Abstract
To establish economically viable production of cyanobacterial bioethanol and commercially valuable bioproducts, mass cultivation is required; however, the exorbitant cost of nutrients is a significant impediment. Currently, potatoes are used extensively in food-processing industries worldwide. These industries produce large quantities of potato peel wastes (PPW), often discarded in the exposed environment, causing ecological problems. As a supplement in the growth medium for cultivation of the marine Synechococcus elongatus BDU 10144, PPW was explored as an inexpensive source of nutrients. Different concentrations of PPW were added to the novel seawater-based medium as a mixotrophic nutritional supplement after physical pretreatment, and biomass and carbohydrate yields were examined for test cyanobacterium. At alkaline pH, PPW supplementation was found to be a promising stimulant for growth and total carbohydrate accumulation, with a marked decline in the cultivation period, doubling carbohydrate synthesis, and significantly increasing bioethanol and co-products production. This study thus demonstrated that 10% PPW supplementation augmented the carbohydrate pool by ~ 2.2-fold and bioethanol yield by ~ 2.3-fold with bioethanol conversion by > 40%. Moreover, the test cyanobacterium was recognized as a prolific producer of high-valued exopolysaccharides (EPS) and mycosporine-like amino acids (MAAs), which were validated using analytical methods like ultraviolet-spectroscopy and high-performance liquid chromatography. Conclusively, PPW could be exploited as a cost-effective, natural, and green nutrient supplement for cyanobacterial mass cultivation, which could aid in devising a sequential strategy for the integrated production of MAAs, EPS, and bioethanol under the “waste-to-wealth” approach.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Global warming, as a result of our long-standing reliance on fossil fuels, has become one of the most pressing problems facing human civilization. As conventional fuel depletes day-to-day and fuel costs continue to rise at an alarming rate, humanity is concerned about the need to develop sustainable alternatives to traditional fuels [1]. Biofuel necessity is expected to increase as a consequence of environmental concerns, population growth, and the necessity to diversify the world’s energy supply. Because the cost of biofuel raw materials has become exorbitant, their worldwide acceptance, universal accessibility, and affordability have all been significantly lowered [2].
Bioethanol may be a potential biofuel and can be produced from any substrate containing starch or sugar. Regarding volume and market value, bioethanol is the most significant biofuel in today’s economy, accounting for 65% of worldwide biofuel generation [3]. Research had previously concentrated on first- and second-generation bioethanol, which employed sugar or starchy feedstocks and agro-wastes by-products as the primary substrate for their production. Cyanobacterial bioethanol can potentially advance the green energy economy significantly [4]. Extensive research is being conducted to expand cyanobacterial bioethanol to a dynamic industrial process, which is still intricate [5]. Scaling up and commercializing cyanobacterial or microalgal bioproducts has been investigated in recent years, but more prudent and step-by-step advancement is required to make it a sustainable economy.
Cyanobacteria are the most fundamental photosynthetic organisms and have phenomenal potential not only for the generation of bioenergy but also for the production of high-valued nutraceuticals, cosmeceuticals, and pharmaceutical products [6, 7]. Cyanobacteria have a high lipid content, are open to metabolic engineering, and include value-added components such as antioxidants [8], exopolysaccharides (EPS) [9], phycocyanin [10], and ultraviolet (UV) protectants [11], making them a prospective feedstock for biorefinery. The domain of biorefinery might flourish ecologically and cost-effectively due to technological improvement. Even though cyanobacteria are highly pertinent for biorefining due to the compositional diversity of their biomass, bioproducts’ recovery from cyanobacteria remains a challenge. Therefore, it is necessary to investigate moderate and sequential extraction methods that retain the value of diverse cell components [12].
Cyanobacteria are microbial factories that are pivotal for the global food chain. Biomass obtained from cyanobacteria is a feasible alternative for the generation of biofuels and has attracted more attention than terrestrial plants because of its exceptional capacity to sequester CO2, rapid growth rate, robust photosynthetic effectiveness, and high sugar content. The food, cosmetic, and pharmaceutical sectors also benefit from the valuable by-products produced by cyanobacteria [13] like pigments, carotenoids, polysaccharides, and UV protectant compounds like scytonemin and mycosporine-like amino acids (MAAs).
MAAs are the most prevalent secondary metabolites and have a maximum absorbance between 310 and 362 nm, shielding cells from damaging UV radiations. Moreover, chemical sunblocks have been proven harmful over the coming decades; researchers are focusing on seeking novel, natural UV-screening molecules derived from plants or microbes. Photosynthetic marine species, such as algae and cyanobacteria, have evolved to produce MAAs to withstand UV radiation better. These are promising alternatives for chemical sunscreens containing present-day sunblock ingredients [11, 13,14,15]. Similarly, another defining characteristic of marine cyanobacterial strains is their ability to generate EPS in response to adverse environmental circumstances. Capsular polysaccharides (CPS) are those sheaths, slimes, or capsules that remain attached to the cell surface, while EPS released by cells to their surrounding environment are known as released polysaccharides (RPS). The amount of RPS and CPS largely depends on the microorganism that produced it and the conditions under which it was cultivated. Cyanobacteria have become more popular as providers of polymeric sugars since these biopolymers commonly have benefits over the polysaccharides currently in use. Compared to polysaccharides derived from plants and Mediterranean macroalgae, EPS of microbial origin introduces promising functionality, reliable physicochemical features, and wide availability. In general, polysaccharides are biotechnologically significant polymeric compounds that may be used to make a broad range of valuable products [4, 16].
Despite this, there are many barriers to large-scale cultivation of cyanobacteria for the production of valuable products, and the high cost of cultivation medium is one of them [17]. As a result, investigations were conducted to explore alternatives to costly nutrient media [17, 18]. The availability of nutrients and water for large-scale production is a critical hindrance, and commercial production appears to be more economically unfeasible [19]. In this aspect, establishing low-cost integrated growth systems tailored for cyanobacteria may substantially improve the development of a biorefinery.
According to the Slade and Bauen assessment [20], if nutrients and water could be acquired at an economical cost, a considerable decrease in the cost of cultivation may be accomplished. One such strategy is to utilize industrial wastewater or agro-wastes as a freshwater and nutrient substitute for microalgal/cyanobacterial cultivation. These food and agro-wastes have been revealed to be rich sources of nutrients and carbohydrates, and as such, they have the potential to be utilized as a culture medium for algal/cyanobacterial cultivation. Investigators suggested that the use of organic biomass feedstock from wastes instead of an expensive analytical grade nutrient medium has the potential to reduce the cost of algal/cyanobacterial biomass production while simultaneously reducing the amount of agricultural and food wastes generated day-to-day [21, 22].
Potato peel waste (PPW) is one such untapped source of inexpensive nutrients for cyanobacterial growth medium. The potato-processing market results in the production of a massive amount of PPW. The management of PPW is a critical concern for the potatoes processing sector, highlighting the need to develop an integrated strategy that is environmentally sustainable. PPW, a zero-value waste, is rich in minerals and other hydrocarbons in the form of sugars, protein, and lipids. As a result, it would be preferable if there was the possibility of designing a cyanobacteria growth medium utilizing discarded PPW [23, 24]. In this regard, more targeted research is necessary to uncover further pathways for using PPW. The most cost-effective technique for allowing large populations of cyanobacteria to flourish is a “microalga factory with seawater,” which may be described as a simple influx of seawater into an existing algal production system [25]. Also, the need for low-cost carbon sources is of paramount significance. Experiments by Hwang et al. [26] reported that food wastes such as papaya and mango peels might be utilized to cultivate Anabaena cylindrica.
Based on these considerations, this study proposes an ecologically sustainable paradigm for maximizing the potential of biomass obtained from the marine cyanobacterial culture to produce multiple high-value products in a single cultivation cycle.
The explicit objectives of this study are:
-
(i)
To investigate discarded potato peel wastes (PPW) as a low-cost exogenous carbon and nutrient source for improving the biomass and carbohydrate accumulation in the marine cyanobacterium Synechococcus elongatus BDU 10144 cultures in order to provide a new vision for the use of PPW in cost-effective cyanobacterial cultivation, and
-
(ii)
to evaluate the potential of the test cyanobacterium for the production of bioethanol vis-à-vis commercially important co-products, viz. exopolysaccharides (EPS) and mycosporine-like amino acids (MAAs) under a sustainable cyanobacterial biorefinery perspective.
2 Materials and methods
2.1 Cyanobacterial strain and growth assessment
The test cyanobacterium chosen for this study was marine Synechococcus elongatus BDU 10144 (hereinafter referred to as S. elongatus) obtained from National Facility for Marine Cyanobacteria (NFMC), Bharathidasan University (BDU), Tiruchirappalli, India, and seed cultures were maintained in standard ASN-III medium [27] as control under laboratory culture conditions. The cyanobacterial cells were incubated under white fluorescent light illumination at 50 µmol photons m−2 s−1 (light intensity) at 25 °C and pH 7.1. To prevent cell adhesion and congregation, the culture flasks were gently shaken every day.
2.2 Potato peel wastes collection and preparation of extracts
PPW was collected from campus dining halls and nearby food shops at the India Institute of Technology, Kharagpur, India. The PPW was then dried in a hot-air oven (60 °C) and pulverized separately to obtain peel powder. The PPW samples were ground into a fine powder with a diameter of less than 1.0 mm using an electric mill and kept in a DURAN® laboratory bottle (250 mL) at 4 °C until assessed.
For this study, a 10.0-g PPW powder sample was suspended in 100 mL of optimized fertilizer-seawater medium (FSW medium) in a 250-mL Erlenmeyer flask and incubated for 24 h at 200 rpm in a shaking incubator at room temperature. The FSW medium is a newly formulated inexpensive fertilizer-seawater-based medium previously optimized in our laboratory using response surface methodology (RSM). The detailed composition of the optimized FSW medium is mentioned in Chandra and Mallick [28]. The combination of FSW and PPW was examined within the scope of this investigation.
