysis, 73% of DPPH radicals inhibited by biosynthesized Hp-AuNPs at the concentration of 50 µg/mL. Similarly, H. pseudofloresii showed 60% of radical scavenging ability. The obtained finding showed moderately significant inhibition compared to standard ascorbic acid (Fig. 3). The antioxidant experiment demonstrated that the H. pseudofloresii mediated AuNPs have a high capacity to scavenge DPPH radicals.
3.7 Antibacterial activity
The green synthesized Hp-AuNPs were tested for their antibacterial ability against some disease-causing bacterial species by the agar well diffusion technique. At varying doses (25, 50, 100 µg/mL), Hp-AuNPs significantly inhibited the expansion of Gram-positive bacteria (Pseudomonas aeruginosa and Lactobacillus) and Gram-negative bacteria like (Staphylococcus aureus and Vibrio cholera) represented in the Fig. 4A. The Hp-AuNPs are more effective against S. aureus (24 mm), Lactobacillus (23 mm), and P. aeruginosa (22 mm) and significantly less effective against V. cholerae (20 nm). This analysis demonstrated the Hp-AuNPs treated pathogens effectively controlled by a dose-dependent manner (Fig. 4B). High concentration of gold nanoparticles induced maximum zones whereas less concentration induced minimum zone; the zone of inhibition confirmed the bactericidal activity of biosynthesized Hp-AuNPs.
3.8 Anticancer activity
3.8.1 Cytotoxic activity (MTT assay)
By using the MTT assay, the cytotoxic activity of Hp-AuNP was evaluated in human lung cancer (A549) cells and glioblastoma cells (LN-18). Hp-AuNP was applied to both cancer cells for the screening cytotoxicity at concentrations ranging from 3 to 50 µg/mL, and the nanodrug-free cells served as the control group. The gold nanoparticles treated cells growth was reduced, and the cells were damaged compared to control cells. The lung cancer cell percentage inhibition was increased from 33.57 to 64.864% based on less to higher concentrations of Hp-AuNPs, and the half inhibitory concentration (IC50) value was found at 19.02 µg/mL (Fig. 5). Likewise, glioma cancer cell inhibition was increased from 32 to 55.4% and its IC50 was found to be 32.46 µg/mL (Fig. 6). This analysis demonstrated the synthesized Hp-AuNPs were effective against lung cancer cells at less IC50 concentration when compared to glioblastoma cell lines.
3.8.2 Clonogenic assay
A colony formation experiment was carried out to measure the inhibition of A549 lung cancer cells proliferative after treatment with Hp-AuNPs. This was accomplished by treating highly colonized cancer cells with three different concentrations (10, 20, and 30 µg/mL) of Hp-AuNPs for 24 h while incubating in the standard culture conditions and then analyzing the inhibition of colony formation. According to the findings, Hp-AuNPs treated cells considerably and dose-dependently reduced the A549 cell colonies when compared with untreated control cells (Fig. 7). When A549 cells were treated with 20 µg/mL of Hp-AuNPs, the colony growth was reduced by more than 50%. Similarly, Hp-AuNPs at 30 µg/mL dose suppressed 90% of lung cancer cell colonies. The outcome of this study demonstrated the tremendous anti-proliferative potential of seaweed-synthesized Hp-AuNPs.
3.8.3 Apoptotic assay
The imbalance between reactive oxygen species and antioxidant levels plays a pivotal role in tumor development. Apoptosis (cell death) in cancer cells may be triggered by an accumulation of reactive oxygen species. The ROS generation assay proved that there was an increased level of ROS in the Hp-AuNPs-treated A549 cell line. In this analysis, green fluorescence was found to be elevated in the A549 cells subjected to 20 µg/mL of Hp-AuNPs compared to the control (Fig. 8A), indicating the onset of apoptosis in lung cancer cells. The Hp-AuNPs effectively induced 73% of apoptotic cells shown in Fig. 8B. This analysis proved that biosynthesized gold nanoparticles have a tendency to induce apoptosis cells.