Next, as a physical treatment procedure, ultrasonication-assisted extraction for release of essential nutrients was conducted by Sonics, Vibra-cell ultrasonic processor, with sound abating chamber (80 amplitude, 10-s pulse, 10 min, 25 °C, 20 Hz, 700 W) [29]. After extraction, the blended residues were subjected to a sieve filtration and then centrifuged at 5000 g for 15 min to remove the small remaining solid residues. Before cultivation, the collected supernatant containing nutrient extracts was filtered with a 0.45-µm syringe-membrane filter. The supernatants hereinafter “PPW extracts” were collected in 250-mL borosilicate glass bottles, and the PPW residues obtained after filtration was collected and dried separately. The PPW extracts were made fresh for each experiment. To minimize nutritional breakdown, the bottles were covered with aluminum foil and preserved in the refrigerator at 4 °C until used. Figure 1a–d shows the different stages of preparation of PPW extracts.
To comprehend the suitability of PPW extracts as a potential exogenous nutrient source, several physicochemical parameters were studied. The dry matter content of discarded raw peels was evaluated by drying samples to a constant weight at 105 °C in an oven. The temperature was kept constant throughout the drying process. The variations in structural changes and nutrient availability before and after treatment were studied using scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR) analyses (see Section 2.4).
2.3 Cyanobacterial cultivation with potato peel waste extracts
To see the effects of PPW extracts as an exogenous low-cost nutritional supplement, the cultures were inoculated in 100 mL of FSW medium with varied PPW concentrations (1.0%, 2.5%, 5.0%, 10.0%, and 20.0% (v/v)). Next, the medium was autoclaved for sterilization. An equal volume of cyanobacterial cultures was used as initial inoculums for conducting experiments. Other cultivation conditions were the same as the pre-culture condition described above. The cultures were manually shaken three to four times a day to ensure that the cells were evenly distributed and to prevent them from settling at the bottom. For comparing the performance of PPW-supplemented cultures, the ASN-III medium was used as the control.
In the subsequent experiments, the ideal concentration of PPW in the FSW medium was selected and henceforth referred to as “PPW-FSW” (Potato Peel Wastes- Fertilizer Seawater) medium, and the pH optimization study at six different pH levels: 6, 7, 8, 9, 10, and 11 were also conducted. To attain the required pH for the investigation, 1 N NaOH and HCL were used. The biomass content was assessed by Rai et al. [30], and the specific growth rate was calculated by the method given by Malakar et al. [31].
2.4 Biochemical analysis and characterization studies
Total carbohydrate contents were estimated by a phenol–sulfuric acid methodology given by Dubois et al. [32]. Using the following equation, the w/w value reflecting cellular content was represented as % dcw (dry cell weight) [10]:
Protein estimation was done using the protocol of Bradford [33]. The ash content of the PPW samples was calculated by the method of Koley et al. [34]. According to the protocols of Hodge and Hofreiter [35] and Miller [36], the starch and reducing sugars were measured, while the cellulose [37] and hemicellulose [38] yield were calculated using the detailed procedure outlined in Chandra et al. [39]. The glycogen was estimated according to the method of Seifter et al. [40] and Deb et al. [10]. The pH of the samples was estimated using a pH meter (Van London Co). Lysine estimation was done according to the method of Qadir et al. [41] using Agilent 1200 (Column – Agilent Zorbax, 4.6 × 150 mm, 5 µm, 100A), methanol as mobile phase (10/90%) phosphate buffer (Na2HPO4), pH 6.0, 25 mM, and a flow rate of 1.0 mL/min with UV detection at 210 nm [42].
The morphological characteristics and ultrastructural changes of the studied samples were analyzed by microscopic examination using a scanning electron microscope (SEM) (Zeiss EVO 60) and a field emission scanning electron microscope (FE-SEM) (Zeiss, Supra 40) at different magnifications. In addition, SEM combined with energy-dispersive X-ray, commonly known as SEM–EDX, was used to investigate the elemental quantification of samples. NICOLET 6700, Thermo Fisher Scientific, was used to measure the Fourier transform infrared radiation (FTIR) spectra in a range of 400–4000 cm−1 at the Central Research Facility (CRF) India Institute of Technology (IIT), Kharagpur. At a ratio of 1:100, tested samples were compressed into potassium bromide (KBr) pellets, and FTIR spectra were taken.
2.5 Extraction of value-added bioproducts from the marine cyanobacterium
2.5.1 Exopolysaccharides from waste supernatant
After the cultures reached the stationary phase, the cultures were centrifuged, and the waste supernatant was collected separately after harvesting the biomass. EPS was extracted using acetone as the precipitating solvent. The precipitated EPS was collected by centrifugation and was dialyzed against Milli-Q water using dialysis membrane-70 (17.5 mm, Hi-media) for 24 h and stored at 4 °C for further analysis. The detailed protocol for extraction is mentioned in Parikh and Madamwar [43]. Characterization studies of freeze-dried EPS samples were conducted using FTIR [43], FE-SEM, and SEM–EDX analysis.
2.5.2 Extraction of mycosporine-like amino acids from methanolic extracts
MAAs were extracted following the protocol of Pathak et al. [44] with slight modifications. The production of MAAs entails three stages: first, the extraction of the MAAs; next, the separation of the MAAs; and lastly, the quantification of the MAAs.
The harvested marine cyanobacterial biomass was freeze-dried with a laboratory freeze dryer (Instrumentation India, Kolkata, India). HPLC-grade methanol was used as extracting solvent and added to the known amount of biomass. The initial extraction phase included homogenizing 1.0 g of cyanobacterial biomass with methanol (5 mL) for a few minutes using a ceramic mortar and pestle before incubating it overnight at 4 °C. Following the extraction step, aliquots were centrifuged (8000 g, 5 min) in clean Eppendorf tubes, and the supernatants were transferred to the fresh vials and evaporated at 45 °C. Residues collected at the bottom were redissolved in 1 mL of sterile double-distilled water, then 100 µL of chloroform was added with moderate vortexing. The pigment-free topmost water layer was carefully taken into clean Eppendorf tubes. After centrifugation, the water extract was filtered using a microcentrifuge syringe-driven filter (13 mm, PVDF, 0.25 m, Moxcare) to obtain partially pure MAAs (8000 g, 5 min). For estimation of yield, the partially purified water extracts collected were transferred to a pre-weighed glass beaker (100 mL) (M1) and kept in an oven at 40 °C till a consistent weight was attained (M2).
The yield and content of partially purified MAA extracts were estimated using the below formulae:
where M1 is the weight of an empty beaker (g), M2 is the weight of a beaker with dried MAA extracts (g), and B is the initial biomass used to extract MAAs (g).
Primarily, for confirmatory studies, UV absorption was used to validate the presence of UV protectants in the aqueous phase [11]. A spectroscopic examination was carried out between the wavelengths of 200 and 700 nm (UV–Vis spectrophotometer, Perkin Elmer, Shelton, USA).
MAA extracts were examined using FTIR spectroscopy to characterize functional groups in the tested samples in a transmittance mode ranging from 400 to 4000 cm−1. A high-performance liquid chromatography (HPLC) system (Agilent 1260, Infinity model with Photodiode Array detector) with a Zorbax SB C18 column (5 m packing; 150 × 4 mm) was used to evaluate partially purified MAAs. A total of 330 nm was used as the detecting wavelength. MAAs were analyzed by injecting 30 µL of sample onto an HPLC column with 0.02% acetic acid (v/v) as mobile phase in Milli-Q water at a flow rate of 1.0 mL/min. Eluted samples were collected using the fraction collector. High-resolution mass spectroscopy (HRMS) spectra were recorded using an Agilent spectrometer 6200 Series. HRMS was recorded on ESI-TOF (electrospray ionization time-of-flight). By comparing the retention periods and absorption spectra to literature, the MAAs were identified [11, 45]. Later on, in the process, the residual cyanobacterial biomass pellets that were collected after the methanolic extraction step were utilized for the production of bioethanol.
2.5.3 Production of bioethanol
Following methanol extraction, collected residual biomass was pretreated with dilute H2SO4 (2 N) to increase the availability of fermentable sugars for fermentation by yeast. Using the process outlined by Deb et al. [10], bioethanol was produced. Under a biorefinery strategy, the integrated approach of producing bioethanol and economically valuable co-products from marine cyanobacterium S. elongatus cultures is shown in Fig. 2.
2.6 Statistical analysis
All tests were conducted in three sets, with each experiment repeated twice to assure reproducibility. Duncan’s new multiple range tests (DNMRT) using MSTAT-C software were used to analyze the statistical data (Plant and Soil Sciences Division, Michigan State University, USA). The data for the three experiments were stated as the mean ± standard deviation. Origin Pro 2022 software was employed to construct graphical illustrations.
3 Results and discussion
3.1 Compositional and morphological examination of potato peel wastes
The investigation initiated with the characterization of PPW in order to gain an understanding of its potential use as an exogenous nutritional source for the cultivation of cyanobacteria. To fully comprehend PPW physicochemical features, it is crucial to examine both the morphological changes and chemical composition of PPW in detail. The investigation of these characteristics would contribute to the formulation of an eco-friendly method for valorizing PPW sustainably and utilizing its full potential.
The PPW extracts were prepared according to Section 2.2. To serve as a low-cost carbon source, PPW must undergo preliminary processing to boost its concentration of readily available nutrients. As shown in Table 1, PPW contained abundant nutrients, including protein, amino acids, and sugars. The proximate analysis of PPW revealed that it was remarkably high in carbohydrates, particularly starch, with a concentration of approximately 50% and 30%, respectively. Although PPW was found to be enriched in starch, it contains only a small amount of fermentable reducing sugar (~ 0.7% dry cell weight (dcw)) in untreated PPW samples (Table 1). The pH of the PPW extracts was recorded to be 5.67 ± 0.2.
Supplementary Figs. S1a and b depict a comparative analysis of changes in the FTIR spectra of PPW before and after ultrasonication-assisted physical treatment. The distinct vibrational modes of the C–C group, 1030 cm−1 for the band of the 1, 4-glycosidic groups, have been identified in both samples, but the bands are sharper in the treated samples. In addition, there are bands at 1630, 2919, and 3424 cm−1 that corresponds to the bond bending stretching vibration of CH2 and C-O–O group, bending vibration of hydroxyl group due to adsorption of water, starching vibration of CH2 group, and hydroxyl groups, respectively. The observed bands are more intense in the treated samples than in untreated samples. Also, the FTIR spectra of pretreated PPW showed a distinct absorption band located at 1321 cm−1 and 1438 cm−1, which is characterized as aliphatic C–H stretching in CH3 [46]. Finally, the C–O–C vibration of the pyranose sugar group was allocated to a strong band between 1200 and 900 cm−1 (carbohydrates). The bands at 1249 cm−1 and 1157 cm−1 are intense only in treated PPW samples (Supplementary Figs. S1a–b).
Furthermore, in the pursuit of key nutrients necessary for cyanobacterial culture, lysine is one such crucial amino acid that has been acknowledged in previous research to contribute favorably to the development of algal cells. Lysine is widely recognized for its function in carbon fixation and activating the Ribulose 1,5-bisphosphate carboxylase/oxygenase (RUBISCO) enzyme [47]. Accordingly, the PPW samples were also analyzed for the presence of lysine using HPLC (Supplementary Fig. S2), and it was discovered that lysine was available in a considerable amount. The earlier investigation also stated the vital role of lysine in carbon fixation and activation of the RUBISCO enzyme under optimal conditions [39]. Hence, under biochemical assessments, it was determined that PPW extracts could be explored as a supplement for the mixotrophic culture of cyanobacterium; nonetheless, further research is required since no study on the influences of PPW on marine cyanobacteria has been conducted hitherto.
Moreover, morphological and structural analyses of untreated and ultrasonicated PPW samples were carried out using an SEM and an FE-SEM at various magnifications. SEM analysis illustrates the abundance of starch in the examined dry PPW samples. Figure 3a–d exhibits the SEM micrograph of the surface morphology of PPW in powder form before and after treatment at varied magnification and scale bar. Figure 3a shows the SEM image at × 500 and 20 µm, whereas Fig. 3b shows the image at × 500 and 30 µm. The two micrographs reveal a richness of intact starch cells, while Fig. 3c demonstrates the disintegration of the intact cellular structures of PPW after pretreatment at × 500, 30 µm. Figure 3d shows a magnified image of the microscopic structure changes occurred after treatments at × 2000 and 10 µm. FE-SEM (low magnification, × 698, 20 µm) micrographs of the PPW powder in Fig. 3e show the typical spherical starch granule morphology, and the high-magnification picture (× 7680, 2 µm) in Fig. 3f displays that the starch granules were spread in groups that create the conventional spherical shape and helps to observe the distinct structure of starch, indicating that PPW is rich in starch (Fig. 3e–f) [48]. SEM–EDX analysis also revealed that PPW contains essential elements like C (62.08%), O (34.30%), Na (0.37%), Si (1.24%), S (1.26%), Cl (0.22%), and K (0.53%) in its composition (Supplementary Fig. S3).
SEM imaging of powdered potato peel wastes (PPW) at different magnification and scale bars. Images before treatment at (a) × 500, 20 µm, and (b) × 500, 30 µm; and images after treatment (c) × 500, 30 µm, and (d) × 2000, 10 µm; (e) The FE-SEM micrograph of PPW shows the presence of starch granules (× 698, 2 µm) and (f) an enlarged image of starch granule spotted in PPW (× 7680, 2 µm). Abbreviations: SEM, scanning electron microscopy; FE-SEM, field emission scanning electron microscopy
The characterization studies unveil that physical pretreatment or hydrolysis methods may be used to transform the features of food wastes to maximize the amount of sugars and other essential minerals released. Thus, after some pretreatment, food/peel wastes could serve as an efficient growth substrate for potential cyanobacterial biorefinery factories [49].
The objective of environmentally sustainable and “green” extraction of the metabolites may be advanced significantly with the use of physical processing measures like milling, sonication, or boiling to disrupt the cells, which could result in a substantial release of sugar and other natural metabolites into the surrounding milieu. The use of ultrasonication provides great reproducibility and, owing to active cooling, can minimize the degradation of thermolabile compounds [29, 50]. This impact is undoubtedly attributable to the decrease in particle size resulting from the application of ultrasound or high pressure. Moreover, physical pretreatment has several benefits over standard chemical and enzymatic methods. The key advantages include reduced extraction and processing time, energy, solvent use, and CO2 emissions. Ultrasound pretreatment could undoubtedly increase the hydrolysis yields from lignocellulosic biomass and the enhancement correlated with decreased lignin content and improved biomass accessibility [51, 52]. The increase in surface area of the solid that occurs as a direct result of the decrease in particle size brought on by the action of ultrasound is directly responsible for the greater mass transfer rate as well as the enhanced extraction rate and yield.
3.2 Effect of varying PPW supplementation on growth and total carbohydrate of S. elongatus
The production of cyanobacteria requires several critical elements, including carbon, which may be found in copious quantities in PPW. This led to investigating the impact of mixotrophy using PPW extracts as a potential nutrient supplement for sustainable cyanobacterial cultivation.
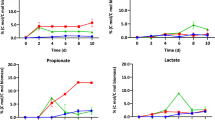
Herein, it was observed that the supplementation of PPW extracts positively influenced cyanobacterial growth and total carbohydrate content. The findings revealed that a range of 5.0–10.0% PPW significantly raised both the biomass yield and total carbohydrate contents of the test cyanobacterium S. elongatus, which were approximately 1.2-fold and 1.5-fold higher than the control, respectively, after 15 days of the incubation period (Fig. 4a–b). The maximum biomass and carbohydrate yield levels were recorded after 15 days of incubation with cultures supplemented with 10% PPW. These values were respectively 1.31 g/L and 508.4 mg/L (Fig. 4a–b). A 1.84-fold rise in biomass productivity was also noticed in comparison with control. Also, the richness of PPW as an exogenous carbon source led to a ~ 15% increase in carbohydrate productivities (Table 2). Also, a declining trend in the cultivation period (from 24 to 15 days) was noted, which ultimately helped achieve higher biomass productivity (Fig. 4a–b).
Moreover, in the current investigation, it was also discovered that at PPW extract (20%) concentration, the test cyanobacterium exhibited a decrease in biomass yield. The plausible justification for this response could be that the organism’s growth was hampered by the high PPW extract concentration. Previous research has shown that high concentrations of waste have a deleterious influence on certain microalgal development. Concentrated waste media are often dark in appearance, limiting light penetration. As a consequence, phototrophic or mixotrophic microalgae, which need light to perform their metabolic activities and function, may decrease their cellular metabolism and proliferation [53, 54].
This finding is consistent with prior studies, indicating that PPW can be used as a potential carbon source for mixotrophic cultivation for marine cyanobacteria in a seawater-based medium [18, 31]. A recent report by Malakar et al. [31] suggests that agricultural wastes like sweet lime and potato peels hydrolysate obtained after acid pretreatment may be utilized as efficient, lucrative, ecologically friendly, and cheap sources for the development of the microalgae isolates. They reported that for Chlorella sorokiniana KMBM K (a green alga), when grown in potato peel hydrolysate, the maximum biomass yield of 2.1 g/L was achieved, and the maximum lipid productivity was observed at 49.93 mg/L/d at a 25% concentration. Similarly, food waste combined with wastewater or food waste alone, such as molasses hydrolysate, may enhance the development of microalgae, according to Yan et al. [55] and Pleissner et al. [56]. Furthermore, it has been shown that date palm waste-enriched media were used to cultivate Chlorella pyrenoidosa and that biomass was found to be improved [57]. Following this, our findings manifestly show that low-value wastes like PPW, both environmentally beneficial and cost-effective carbon sources, should be examined as growth propellers to make biofuels economically feasible.
Additionally, recovering essential fertilizer components from biological waste is a potential approach. Better waste management may permit the recovery of valuable substances from biodegradable waste. According to the circular economy notion, nutrients should be recycled. PPW has been effectively used in the production of biofertilizers. As a result, the residues recovered following nutrient extraction have the potential to be utilized further as prospective feedstocks for biofertilizers or animal feed [58].
Since the optimal pH for each species of cyanobacteria is different, it was necessary to conduct research on the growth and carbohydrate accretion of S. elongatus under different initial pH levels of the culture medium (Fig. 4c). Changes in the initial pH of the culture and nutrient supplements in the optimized FSW medium not only increased the cellular carbohydrate content (%dcw) but also led to a significant increase in its amount (mg/L) by improving the growth of the test cyanobacterium. A biomass yield of 1.87 g/L was achieved at pH 9.0 compared to 1.14 g/L at a pH of control (pH 7.1) (Fig. 4c). Further increasing the initial pH of the medium to 10.0 resulted in a 35% decrease in carbohydrate content, demonstrating that pH 9 is the most conducive of all. The total carbohydrate production was maximal at this pH on the 15th day of the cultivation period (742.1 mg/L), which was substantially higher than the value recorded on the 24th day in control (334 mg/L). Increasing the initial pH to 10 had a detrimental effect, resulting in a decreased biomass and carbohydrate yield of 1.53 g/L and 533.1 mg/L, respectively. The highest carbohydrate accretion (40%) was attained with the initial pH set to 9, compared to 29% under the control treatment (Fig. 4c). Conclusively, in a biomass and carbohydrate production comparison, marine cyanobacteria grown in PPW medium under mixotrophic conditions produced twofold more biomass and 2.5-fold more carbohydrates than those grown in standard ASN-III medium. From here on, the optimized medium containing 10%-PPW in the FSW medium at pH 9 would be referred to as the “PPW-FSW” (Potato Peel Wastes-Fertilizer Seawater) medium.