4 Discussion
Nanoparticles, particularly green synthesized gold nanoparticles have emerged to receive a lot of interest in recent times, because of the increased applications in the pharmacological field as a nano drug [29]. It is essential to enhance environmentally acceptable techniques of nanoparticle synthesis using toxic-free marine resources. Recently, there has been a rise in activity all over the globe in the search for novel therapeutic medications derived from seaweeds to treat bacterial infections and cancer [30]. Therefore, in this study, the gold nanoparticles were developed from an aqueous extract of H. pseudofloresii, a red seaweed species, and then, we used a variety of in vitro assays to examine its broad spectrum of biological activities, including antioxidant, antibacterial, and anticancer effects. The optical properties of manufactured Hp-AuNPs were investigated using UV–vis spectroscopy, which identified an SPR peak at 545 nm as a result of a reduction process involving chloroauric acid and seaweed extract. This signal was caused by the conversion of gold ions to gold nanoparticles and surface plasmon resonance factors of gold [31]. The polyphenolic compounds present in the red seaweed species serve as a capping agent and help to stabilize the nanoparticles [32]. The presence of phenolic groups and protein molecule in the biosynthesized Hp-AuNPs were verified by FTIR analysis. The average size of H. pseudofolresii synthesized gold nanoparticles was found to be 27 nm using scanning electron microscopy, and the Hp-AuNP crystalline structure was confirmed by XRD analysis. Significantly similar results were found in the previously synthesized AuNPs from various seaweed species such as Lobophora variegada [33], Halymenia dilatata [22], and Champia parvula [24]. However, compared to previous reports, the Hp-AuNPs were synthesized rapidly through green synthesis technique.
An easy and effective technique of DPPH radical scavenging tests was used in order to investigate the anti-oxidant capacities of both the H. pseudofolresii extract and Hp-AuNPs. DPPH is of stable free radicals; oxidative stress occurs due to excess levels of free radicals accumulated in the body, which may cause harm to healthy cells [34]. Multiple types of research highlighted the anti-oxidant capabilities of edible plants that included a high concentration of phenolic chemicals. Phenolic components found in plants are one of the most important classes of antioxidants and free radical scavengers [35]. Seaweeds are a rich source of antioxidant molecules that could reduce the accumulation of free radicals in the body. Here, biosynthesized gold nanoparticles showed more effective antioxidant potential than seaweed extract. The synthesized AuNPs have a wide surface area, which serves to accelerate the absorption of phytochemical compounds; as a result, the antioxidant activity was greater in the produced particles than the extract [36].
In spite of remarkable development in medical treatment, bacterial diseases remain a critical risk to human health [37]. Gold nanoparticles have a kind of features, including their size, their ability to adhere to a wide variety of molecules, and their optical properties; such particles are an excellent prospect for both chemical and biological applications [38]. In addition, naturally occurring substances in seaweeds have been studied as a possible source of novel therapeutic medicines for a range of bacterial infections [39]. Hence, in the present research, agar well diffusion methodology was used to assess the antimicrobial activity of the synthesized Hp-AuNP against gram-positive bacteria such as Lactobacillus, S. aureus, and two gram-negative bacteria P. aeruginosa and V. cholerae. Among the above pathogens, Gram-positive bacteria S. aureus showed a higher zone of inhibition compared to others. It is because these bacteria’s cell wall composition is different. Gram-negative bacteria’s outer membrane has a very strong negative charge because of the presence of phospholipids and lipopolysaccharide, whereas the cell wall of Gram-positive bacteria is negatively charged because phosphate is a component of its cell wall composition [40]. The inhibition zone regulates; when metal ions in a solution interact with a bacterial cell, they become uniformly distributed across the area around the bacterial cell, with no particular localization [41]. The antibacterial action of Hp-AuNPs is caused by the denaturation of a bacterial cell wall, the slowdown of the respiratory function, the destruction of the outer membrane, and the suppression of pathogenic bacteria’s intracellular ATP generation. Additionally, the AuNPs had a long-lasting electrostatic attraction to the negatively charged bi-layer; hence, the particles were able to readily migrate into the cells and cause cell death [42].
Nanoparticles (Hp-AuNP) will develop into highly effective drug delivery methods for a variety of illnesses, particularly cancer by cell apoptosis. In tumor endothelial cells, nanoparticles can typically accumulate in non-specific ways, such as passive targeting via the increased permeability and retention (EPR) effect. One of the biggest breakthroughs in the creation of tailored anticancer therapy was the discovery of the EPR effect [43]. The gold ions in Hp-AuNP can inhibit DNA replication enzymes, which prevents the S-phase of the cell cycle from occurring [44]. H. pseudofloresii produced Hp-AuNPs was evaluated for their in vitro cytotoxic potential against human lung cancer and glioma cancer cells. The Hp-AuNP was tested in vitro, and the results indicated possible cytotoxicity in a dose-dependent manner. Hp-AuNP (19.02 µg/mL) was determined to be the half-inhibitory concentration for lung cancer, and for glioblastoma IC50 was found to be 32.46 µg/mL. Recent studies show that cytotoxicity depends on the stabilizing agent and size of the Hp-AuNP [45]. This study showed Hp-AuNPs have a potent anti-proliferation effect on the A549 lung cancer cell. Our research confirms previous reports that gold nanoparticles produced from Magnolia officinalis [46], Pleuropterus multiflorus [47], and Caulepra racemose [48] have a strong cytotoxic impact on A549 cancer cells. Cancer cells depend greatly on an excessive production of reactive oxygen species, which contributes to the malfunctioning of mitochondrial, peroxidase, and oxidase activities while enhancing metabolic performance [49]. Additionally, ROS production was assessed by H2DCFDA labeling in lung cancer cells that had been treated with Hp-AuNPs. Because of their exposure to Hp-AuNPs, cancer cells have been reported to have higher levels of reactive oxygen species. In this study, the biosynthesized Hp-AuNPs effectively induces apoptosis in lung cancer cells and control the cancer cell colony formation.
5 Conclusion
This study describes a simple, rapid, and biological synthesis of gold nanoparticles from marine red algae Halymenia pseudofloresii. The reduction of gold ions to gold nanoparticles showed SPR peak at 545 nm which was initially confirmed by UV-spectroscopy. Scanning electron microscopy showed the average size of Hp-AuNPs was 27 nm, and their shapes were mostly cubic, rectangular, and irregular. The crystalline character of Hp-AuNPs is confirmed by Bragg’s reflection pattern of XRD. In Hp-AuNP synthesis, the bioactive molecules in the seaweed extract acted as a capping agent. The FTIR analysis identified the biomolecules such as phenol and protein groups presented in the gold nanoparticles. Due to the presence of bioactive molecules, the Hp-AuNPs effectively suppress the DPPH radicals. The antibacterial studies proved that Hp-AuNPs effectively destroy the Gram-positive microbe S. aureus and Lactobacillus. The Hp-AuNPs exhibited effective cytotoxic activity against A549 and LN-18 cells. The clonogenic and apoptosis assay revealed lung cancer cell proliferation arrest when treated with Hp-AuNPs. The findings indicate that red seaweed extract successfully acted as a reducing agent of AuNPs, which have natural antibacterial, antioxidant, and anticancer properties. This research demonstrated the H. pseudofloresii extract synthesized AuNPs might be used as a source for new therapeutic agents in the biomedical field, with the goal of preventing a wide variety of human diseases.