When carbohydrate component analysis was performed in the PPW-FSW medium, the maximum yields of reducing sugars, starch, glycogen, cellulose, and hemicellulose accumulation were documented to be (in mg/L) 289.6, 116.4, 81.7, 52.0, and 66.8, respectively, whereas the yields for control were (in mg/L) 110.2, 43.4, 26.7, 110.2, and 16.7, respectively. Interestingly, the yield of reducing sugars was found to be 2.6-fold greater in the PPW-FSW medium than in the control. In addition, the S. elongatus cyanobacterium was determined to contain a high percentage of reducing sugars compared to much lower amounts of hemicellulose and cellulose in the test cyanobacterium, ensuring the availability of simple fermentable sugars for bioethanol production.
Biofuel production would be more expensive and more difficult to commercialize if mixotrophic culture is not practicable due to high organic carbon costs [59]. In this regard, food wastes have not been extensively explored for cyanobacterial cultivation. However, it is well known that the inclusion of inorganic and organic carbon sources in the growth medium has a substantial impact on algal/cyanobacterial biomass production, which affects the biochemical characteristics of cyanobacteria [9, 60]. Table 3 presents a comparative account of food waste used in the cultivation of algae species as low-cost feedstocks reported in previous studies. Not many exclusive studies have been conducted on marine cyanobacteria using food waste as a nutrient source [61,62,63,64].
To maximize biomass output, potato peel wastewater-based mixotrophic cultivation is a promising technique to integrate low-cost carbon and other vital nutrients into the culture medium. The inherent carbohydrate content of cyanobacteria prompted researchers to focus on using cyanobacteria as a feedstock for the production of bioethanol. As the amount of sugar in cyanobacterial biomass is indispensable for bioethanol production, a comprehensive study is needed in this area to curve a strategy to enhance carbohydrate accumulation. Cyanobacterial polysaccharides are usually in the form of reducing sugars, starch, or glycogen. Also, cyanobacterial-derived biomass has gained worldwide attention due to the lack of lignin as a cellular component. Bioethanol production is most effective when using reducing sugars as a fermentable substrate. When fermented with the help of organisms like yeast, they may be efficiently transformed into bioethanol. Since glucose is a readily fermentable reducing sugar, the fermentation process may go considerably quicker due to the yeast’s capacity to break it down. The correlation between the increase in photosynthetic activity of the test cyanobacterium and its enhanced growth and carbohydrate accumulation with increasing initial pH is plausible. This observation might be attributed to the fact that the enzymes involved in the CO2 assimilation pathway are more effective at alkaline pH. For instance, when the environment’s pH is alkaline, carbamoyllysine, a decisive step in activating RUBISCO, happens at an accelerated pace. First, lysine binds to the RuBP binding site and then awaits carbamation to activate RUBISCO. RUBISCO activase removes one proton from lysine, generating a binding pocket for CO2. Khalil et al. [65] discovered that the same pH (pH 9) promoted carbohydrate accumulation in C. ellipsoidea. However, the steady decline noticed at pH 10 may be a manifestation of the organism’s inability to utilize carbonate ion (CO32−), which is the most common inorganic carbon at this pH, as bicarbonate (HCO3) is the preferred form for most microalgae/cyanobacteria to assimilate for photosynthesis.
3.3 Extraction of commercially important bioproducts from marine cyanobacterial cultures
Natural bioproducts derived from cyanobacteria, which have complex chemical structures and potent biological activity, are diverse and lucrative and possess extreme commercial importance [7, 66]. In general, the conditions within which marine cyanobacteria are cultivated may result in substantial differences in the types of metabolites produced by these organisms [25, 67]. Biorefineries using cyanobacteria have been effective in the past because they prioritized multi-product recovery and optimization of culture conditions [68].
3.3.1 Extraction of exopolysaccharides from discarded supernatant
Figure 5a displays precipitate EPS harnessed from PPW-FSW medium supernatant using acetone as the extracting solvent. After being freeze-dried, the EPS isolated from the test cyanobacterium emerged in the form of white cottony flakes (Fig. 5b). It was discovered that the PPW-FSW cultures increased EPS production by about ~ 1.7-fold more than the control medium. The EPS production in terms of yield was recorded to be 181.3 mg/L for control and 297.8 mg/L for PPW-FSW medium, which was around 1.6-fold higher than the control condition (Table 4).
The surface morphology of EPS produced by test cyanobacterial culture was investigated using FE-SEM analysis (Fig. 5c). It has been discovered that EPS has a higher density and greater rigidity, which leads to aggregates having the appearance of thin threads and even meshwork in some areas. The absence of a condensed crisscross pattern in EPS recovered from filamentous species possibly would be attributable to the test cyanobacterium being unicellular compared to those extracted from long filament-shaped cyanobacterial species. Indeed, a multitude of variables impacts the structure, content, and viscosity of EPS, including the composition of the culture media, carbon and nitrogen sources, and species type.
FTIR spectroscopy was used to validate produced cyanobacterial EPS. The FTIR spectrum of EPS with distinctive peaks is shown in Fig. 5d. Stretching and bending modes of vibration with a single functional group are typically linked to the vibration of a neighboring group as well as the number of substitutions occurring itself on the molecule. The peaks of two or more functional groups in the same area of the IR spectra shift or overlap as a function of this. A peak at 3444.2 cm−1 corresponds to the O–H stretching frequency, and minor absorptions at 2928.4 cm−1 and 1551.9 cm−1 correspond to the asymmetric and symmetrical C–H stretch vibrations of aliphatic CH2 and C–C of aromatic groups (in ring) (Fig. 5d). Intense absorption at 1653.6 cm−1 might have resulted from the medium stretch vibration of the carboxylate group, raising uncertainties about the occurrence of uronic acid in the hydrolyzed EPS [69]. Furthermore, there is absorption at 1145.0 cm−1 and 1114.1 cm−1 that might be due to the existence of a sulfate group as S = O and C–O–S [16, 43]. The elemental analysis of EPS further verified the presence of sulfur in the EPS samples (Supplementary Fig. S4).
3.3.2 Extraction of mycosporine-like amino acids from harvested cyanobacterial biomass
It has been discovered that certain species of cyanobacteria naturally generate UV protectant MAAs under specific environments [14]. The pictorial representation of extraction steps of MAAs is depicted in Fig. 6a–d. Initial validation of MAAs in the extract was confirmed by spectroscopic evaluation of the methanolic extracts, which revealed an absorption peak of UV-B absorbing compounds (in the range of 300–362 nm). MAA extraction procedures have evolved over the years to include a wide range of solvents, extraction temperatures, and durations.
a Methanolic extracts containing mycosporine-like amino acids (MAAs) extracted from unicellular marine Synechococcus elongatus BDU 10144 biomass (b–c) dried particles of MAAs, and (d) partly purified MAAs dissolved in water, (e) UV–Vis absorbance spectrum of extracted partially purified MAAs showing a confirmatory peak at 340 nm, and (f) HPLC analysis showing six distinct peaks of partially purified MAAs at 330 nm
Factors like incubation period, harvesting of biomass, temperature, solvent ratios, and culture environment all have an effect on the extraction efficiencies and concentrations of MAAs. In recent times, the extraction of MAAs has been carried out using methanol and ethanol in concentrations ranging from 20 to 80%. Herein, 100% methanol provided the maximum yield after overnight incubation at 4 °C. It was discovered that the control culture produced 3.23 mg/g of MAAs, whereas the PPW-FSW culture produced 6.28 mg/g of MAAs. In addition, the MAA content was determined to be 0.323% for the control cultures and 0.628% for the PPW-FSW cultures, respectively.
Spectrophotometric analysis of the absorption spectra of cyanobacteria extracts showed a peak at 340 nm, signifying the presence of MAAs (Fig. 6e) [44]. Next, using HPLC and FTIR spectroscopy, researchers were able to study and analyze MAAs, which are photoprotective naturally occurring substances in cyanobacteria [45]. In this study, HPLC chromatograms revealed a variety of peaks with various retention times (RT) corresponding to distinct MAAs (Fig. 6f). HPLC chromatograms of partly purified aqueous MAA extract revealed the presence of six distinct peaks that were separated and examined spectrophotometrically, revealing peaks at 1.433 (328 nm), 1.842 (334 nm), 2.455 (337 nm), 5.941 (330 nm), 6.621 (320 nm), and 17.429 min (320 nm). In order to determine the m/z of the HPLC-purified sample, HRMS spectra were obtained and analyzed. Using ESI-TOF, it was determined that the peak for palythine-serine in the tested sample has a molecular weight of 274.27, a molecular formula of C11H18N2O6. The spectrum analysis revealed that the peak for palythine-serine had an m/z of 275.12 and an absorption wavelength of 320 nm [70, 71] (Supplementary Fig. S6).
The existence of MAAs was further verified by FTIR analysis, which revealed four significant bands. In the FTIR spectrum, a broad peak of 3200–3600 cm−1 designates the occurrence of an OH group, a band of 3100–3020 signifies C–H with sp2 hybridization, a band of 1386 suggests the existence of a carboxylic groups, and 1342–1266 and 1250–1020 cm−1 specify the presence of a C-N aromatic and a C-N aliphatic, respectively. The existence of MAAs was corroborated by FTIR analysis of partly purified samples, which exhibited prominent peaks of functional groups present at four significant spectral bands (3439, 2934, 1633, and 1384) (see Supplementary Fig. S5). A comparison was made between the FTIR bands of the MAAs and those of previous research works [72]. These findings validate the presence of MAAs in the marine S. elongatus [72,73,74]. The wide variety of MAAs observed in marine organisms is owing to the substitution of amino acids for mycosporine-glycine, Porphyra-334, shinorine, and other MAAs [72, 75].