References
Clarance P, Luvankar B, Sales J, Khusro A, Agastian P, Tack JC, Al Khulaifi MM, Al-Shwaiman HA, Elgorban AM, Syed A, Kim HJ (2020) Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J Biol Sci 27(2):706–712. https://doi.org/10.1016/j.sjbs.2019.12.026
Viswanathan S, Palaniyandi T, Shanmugam R, Rajendran BK, Sivaji A (2022) Biomedical potential of silver nanoparticles capped with active ingredients of Hypnea valentiae, red algae species. Part Sci Technol 40(6):686–696. https://doi.org/10.1080/02726351.2021.1992059
Sandhiya V, Gomathy B, Sivasankaran MR, Thirunavukkarasu P, Mugip Rahaman A, Asha S (2021) Green synthesis of silver nanoparticles from Guava (Psidium guajava Linn) leaf for antibacterial, antioxidant and cytotoxic activity on HT-29 cells (Colon cancer). Ann Romanian Soc Cell Biol 25(6):20148–20163 (https://www.annalsofrscb.ro/index.php/journal/article/view/10001)
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R (2021) Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 20(2):101–124. https://doi.org/10.1038/s41573-020-0090-8
Hammami I, Alabdallah NM (2021) Gold nanoparticles: synthesis properties and applications. J King Saud Univ-Sci 33(7):101560. https://doi.org/10.1016/j.jksus.2021.101560
Babu B, Palanisamy S, Vinosha M, Anjali R, Kumar P, Pandi B, Tabarsa M, You S, Prabhu NM (2020) Bioengineered gold nanoparticles from marine seaweed Acanthophora spicifera for pharmaceutical uses: antioxidant, antibacterial, and anticancer activities. Bioprocess Biosyst Eng 43(12):2231–2242. https://doi.org/10.1007/s00449-020-02408-3
D’Acunto M, Cioni P, Gabellieri E, Presciuttini G (2021) Exploiting gold nanoparticles for diagnosis and cancer treatments. Nanotechnology 32(19):192001. https://doi.org/10.1088/1361-6528/abe1ed
Taha RH (2022) Green synthesis of silver and gold nanoparticles and their potential applications as therapeutics in cancer therapy; a review. Inorg Chem Commun 109610. https://doi.org/10.1166/jbn.2022.3420
Bade BC, Cruz CSD (2020) Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med 41(1):1–24. https://doi.org/10.1016/j.ccm.2019.10.001
Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, Mariotto AB, Lowy DR, Feuer EJ (2020) The effect of advances in lung-cancer treatment on population mortality. N Engl J Med 383(7):640–649. https://doi.org/10.1056/NEJMoa1916623
Pranav P, Palaniyandi T, Baskar G, Ravi M, Rajendran BK, Sivaji A, Ranganathan M (2022) Gene expressions and their significance in organoid cultures obtained from breast cancer patient-derived biopsies. Acta Histochem 124(5):151910. https://doi.org/10.1016/j.acthis.2022.151910
Aghebati-Maleki A, Dolati S, Ahmadi M, Baghbanzhadeh A, Asadi M, Fotouhi A, Yousefi M, Aghebati-Maleki L (2020) Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol 235(3):1962–1972. https://doi.org/10.1002/jcp.29126
Carrasco-Esteban E, Domínguez-Rullán JA, Barrionuevo-Castillo P, Pelari-Mici L, Leaman O, Sastre-Gallego S, López-Campos F (2021) Current role of nanoparticles in the treatment of lung cancer. J Clin Transl Res 7(2):140. https://doi.org/10.18053/jctres.07.202102.005
Mmola M, Roes-Hill ML, Durrell K, Bolton JJ, Sibuyi N, Meyer ME, Beukes DR, Antunes E (2016) Enhanced antimicrobial and anticancer activity of silver and gold nanoparticles synthesised using Sargassum incisifolium aqueous extracts. Molecules 21(12):1633. https://doi.org/10.3390/molecules21121633
Zhu X, Radovic-Moreno AF, Wu J, Langer R, Shi J (2014) Nanomedicine in the management of microbial infection–overview and perspectives. Nano Today 9(4):478–498. https://doi.org/10.1016/j.nantod.2014.06.003
Farzin A, Etesami SA, Quint J, Memic A, Tamayol A (2020) Magnetic nanoparticles in cancer therapy and diagnosis. Adv Healthcare Mater 9(9):1901058. https://doi.org/10.1002/adhm.201901058
Rónavári A, Igaz N, Adamecz DI, Szerencsés B, Molnar C, Kónya Z, Pfeiffer I, Kiricsi M (2021) Green silver and gold nanoparticles: Biological synthesis approaches and potentials for biomedical applications. Molecules 26(4):844. https://doi.org/10.3390/molecules26040844
Vemuri SK, Banala RR, Mukherjee S, Uppula P, Subbaiah GPVAVGR, Malarvilli T (2019) Novel biosynthesized gold nanoparticles as anti-cancer agents against breast cancer: Synthesis, biological evaluation, molecular modelling studies. Mat Sci Eng: C 99:417–429. https://doi.org/10.1016/j.msec.2019.01.123
El-Kassas HY, El-Sheekh MM (2014) Cytotoxic activity of biosynthesized gold nanoparticles with an extract of the red seaweed Corallina officinalis on the MCF-7 human breast cancer cell line. Asian Pac J Cancer Prev 15(10):4311–4317. https://doi.org/10.7314/APJCP.2014.15.10.4311
Chellapandian C, Ramkumar B, Puja P, Shanmuganathan R, Pugazhendhi A, Kumar P (2019) Gold nanoparticles using red seaweed Gracilaria verrucosa: green synthesis, characterization and biocompatibility studies. Process Biochem 80:58–63. https://doi.org/10.1016/j.procbio.2019.02.009
Algotiml R, Gab-Alla A, Seoudi R, Abulreesh HH, El-Readi MZ, Elbanna K (2022) Anticancer and antimicrobial activity of biosynthesized Red Sea marine algal silver nanoparticles. Sci Rep 12(1):1–18. https://doi.org/10.1038/s41598-022-06412-3
Vinosha M, Palanisamy S, Muthukrishnan R, Selvam S, Kannapiran E, You S, Prabhu NM (2019) Biogenic synthesis of gold nanoparticles from Halymenia dilatata for pharmaceutical applications: Antioxidant, anti-cancer and antibacterial activities. Process Biochem 85:219–229. https://doi.org/10.1016/j.procbio.2019.07.013
Manam D, Kiran V, Murugesan S (2013) Biogenic silver nanoparticles by Halymenia poryphyroides and its in vitro anti-diabetic efficacy. J Chem Pharm Res 5(12):1001–1008 (https://ssrn.com/abstract=3498917)
Viswanathan S, Palaniyandi T, Kannaki P, Shanmugam R, Baskar G, Rahaman AM, Paul LTD, Rajendran BK, Sivaji A (2022) Biogenic synthesis of gold nanoparticles using red seaweed Champia parvula and its anti-oxidant and anticarcinogenic activity on lung cancer. Part Sci Technol 1–9. https://doi.org/10.1080/02726351.2022.2074926
Sandhiya V, Thirunavukkarasu P, Gomathy B, Sridhar M, Rajeshkumar S, Ravi M, Asha S (2021) Agnp-Hp synthesized using red marine algae Halymenia pseudofloresii and its pharmacological activities. Ann Rom Soc Cell Biol 25(6):19433–50 (https://annalsofrscb.ro/index.php/journal/article/view/9758)
Palaniyandi T, Reddy RK, Natrajan S, Ranganathan M, Hari R, Sivaji A, Viswanathan S (2021) Anti-proliferative effect of Ceriopsdecandra derived fraction against Human gastric cancer on AGS cell line. Ann Rom Soc Cell Biol 25(6):20422–20448 (https://www.annalsofrscb.ro/index.php/journal/article/view/10098)
Jung J, Park SJ, Chung HK, Kang HW, Lee SW, Seo MH, Park HJ, Song SY, Jeong SY, Choi EK (2012) Polymeric nanoparticles containing taxanes enhance chemoradiotherapeutic efficacy in non-small cell lung cancer. Int J RadiatOncol* Biol* Phys 84(1):e77–e83. https://doi.org/10.1016/j.ijrobp.2012.02.030