3.3.3 Production of bioethanol
The need for fuels in human civilization is unquenchable, and at present, the supply of liquid fuels across the globe is effectively contingent on petroleum. The rising price of feedstocks and the mechanisms for converting biomass to monomeric sugars, particularly the cost incurred for the pretreatment and hydrolysis, is a significant impediment to the cost-competitive production of biofuels; therefore, boosting conversion yield is essential for counterbalancing feedstock cost.
In the current investigation, the residual biomass collected after the methanol extraction (for MAAs production) was fermented to produce bioethanol. It is apparent that the increased carbohydrate accretion that occurred under the optimal PPW-FSW medium was a contributing factor in the increase in bioethanol production from 143.9 (control) to 326.5 mg/L. A rise in bioethanol conversion from 43.1 to 44.3% was also observed as a result of the increased supply of fermentable sugar. Accordingly, the bioethanol conversion rate, which measures the percentage of total carbohydrates converted into ethanol, remained relatively stable. In the experimental conditions studied, the conversion rate was between 43 and 44%, demonstrating that biomass could be efficiently used utilizing PPW-FSW medium as a growth medium for the test cyanobacterium for sustainable bioethanol production.
FTIR spectroscopy has emerged in the recent decade as a robust method for studying biological materials. Molecular functional group composition and structure may be identified by measuring the location, width, and intensity of infrared light absorption. Supplementary Fig. S7 illustrates the FTIR spectrum of bioethanol produced from marine test cyanobacterium biomass cultivated using the PPW-FSW medium. In the spectrum, the O–H stretch, C-H stretch, C–C stretch, and C-O stretch of ethanol can all be seen quite clearly (Supplementary Table S1). In the infrared spectrum of ethanol, the O–H stretches contribute to the broadest peak, which is analogous to the O–H bonds in water. The bands of the recorded FTIR spectra are compared to those found in the reference literature to determine the properties of the samples [25]. Besides, these findings imply that growing marine species in a PPW-FSW medium increases carbohydrate pool relative to bioethanol production and that PPW has the potential to be used in the mass cultivation of marine cyanobacterial species. Additionally, most of the income generated by selling the high-value bioproducts would improve the possibilities of the devised technology for the cyanobacterial refinery.
3.4 Commercial importance of high-value bioproducts
Cyanobacteria offer enormous biofuel and value-added product recovery prospects at the industrial scale. Algae and cyanobacteria found in marine environments are pivotal producers of high-value bioproducts. The high expense of cultivating cyanobacteria/microalgae might be mitigated by substituting out the chemical growth medium for a more economical alternative [68].
EPS produced by microorganisms are a structurally diverse range of polymers. Since EPS produced by different organisms has various properties, it has multifarious industrial applications. EPS is primarily employed in industries as a gelling and thickening agent that suspends or stabilizes the aqueous phase. In light of this, the discovery of new microbial strains that produce the highest quantity of innovative polysaccharides have been a significant focus in recent years. Considering that nearly all microbes possess the genetic and metabolic machinery for producing polysaccharides under specific circumstances, there is a need for high-throughput screening techniques that can help in identifying novel variants of microbial EPS with properties that are superior to those already described. It has also been emphasized that despite the vast number of bacterial EPS that have been chemically characterized, only a handful of those are under commercial usage [76]. According to a recent pilot-scale study conducted under outdoor conditions, Spirulina sp. LEB-18 produced crude EPS during all stages of biomass development, and the amount produced was around 10 times more than the biomass concentration of Spirulina sp. at the end of the cultivation cycle [77].
In addition, EPS from microbiological sources is commonly used in food (jelly, cakes, ice creams, confections, dressing, sauce, appetizers), healthcare (capsule coating, anti-tumor medications), fabrics (printing, dye, and pigment suspensions), cosmetics (moisturizer, stabilizer), detergents, oil recovery, and so forth. Microalgae-based bio-product development has progressed throughout time, adding significant worth to the market. In the market, microalgal biomass is now sold for about €1000/ton. Recent techno-economical report on the sales’ value of microalgal polysaccharide applications predicted (in €/ton) 2,000,000.0, 10,000.0, 2500.0, and 400.0 for immune-stimulant, plant growth stimulator, moisturizer in cosmetics, and biofuel manufacturers, respectively [78].
Also, biochemicals generated by algae are regarded as natural gifts since their supply is perpetually regenerated by the energy from the sun. MAAs are typical representations of these natural gifts generally found in marine species. MAAs have been investigated commercially as sun protection agents for the skin. Environmentally and dermatologically harmful sunscreen filters are receiving increasing disapproval. The MAAs are a remarkable example of an algal-secondary metabolite implicated in photoprotection (MAAs). The use of conventional sunscreens has been linked to several adverse health effects, and recent studies on the econometrics of the commercial sunscreen industry have shown a shift away from the use of synthetic chemicals. So, MAAs are promising functional ingredients used for novel cosmeceuticals (cosmetic products with health benefits). MAAs are currently commercially available as Helioguard®365. Helioguard®365 cosmetic reagent is a safe and natural sunscreen containing Porphyra umbilicalis-isolated MAAs, shinorine, and Porphyra-334. In vivo testing on ten human participants revealed that Helioguard®365 (2% concentration) increased the SPF value of sunscreen from 7.2 to 8.2 [79]. Helionori® is another commercially active natural sunscreen product derived from the red algae P. umbilicalis that contains Palythine, Porphyra-334, and Shinorine [15]. Since there are few MAAs-based sunscreens on the market, there is still a long way to go before naturally derived sunscreens, such as MAAs, are widely accepted. In the biotechnology sector, MAAs are attractive metabolites because of their strong photostability over a broad range of temperatures and pH and their antioxidant abilities [75]. In addition to their potential efficacy against actinic erythema, MAAs can shield humans from additional biological repercussions, such as immunosuppression or photooxidative damages. UV-B rays are the primary cause of skin cancer, and in the photoaging process, the usage of mycosporine-based sunscreens has increased dramatically over the last several decades. New findings show that the incidence of non-melanoma skin cancer (NMSC) has grown intensely during the preceding times. It has been reported that the incidence of NMSC may be vividly lowered by utilizing a natural sun blocker made from algae/cyanobacteria [80].
There is no denying that the cosmetics market is one that is continually expanding and one that requires intensive reinvention. The expansion of these enterprises, as well as potential profitability, is shown by the economic evaluations that are based on particular reports. An average woman, in her lifetime, would spend close to $15,000 on products related to the cosmetics industry. According to Eurostat, the global cosmetics business envisaged a total revenue of 170 billion dollars each year. In 2016, the market for cosmetics in Europe was valued at 77 billion euros, which was followed by the markets in the USA and Brazil [80, 81]. The expanding market demand for natural sunscreen agents and MAAs-based products is a result of their diversified functions and expanded applications [81]. The market for these goods is indeed developing, and the aquaculture-based sector faces the additional task of cultivating biomass to generate polysaccharides and mycosporine-containing algal/cyanobacterial isolates on a mass scale.
3.5 Economic evaluation of cyanobacterial-based biorefinery
The feasibility of any process using an integrated scientific approach can be established by evaluating the economic investment in terms of net present value. The economic viability of a large-scale biorefinery is among the most critical issues limiting its practical implementation. A comprehensive understanding of the technological and economic components of the approach is required to assess its long-term feasibility [7].
Considering the direct costs of a biomass-based biorefinery, the growth medium of the test organism that serves as the raw material for bioproducts generation incurs enormous expenditures. Due to species-specific operating requirements and product yield variances, biorefinery costs differ considerably for diverse species depending upon variable factors like mode of cultivation, extraction procedures, and downstream processing [68]. The expenses for utilities and other inputs were often the major contributing expenses in the studied single product value chain configurations. Under the cultivation factor, in addition to the exorbitant cost of analytical chemicals, several inorganic compounds pose disposal challenges and have a detrimental impact on the environment, as many are not eco-friendly or biodegradable.
Using a potent waste such as potato peel as a nutrient source in conjunction with an inexpensive seawater-based medium for the cultivation of the test cyanobacterium could reduce the upstream cost and contribute to increasing biomass productivity relative to carbohydrates from a biorefinery standpoint. Also, substituting seawater in lieu of freshwater reduces the dependence on potable water used in the cultivation medium for cyanobacteria, thereby reducing the water footprint.
Accordingly, utilizing the current method as an integrated biorefinery would strengthen the economy. In this study, the low-cost PPW-FSW medium containing potato peels, agricultural fertilizers, and seawater was developed for cultivation with an anticipated 36-fold reduction in cultivation medium costs and a projected yield of 1.81 kg of biomass (1.6-times superior to the analytical-grade ASN-III medium cultures). The production of co-products rose by around 1.6- and 1.9-fold for EPS and MAAs, respectively, when PPW extracts were used as an exogenous nutrient source. Additionally, 2.3-fold more bioethanol was produced from PPW-FSW cultivated cyanobacterial biomass than in control (Table 5). Moreover, as the wastes used to formulate the PPW-FSW medium were gathered from the near proximity of the experimental site, the transportation costs may also be deemed to be minimal, and the processing cost for PPW was calculated according to Bagchi et al. [82] and Silva et al. [83]. Chandra and Mallick provide a detailed breakdown of the cost of ASN-III medium and FSW medium [28]. Eventually, there possibly will be an opportunity for further upstream cost reduction by optimizing biomass processing steps and bioproduct extraction parameters [68].
Table 5 shows the projected output of bioethanol, MAAs, and EPS that could be achieved from a culture of S. elongatus if the culture volume was scaled-up to 1000 L. Considering the high commercial interest in MAAs, in the PPW-FSW medium, 6.2 mg/g MAAs can be produced from 1.0 g of biomass, which can be expected to be 6.2 g utilizing 1.0 kg of biomass (Table 5). Currently, for economic reasons, the minimum order quantity of MAAs in the commercial market is established at 1.0 g; prices are often costly and take months to prepare, depending on the technological difficulties. MAA yield might be improved further by improving extraction parameters, which would undoubtedly strengthen overall market potential. If MAAs are commercialized, the selling price would assuredly boost economic empowerment [13, 68].
3.6 Approach to holistic biorefinery and circular bioeconomy
The prerequisites for the environmentally responsible production of biofuels would potentially be achieved by the development of biorefineries. The word “biorefinery” refers to an integrative and multifunctional concept that incorporates the use of biomass for the purpose of the sustainable production of a plethora of intermediates and products, as well as the complete possible use of all feedstock components. The extraction of value-added bioproducts along with biofuel feedstock from cyanobacteria-based biorefineries might be enhanced by process integration [84]. The following aspects must be considered: cyanobacterial strain, extraction procedure, and industrial by-products to construct the biorefinery framework [66]. A renewed emphasis is laid on lessening the ecological footprint and establishing a sustainable supply of renewable biomass resources. The biorefinery is already on the verge of expanding the bioeconomy due to the integrated exploitation of algal/cyanobacterial biomass [85].
Cyanobacterial species from various spots can synthesize bioactive substances, but the economic viability is questionable due to the costs associated with the organism’s cultivation, harvesting, and dewatering. In this regard, the algal/cyanobacterial biorefinery provides a superior alternative for cost-reduction while maximizing recovery. Accordingly, the primary focus is to get a maximal accumulation of value-added bioproducts from cyanobacteria by improving their cultivation methods and extraction parameters. The fundamental concept behind the optimization of culture conditions is to raise the biomass of the algae/cyanobacteria under consideration and consequently increase the product’s value.
In the same vein, it is necessary to reconfigure all the limiting factors that prevent the successful establishment of a cultivation system. It is well acknowledged that the notion of a circular bioeconomy is gathering traction as an integral aspect of green technology [7]. Incidentally, the pioneering effort of the circular bioeconomy is to integrate green technology to produce commercially viable products from zero-value waste [68].
Moreover, pollution caused by improper solid-waste management is a worldwide crisis. Environment contamination from untreated agro-waste, food, or livestock waste in landfills is a significant contributor to global warming and climate change under the canopy of waste mismanagement [53, 59]. Open disposals and waste incineration are prime waste treatment and ultimate disposal procedures used in low-income nations. The application of heterogeneous substances derived from wastes not only reduces the cost of feedstocks but also promotes waste management, controls pollution, ensures accessibility and non-toxicity, and engenders less destructive ecological impacts [86].
Also, integrating the biorefinery idea with wastewater treatment allows for more effective usage of algal/cyanobacterial biomass, decreases total residual waste component, and promotes enduring economic viability. Cyanobacterial research is also gaining great importance due to its unique role in bioremediation and wastewater treatment. With the addition of a small amount of wastewater, inexhaustible seawater has the potential to optimize algal production so that bioenergy production may flourish cost-effectively and sustainably [25, 84]. Because marine S. elongatus possesses an efficient mechanism for adapting to the saline environment in seawater, the polysaccharides, proteins, lipids, and pigments that can be extracted from S. elongatus biomass have the potential to be used in the production of value-added bioproducts, which in turn can improve the economics of biorefineries. Figure 7 displays the potential contribution of marine cyanobacteria to a sustainable biorefinery and circular bioeconomy.
Even though cyanobacterial biorefineries with multiple product recoveries may effectively valorize biomass with little waste output, cyanobacterial biomass-based biorefineries encounter a number of obstacles and roadblocks. Most of them are associated with difficult-to-manage industrial processes and downstream methods.
Employing waste potato peels as an additional source of nutrients with seawater for bioethanol production is an opportunity to democratize sustainable bioenergy generation. Nevertheless, several factors influence the compositional characteristics and processing of potato peels. These include the kind of potato, the time of the year, and the location of the farm where the potato was cultivated. Also, the collection, storage, and transportation of PPW are impacted by their high moisture content. During the storage process, open PPW storage and the onset of fermentation that may occur during the storing period can considerably affect the PPW’s starch content. Also, a lack of information on processing, management, and transportation methods can impact the long-term storage of PPW. As a result, PPW drying processes that are efficient and cost-effective must be developed. In the laboratory, the methods for extracting vital nutrients from PPW were viable, but they need to be scaled up for commercial applications. For this, a continuous and cost-effective supply of PPW is necessary for industrial-scale production of value-added products. The consistent supply of PPW, storage management, and the separation of desirable components are the key barriers to scaling up, and further exploration is needed in the future in such areas [87].
Despite the importance of bioethanol in the energy revolution and the integration of the water-food-energy nexus, bioethanol from microalgal/cyanobacterial species remains a technological barrier [84]. Future research should also focus on comparing cyanobacterial bioethanol to commercial ethanol, given that cyanobacterial bioethanol may contain inorganic contaminants and organic chemicals at the end of the production process that can have a serious impact on its quality, such as reducing combustion efficiency or compromising engine performance [88].
It is necessary to suggest novel strategies to reduce challenging tailbacks to transform the cyanobacterial biorefinery into an economically viable, minimally waste-generating multi-product biorefinery. Lack of research funding, small-scale operations, and regulatory implementation challenges all contribute to the fact that cyanobacteria are not yet widely available for commercial utilization. Cyanobacterial cultivation and downstream processing might need more opportunities and possibilities from contributors. It is also critical to overcome bottlenecks in the existing fragmented residual biomass and waste supply chains to assure sustainable biomass supply in an economically viable manner. These include the development of robust business models that allow the biomass supply chain to be well-coordinated, particularly in developing and less developed countries, to improve process efficiency, raise awareness among the public, and provide incentives so that crop waste residues are channeled to bioenergy production rather than being burned and discarded [85, 89].
4 Conclusion
Cyanobacteria are recognized as a quintessential feedstock for bioenergy and bioproducts production, although cyanobacterial biomass-based bioethanol production has miles to go before achieving commercial viability. Considering this, in the present study, a dual strategy for waste valorization vis-à-vis multi-product cyanobacterial biorefinery with minimal waste output has been demonstrated. Biorefinery may be achieved by employing potato peels as a low-cost source of nutrients for the cultivation of unicellular marine Synechococcus elongatus BDU 10144 to produce bioethanol and valuable co-products simultaneously. The addition of a little quantity of wastewater to inexhaustible seawater has the potential to strengthen cyanobacterial productivity, thereby doubling bioethanol production and flourishing economically and sustainably. The PPW-FSW medium developed using low-cost fertilizer, potato peels, and seawater would not only eliminate the need for freshwater and analytical-grade chemicals but also provide a cost-effective avenue for the mass production of cyanobacterial biomass.
Furthermore, under a multi-product biorefinery technique, S. elongatus biomass was used to extract bioethanol, MAAs, and EPS in a single cultivation cycle. Intriguingly, analytical methods such as FTIR, HPLC, and SEM for characterization were demonstrated to be practical and accurate methods, and they may be used effectively commercially for providing chemical and visual validation of extracted bioproducts. There has been no scientific literature describing biorefinery production of ethanol and economically relevant MAAs and EPS from marine cyanobacteria using PPW as a low-cost nutritional supplement, which adds distinctiveness to the current work. However, it still has limitations because most findings are based only on laboratory research. Even if the method of extracting value-added products is well developed in the laboratory, their industrial production remains challenging, and further pilot-scale studies for better understanding are needed. Another critical aspect that requires additional investigation in future studies is the comparison of commercial ethanol to bioethanol derived from cyanobacteria. For this, exploration of a multi-product biorefinery under a pilot scale could provide viable, cost-effective, and environment-friendly solutions to a significant degree for existing commercialization challenges. Hence, this study demonstrates a state-of-the-art approach for holistic cyanobacterial biorefinery, which leverages discarded potato peels to facilitate the mixotrophic cultivation of the marine S. elongatus with a goal of increasing the amount of biomass and carbohydrate accessible for the production of sustainable bioethanol and industrially important bioproducts. On a concluding note, this investigation presents a novel paradigm for producing high-value bioproducts from cyanobacterial biomass cultivated in renewable seawater, exploiting low-cost waste with detrimental environmental impact.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Silva CE de F, Bertucco A (2016) Bioethanol from microalgae and cyanobacteria: a review and technological outlook. Process Biochem 51:1833–1842. https://doi.org/10.1016/j.procbio.2016.02.016