Acharya D, Satapathy S, Somu P, Parida UK, Mishra G (2021) Apoptotic effect and anticancer activity of biosynthesized silver nanoparticles from marine algae Chaetomorpha linum extract against human colon cancer cell HCT-116. Biol Trace Elem Res 199(5):1812–1822. https://doi.org/10.1007/s12011-020-02304-7
Wang L, Xu J, Yan Y, Liu H, Li F (2019) Synthesis of gold nanoparticles from leaf Panax notoginseng and its anticancer activity in pancreatic cancer PANC-1 cell lines. Artif Cells Nanomed Biotechnol 47(1):1216–1223. https://doi.org/10.1080/21691401.2019.1593852
Fu Y, Xie D, Zhu Y, Zhang X, Yue H, Zhu K, Pi Z, Dai Y (2022) Anti-colorectal cancer effects of seaweed-derived bioactive compounds. Front Med 9:988507. https://doi.org/10.3389/fmed.2022.988507
Alegria EC, Ribeiro AP, Mendes M, Ferraria AM, Rego AMBD, Pombeiro AJ (2018) Effect of phenolic compounds on the synthesis of gold nanoparticles and its catalytic activity in the reduction of nitro compounds. Nanomaterials 8(5):320. https://doi.org/10.3390/nano8050320
Roseline TA, Murugan M, Sudhakar MP, Arunkumar K (2019) Nanopesticidal potential of silver nanocomposites synthesized from the aqueous extracts of red seaweeds. Environ Technol Innov 13:82–93. https://doi.org/10.1016/j.eti.2018.10.005
Francis PK, Sivadasan S, Avarachan A. and Gopinath A (2019) A novel green synthesis of gold nanoparticles using seaweed Lobophora variegata and its potential application in the reduction of nitrophenols. Part Sci Technol. https://doi.org/10.1080/02726351.2018.1547340
Torres SK, Campos VL, León CG, Rodríguez-Llamazares SM, Rojas SM, Gonzalez M, Smith C, Mondaca MA (2012) Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J Nanopart Res 14(11):1–9. https://doi.org/10.1007/s11051-012-1236-3
Palaniyandi T, Sivaji A, Thirugnanasambandam R, Durairaj P, Reddy RK, Vishwanathan S (2020) Isolation and identification of anti-oxidant fraction from active extract of Rhizophora mucronata Poir. leaves. Pharm Chem J 54(4):380–385
Martínez-Cabanas M, López-García M, Rodríguez-Barro P, Vilariño T, Lodeiro P, Herrero R, Barriada JL, Sastre de Vicente ME (2021) Antioxidant capacity assessment of plant extracts for green synthesis of nanoparticles. Nanomaterials 11(7):1679. https://doi.org/10.3390/nano11071679
Ruidas B, Som Chaudhury S, Pal K, Sarkar PK, Das Mukhopadhyay C (2019) A novel herbometallic nanodrug has the potential for antibacterial and anticancer activity through oxidative damage. Nanomedicine 14(9):1173–1189. https://doi.org/10.2217/nnm-2018-0187
Krishnamoorthi R, Bharathakumar S, Malaikozhundan B, Mahalingam PU (2021) Mycofabrication of gold nanoparticles: optimization, characterization, stabilization and evaluation of its antimicrobial potential on selected human pathogens. Biocatalysis Agric Biotechnol 35:102107. https://doi.org/10.1016/j.bcab.2021.102107
Singh RP, Kumari P, Reddy CRK (2015) Antimicrobial compounds from seaweeds-associated bacteria and fungi. Appl Microbiol Biotechnol 99(4):1571–1586. https://doi.org/10.1007/s00253-014-6334-y
Mahmood H, Hussain SB, Nosheen A, Mahmood T, Shafique M, Ul-Haq N, Haq AU (2021) Antibacterial activities of gold nanoparticles synthesized by citrus limonum fruit extract. Pak J Bot 53(6):2305–2310. https://doi.org/10.30848/PJB2021-6(36