Athar M, Zaidi S (2020) A review of the feedstocks, catalysts, and intensification techniques for sustainable biodiesel production. J Environ Chem Eng 8:104523. https://doi.org/10.1016/j.jece.2020.104523
Sadhukhan J, Martinez-Hernandez E, Amezcua-Allieri MA et al (2019) Economic and environmental impact evaluation of various biomass feedstock for bioethanol production and correlations to lignocellulosic composition. Bioresour Technol Rep 7:100230. https://doi.org/10.1016/j.biteb.2019.100230
Olgu EJ, Gloria S, Arias-olgu II et al (2022) Microalgae-based biorefineries: challenges and future trends to produce carbohydrate enriched biomass, high-added value products and bioactive compounds. Biology 11:1146. https://doi.org/10.3390/biology11081146
Greetham D, Zaky A, Makanjuola O, Du C (2018) A brief review on bioethanol production using marine biomass, marine microorganism and seawater. Curr Opin Green Sustain Chem 14:53–59. https://doi.org/10.1016/J.COGSC.2018.06.008
Thajuddin N, Subramanian G (2005) Cyanobacterial biodiversity and potential applications in biotechnology. Curr Sci 89:47–57. http://www.jstor.org/stable/24110431. Accessed 11 Mar 2022
Chandra R, Iqbal HM, Vishal G et al (2019) Algal biorefinery: a sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour Technol 278:346–359. https://doi.org/10.1016/j.biortech.2019.01.104
Guerreiro A, Andrade MA, Menezes C et al (2020) Antioxidant and cytoprotective properties of cyanobacteria: potential for biotechnological applications. Toxins (Basel) 12:548. https://doi.org/10.3390/toxins12090548
Deb D, Mallick N, Bhadoria PBS (2021) Engineering culture medium for enhanced carbohydrate accumulation in Anabaena variabilis to stimulate production of bioethanol and other high-value co-products under cyanobacterial refinery approach. Renew Energy 163:1786–1801. https://doi.org/10.1016/j.renene.2020.10.086
Deb D, Mallick N, Bhadoria PBS (2019) Analytical studies on carbohydrates of two cyanobacterial species for enhanced bioethanol production along with poly-β-hydroxybutyrate, C-phycocyanin, sodium copper chlorophyllin, and exopolysaccharides as co-products. J Clean Prod 221:695–709. https://doi.org/10.1016/j.jclepro.2019.02.254
Rastogi RP, Incharoensakdi A (2014) Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol Ecol 87:244–256. https://doi.org/10.1111/1574-6941.12220
Chandra R, Das P, Vishal G, Nagra S (2019) Factors affecting the induction of UV protectant and lipid productivity in Lyngbya for sequential biorefinery product recovery. Bioresour Technol 278:303–310. https://doi.org/10.1016/j.biortech.2019.01.084
Singh R, Parihar P, Singh M et al (2017) Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: current status and future prospects. Front Microbiol 8:1–37. https://doi.org/10.3389/fmicb.2017.00515
Sen S, Mallick N (2021) Mycosporine-like amino acids: algal metabolites shaping the safety and sustainability profiles of commercial sunscreens. Algal Res 58:102425. https://doi.org/10.1016/j.algal.2021.102425
Singh A, Čížková M, Bišová K, Vítová M (2021) Exploring mycosporine-like amino acids (Maas) as safe and natural protective agents against UV-induced skin damage. Antioxidants 10:683. https://doi.org/10.3390/antiox10050683
Delattre C, Pierre G, Laroche C, Michaud P (2016) Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol Adv 34:1159–1179. https://doi.org/10.1016/j.biotechadv.2016.08.001
de Castro GFPS, Rizzo RF, Passos TS et al (2015) Biomass production by Arthrospira platensis under different culture conditions. Food Sci Technol 35:18–24. https://doi.org/10.1590/1678-457X.6421
Jiang L, Sun J, Nie C et al (2019) Filamentous cyanobacteria triples oil production in seawater-based medium supplemented with industrial waste: monosodium glutamate residue. Biotechnol Biofuels 12:53. https://doi.org/10.1186/s13068-019-1391-1
Özçimen D, Inan B (2015) An overview of bioethanol production from algae. In: Biofuels - Status and Perspective. IntechOpen, London. https://doi.org/10.5772/58662
Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 53:29–38. https://doi.org/10.1016/j.biombioe.2012.12.019
Katiyar R, Gurjar BR, Kumar A et al (2019) A novel approach using low-cost Citrus limetta waste for mixotrophic cultivation of oleaginous microalgae to augment automotive quality biodiesel production. Environ Sci Pollut Res 26:16115–16124. https://doi.org/10.1007/s11356-019-04946-0
Ji MK, Yun HS, Park S et al (2015) Effect of food wastewater on biomass production by a green microalga Scenedesmus obliquus for bioenergy generation. Bioresour Technol 179:624–628. https://doi.org/10.1016/j.biortech.2014.12.053
Taskila S, Ahokas M, Sotaniemi V-H et al (2018) Conversion of potato peel waste to single cell protein by an acidophilic fungus. J Water Resour Prot 10:522–532. https://doi.org/10.4236/jwarp.2018.105028
Jekayinfa SO, Linke B, Pecenka R (2015) Biogas production from selected crop residues in Nigeria and estimation of its electricity value. Int J Renew Energy Technol 6:101. https://doi.org/10.1504/ijret.2015.068593
Pei H, Jiang L (2018) Mixing seawater with a little wastewater to produce bioenergy from limnetic algae. Trends Biotechnol 36:480–483. https://doi.org/10.1016/j.tibtech.2017.12.002
Hwang TY, Qi HA, Kin CM et al (2021) Potential of fruit peel waste in growing cyanobacteria Anabaena cylindrica. Int J Technol 12:711–719. https://doi.org/10.14716/ijtech.v12i4.4852
Rippka R, Deruelles J, Waterbury JB et al (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111:1–61. https://doi.org/10.1099/00221287-111-1-1
Chandra N, Mallick N (2022) Co-production of bioethanol and commercially important exopolysaccharides from the marine cyanobacterium Synechococcus elongatus BDU 10144 in a novel low-cost seawater-fertilizer-based medium. Int J Energy Res 46:13487–13510. https://doi.org/10.1002/er.8069
Chemat F, Rombaut N, Sicaire AG et al (2017) Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem 34:540–560. https://doi.org/10.1016/j.ultsonch.2016.06.035
Rai LC, Mallick N, Singh JB, Kumar HD (1991) Physiological and biochemical characteristics of a copper tolerant and a wild type strain of Anabaena doliolum under copper stress. J Plant Physiol 138:68–74. https://doi.org/10.1016/S0176-1617(11)80732-7
Malakar B, Das D, Mohanty K (2022) Utilization of waste peel extract for cultivation of microalgal isolates: a study of lipid productivity and growth kinetics. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02313-7
Dubois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Koley S, Mathimani T, Bagchi SK et al (2019) Microalgal biodiesel production at outdoor open and polyhouse raceway pond cultivations: a case study with Scenedesmus accuminatus using low-cost farm fertilizer medium. Biomass Bioenergy 120:156–165. https://doi.org/10.1016/j.biombioe.2018.11.002
Hodge JE, Hofreiter BT (1962) Determination of reducing sugars and carbohydrates. In: Whistler RL, Wolfrom ML (eds) Methods in Carbohydrate Chemistry. Academic Press, New York, pp 380–394
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Updegraff DM (1969) Semimicro determination of cellulose in biological materials. Anal Biochem 32:420–424. https://doi.org/10.1016/S0003-2697(69)80009-6
Ververis C, Georghiou K, Danielidis D et al (2007) Cellulose, hemicelluloses, lignin and ash content of some organic materials and their suitability for use as paper pulp supplements. Bioresour Technol 98:296–301. https://doi.org/10.1016/j.biortech.2006.01.007
Chandra N, Shukla P, Mallick N (2020) Role of cultural variables in augmenting carbohydrate accumulation in the green microalga Scenedesmus acuminatus for bioethanol production. Biocatal Agric Biotechnol 26:101632. https://doi.org/10.1016/j.bcab.2020.101632
Seifter S, Dayton S, Novic B, Muntwyler E (1950) The estimation of glycogen with the anthrone reagent. Arch Biochem 25:191–200
Qadir MA, Ahmed M, Hussain WA, Tahir MS (2015) Development and validation of new HPLC method for simultaneous estimation of l-lysine hydrochloride and l-carnitine-l-tartrate in pharmaceutical dosage form. Indian J Pharm Sci 77:434–438. https://doi.org/10.4103/0250-474X.164772
Sielc (2021) Lysine _ SIELC. In: https://sielc.com/Compound-Lysine.html. Accessed 21 April 2022
Parikh A, Madamwar D (2006) Partial characterization of extracellular polysaccharides from cyanobacteria. Bioresour Technol 97:1822–1827. https://doi.org/10.1016/j.biortech.2005.09.008
Pathak J, Rajneesh R, Sonker AS et al (2015) Isolation and partial purification of scytonemin and mycosporine-like amino acids from biological crusts. J Chem Pharm Res 7:362–371
Carreto JI, Carignan MO, Montoya NG (2005) A high-resolution reverse-phase liquid chromatography method for the analysis of mycosporine-like amino acids (MAAs) in marine organisms. Mar Biol:237–252. https://doi.org/10.1007/s00227-004-1447-y
Singh R, Singh S, Trimukhe KD et al (2005) Lignin-carbohydrate complexes from sugarcane bagasse: Preparation, purification, and characterization. Carbohydr Polym 62:57–66. https://doi.org/10.1016/j.carbpol.2005.07.011
Stec B (2012) Structural mechanism of RuBisCO activation by carbamylation of the active site lysine. Proc Natl Acad Sci 109:18785–18790. https://doi.org/10.1073/PNAS.1210754109
Hasanin MS (2021) Simple, economic, ecofriendly method to extract starch nanoparticles from potato peel waste for biological applications. Starch 73:2100055. https://doi.org/10.1002/star.202100055
Malakar B, Das D, Mohanty K (2020) Optimization of glucose yield from potato and sweet lime peel waste through different pretreatment techniques along with enzyme assisted hydrolysis towards liquid biofuel. Renew Energy 145:2723–2732. https://doi.org/10.1016/j.renene.2019.08.037