Sathiyaraj S, Suriyakala G, Gandhi AD, Babujanarthanam R, Almaary KS, Chen TW, Kaviyarasu K (2021) Biosynthesis, characterization, and antibacterial activity of gold nanoparticles. J Infect Public Health 14(12):1842–1847. https://doi.org/10.1016/j.jiph.2021.10.007
Tao C (2018) Antimicrobial activity and toxicity of gold nanoparticles: research progress, challenges and prospects. Lett Appl Microbiol 67(6):537–543. https://doi.org/10.1111/lam.13082
Maccora D, Dini V, Battocchio C, Fratoddi I, Cartoni A, Rotili D, Castagnola M, Faccini R, Bruno I, Scotognella T, Giordano A (2019) Gold nanoparticles and nanorods in nuclear medicine: a mini review. Appl Sci 9(16):3232. https://doi.org/10.3390/app9163232
Biresaw SS, Damte SM, Taneja P (2020) Green synthesized silver nanoparticles: a promising anticancer agent. Int J Nanosci 19(04):1950027. https://doi.org/10.1142/S0219581X19500273
Vijayakumar S (2019) Eco-friendly synthesis of gold nanoparticles using fruit extracts and in vitro anticancer studies. J Saudi Chem Soc 23(6):753–761. https://doi.org/10.1016/j.jscs.2018.12.002
Zheng Y, Zhang J, Zhang R, Luo Z, Wang C, Shi S (2019) Gold nano particles synthesized from Magnolia officinalis and anticancer activity in A549 lung cancer cells. Artif Cells Nanomedand Biotechnol 47(1):3101–3109. https://doi.org/10.1080/21691401.2019.1645152
Castro-Aceituno V, Abbai R, Moon SS, Ahn S, Mathiyalagan R, Kim YJ, Kim YJ, Yang DC (2017) Pleuropterus multiflorus (Hasuo) mediated straightforward eco-friendly synthesis of silver, gold nanoparticles and evaluation of their anti-cancer activity on A549 lung cancer cell line. Biomed Pharmacother 93:995–1003. https://doi.org/10.1016/j.biopha.2017.07.040
Pitchai P, Subramani P, Selvarajan R, Sankar R, Vilwanathan R, Sibanda T (2022) Green synthesis of gold nanoparticles (AuNPs) using Caulerpa racemosa and evaluation of its antibacterial and cytotoxic activity against human lung cancer cell line. Arab J Basic Appl Sci 29(1):351–362. https://doi.org/10.1080/25765299.2022.2127510
Sypniewski D, Szkaradek N, Loch T, Waszkielewicz AM, Gunia-Krzyżak A, Matczyńska D, Sołtysik D, Marona H, Bednarek I (2018) Contribution of reactive oxygen species to the anticancer activity of aminoalkanol derivatives of xanthone. Invest New Drugs 36(3):355–369. https://doi.org/10.1007/s10637-017-0537-x
Acknowledgements
We gratefully acknowledge Er. A. C. S. Arun Kumar, President, Dr. M.G.R Educational and Research Institute University, for providing the necessary facilities, and we acknowledge the Nanotechnology Research Centre (NRC), SRMIST, for providing an instrumentation facility for nanoparticle characterization.
Author information
Authors and Affiliations
Contributions
Conceptualization: Thirunavukkarasu Palaniyandi; methodology: Sandhiya Viswanathan, Gomathy Baskar, Mugip Rahaman Abdul Wahab; formal analysis and investigation: HemaPreethi Surendran, Asha Sivaji; writing — original draft preparation: Pranav Prabhakaran, Sandhiya Viswanathan; writing — review and editing: Thirunavukkarasu Palaniyandi, Saravanan Kumarasamy; supervision: Maddaly Ravi, Barani Kumar Rajendra, Meivelu Moovendhan.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Palaniyandi, T., Viswanathan, S., Prabhakaran, P. et al. Green synthesis of gold nanoparticles using Halymenia pseudofloresii extracts and their antioxidant, antimicrobial, and anti-cancer activities. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03873-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03873-y