Nemer G, Louka N, Vorobiev E et al (2021) Mechanical cell disruption technologies for the extraction of dyes and pigments from microorganisms: a review. Fermentation 7:1–17. https://doi.org/10.3390/fermentation7010036
Bussemaker MJ, Zhang D (2013) Effect of ultrasound on lignocellulosic biomass as a pretreatment for biorefinery and biofuel applications. Ind Eng Chem Res 52:3563–3580. https://doi.org/10.1021/ie3022785
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4:1–19. https://doi.org/10.1186/s40643-017-0137-9
Lau KY, Pleissner D, Lin CSK (2014) Recycling of food waste as nutrients in Chlorella vulgaris cultivation. Bioresour Technol 170:144–151. https://doi.org/10.1016/j.biortech.2014.07.096
Sloth JK, Jensen HC, Pleissner D, Eriksen NT (2017) Growth and phycocyanin synthesis in the heterotrophic microalga Galdieria sulphuraria on substrates made of food waste from restaurants and bakeries. Bioresour Technol 238:296–305. https://doi.org/10.1016/j.biortech.2017.04.043
Yan D, Lu Y, Chen YF, Wu Q (2011) Waste molasses alone displaces glucose-based medium for microalgal fermentation towards cost-saving biodiesel production. Bioresour Technol 102:6487–6493. https://doi.org/10.1016/j.biortech.2011.03.036
Pleissner D, Lam WC, Sun Z, Lin CSK (2013) Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresour Technol 137:139–146. https://doi.org/10.1016/j.biortech.2013.03.088
El-Awady R, El-Sayed AE, El-Zabalawy K, El-Mohandes M (2020) Bio-mass production of Chlorella vulgaris grown on date wastes under different stress conditions. Al-Azhar J Agric Res 45:62–75. https://doi.org/10.21608/ajar.2020.149423
Javed A, Ahmad A, Tahir A et al (2019) Potato peel waste—its nutraceutical, industrial and biotechnological applications. AIMS Agric Food 4:807–823. https://doi.org/10.3934/agrfood.2019.3.807
Bhatnagar A, Chinnasamy S, Singh M, Das KC (2011) Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl Energy 88:3425–3431. https://doi.org/10.1016/j.apenergy.2010.12.064
Goswami RK, Mehariya S, Karthikeyan OP, Verma P (2022) Influence of carbon sources on biomass and biomolecule accumulation in Picochlorum sp. cultured under the mixotrophic condition. Int J Environ Res Public Health 19:3674. https://doi.org/10.3390/ijerph19063674
Liang Y, Sarkany N, Cui Y et al (2010) Use of sweet sorghum juice for lipid production by Schizochytrium limacinum SR21. Bioresour Technol 101:3623–3627. https://doi.org/10.1016/j.biortech.2009.12.087
Park WK, Moon M, Kwak MS et al (2014) Use of orange peel extract for mixotrophic cultivation of Chlorella vulgaris: increased production of biomass and FAMEs. Bioresour Technol 171:343–349. https://doi.org/10.1016/j.biortech.2014.08.109
Gupta A, Abraham RE, Barrow CJ, Puri M (2015) Omega-3 fatty acid production from enzyme saccharified hemp hydrolysate using a novel marine thraustochytrid strain. Bioresour Technol 184:373–378. https://doi.org/10.1016/j.biortech.2014.11.031
Amit KG, U, (2019) Utilization of kinnow peel extract with different wastewaters for cultivation of microalgae for potential biodiesel production. J Environ Chem Eng 7:103135. https://doi.org/10.1016/j.jece.2019.103135
Khalil ZI, Asker MMS, El-Sayed S, Kobbia IA (2010) Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea. World J Microbiol Biotechnol 26:1225–1231. https://doi.org/10.1007/s11274-009-0292-z
Deb D, Mallick N, Bhadoria PBS (2022) A waste ‑ to ‑ wealth initiative exploiting the potential of Anabaena variabilis for designing an integrated biorefinery. Sci Rep:1–19. https://doi.org/10.1038/s41598-022-13244-8
Maity S, Mallick N (2022) Trends and advances in sustainable bioethanol production by marine microalgae: a critical review. J Clean Prod 345:131153. https://doi.org/10.1016/j.jclepro.2022.131153
Prabha S, Vijay AK, Paul RR, George B (2022) Cyanobacterial biorefinery: towards economic feasibility through the maximum valorization of biomass. Sci Total Environ 814:152795. https://doi.org/10.1016/j.scitotenv.2021.152795
De Philippis R, Margheri MC, Materassi R, Vincenzini M (1998) Potential of unicellular cyanobacteria from saline environments as exopolysaccharide producers. Appl Environ Microbiol 64:1130–1132. https://doi.org/10.1128/aem.64.3.1130-1132.1998
Carignan MO, Cardozo KHM, Oliveira-silva D et al (2009) Palythine – threonine, a major novel mycosporine-like amino acid (MAA) isolated from the hermatypic coral Pocillopora capitata. J Photochem Photobiol B Biol 94:191–200. https://doi.org/10.1016/j.jphotobiol.2008.12.001
Teai TT, Rahanvelomanana P, Bianchini J, Faure R et al (1997) Structure de Deux Nouvelles lminomycosporines lsolées de Pocillopora eydouxi. Tetrahedron Lett 38:5799–5800. https://doi.org/10.1016/S0040-4039(97)01281-1
Richa SRP (2015) Biochemical characterization of sunscreening mycosporine-like amino acids from two Nostoc species inhabiting diverse habitats. Protoplasma 252:199–208. https://doi.org/10.1007/s00709-014-0674-4
Whitehead K, Hedges JI (2003) Electrospray ionization tandem mass spectrometric and electron impact mass spectrometric characterization of mycosporine-like amino acids. Rapid Commun Mass Spectrom 17:2133–2138. https://doi.org/10.1002/rcm.1162
Yoshiki M, Tsuge K, Tsuruta Y et al (2009) Production of new antioxidant compound from mycosporine-like amino acid, porphyra-334 by heat treatment. Food Chem 113:1127–1132. https://doi.org/10.1016/j.foodchem.2008.08.087
Rosic NN, Braun C, Kvaskoff D (2015) Extraction and analysis of mycosporine-like amino acids in marine algae. Methods Mol Biol 1308:119–129. https://doi.org/10.1007/978-1-4939-2684-8_6
Jindal N, Khattar JS, Singh DP (2021) Isolation of microbial polysaccharides. In: Oliveira J, Radhouani H, Reis RL (eds) Polysaccharides of Microbial Origin: Biomedical Applications. Springer International Publishing, Cham, pp 1–14. https://doi.org/10.1007/978-3-030-35734-4_27-1
de Jesus CS, de Jesus AD et al (2019) Pilot-scale isolation and characterization of extracellular polymeric substances (EPS) from cell-free medium of Spirulina sp. LEB-18 cultures under outdoor conditions. Int J Biol Macromol 124:1106–1114. https://doi.org/10.1016/j.ijbiomac.2018.12.016
Barsanti L, Gualtieri P (2018) Is exploitation of microalgae economically and energetically sustainable? Algal Res 31:107–115. https://doi.org/10.1016/j.algal.2018.02.001
Mibelle Group Biochemistry (2010) Helioguard 365 Switzerland. Helioguard™ 365 2006:1–11. https://mibellebiochemistry.com/helioguardtm-365. Accessed 12 May 2022
Bjorn LO, Widell S, Wang T (2002) Evolution of UV-B regulation and protection in plants. Adv Space Res 30:1557–1562. https://doi.org/10.1016/S0273-1177(02)00371-X
Bhatia S, Garg A, Sharma K, Kumar S et al (2011) Mycosporine and mycosporine-like amino acids: a paramount tool against ultra violet irradiation. Pharmacogn Rev 5:138–146. https://doi.org/10.4103/0973-7847.91107
Bagchi SK, Rao PS, Mallick N (2015) Development of an oven drying protocol to improve biodiesel production for an indigenous chlorophycean microalga Scenedesmus sp. Bioresour Technol 180:207–213. https://doi.org/10.1016/j.biortech.2014.12.092
de Silva CE, F, Meneghello D, et al (2020) Pretreatment of microalgal biomass to improve the enzymatic hydrolysis of carbohydrates by ultrasonication: yield vs energy consumption. J King Saud Univ Sci 32:606–613. https://doi.org/10.1016/j.jksus.2018.09.007
Pessôa LC, Deamici KM, Pontes LAM et al (2021) Technological prospection of microalgae-based biorefinery approach for effluent treatment. Algal Res 60:102504. https://doi.org/10.1016/j.algal.2021.102504
Rajvanshi M, Podili R, Dwivedi V et al (2022) Waste biomass to bioenergy: a compressive review. In: Prakash K, Sarangi LB (eds) Biotechnology for Waste Biomass Utilization, 1st edn. Apple Academic Press, New York, pp 1–28. https://doi.org/10.1201/9781003277187
Alagumalai A, Mahian O, Hollmann F, Zhang W (2021) Environmentally benign solid catalysts for sustainable biodiesel production: a critical review. Sci Total Environ 768:144856. https://doi.org/10.1016/j.scitotenv.2020.144856
Pathak PD, Mandavgane SA, Puranik NM et al (2018) Valorization of potato peel: a biorefinery approach. Crit Rev Biotechnol 38:218–230. https://doi.org/10.1080/07388551.2017.1331337
Sánchez C, Santos S, Sánchez R et al (2020) Profiling of organic compounds in bioethanol samples of different nature and the related fractions. ACS Omega 5:20912–20921. https://doi.org/10.1021/acsomega.0c02360
Show PL (2022) Global market and economic analysis of microalgae technology: status and perspectives. Bioresour Technol 357:127329. https://doi.org/10.1016/j.biortech.2022.127329
Acknowledgements
The first author gratefully acknowledges Dr. N. D. Pradeep Singh and Mr. Amit Kumar Singh for their kind help in analytical studies. The first author would like to extend immense gratitude to Ms. Lazumla Sherpa, Mr. Yash Sharma, Mr. Zaki Ahmed, Ms. Sudatta Maity, Mr. Karan, and Mr. Pawan for their helpful suggestions and advice in preparing the manuscript.
Funding
The authors are thankful to the Indian Institute of Technology Kharagpur, India, for providing financial aid and research facilities in the form of a Senior Research Fellowship.
Author information
Authors and Affiliations
Contributions
Neha Chandra: writing—original draft, investigation, methodology, validation, data curation.
Nirupama Mallick: conceptualization, writing—review & editing, visualization, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
All the authors have read and agreed with the ethics for publishing the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandra, N., Mallick, N. A synergistic “waste-to-wealth” approach towards a cyanobacterial biorefinery via valorizing potato peels for the cultivation of marine Synechococcus elongatus. Biomass Conv. Bioref. 14, 13135–13156 (2024). https://doi.org/10.1007/s13399-022-03281-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03281-8