Abstract
Mannosylerythritol lipids (MELs) are biosurfactants with excellent biochemical properties and a wide range of potential applications. However, high production costs, low productivity and unsatisfactory scale-up production have hampered commercial adoption. Herein, we report for the first time the β-galactosidase production by Moesziomyces spp. from different sugars (D-galactose, D-glucose and D-lactose), with D-galactose being the best β-galactosidase inducer, with 11.2 and 63.1 IU/mgbiomass, for Moesziomyces aphidis 5535 T and Moesziomyces antarcticus 5048 T, respectively. The production of this enzyme allows to break down D-lactose and thus to produce MEL directly from D-lactose or cheese whey (a cheese industry by-product). Remarkably, when CW was used as sole media component (carbon and mineral source), in combination with waste frying oil, MEL productivities were very close (1.40 and 1.31 gMEL/L/day) to the ones obtained with optimized medium containing yeast extract (1.92 and 1.50 gMEL/gsusbtrate), both for M. antarcticus and M. aphidis. The low-cost, facile and efficient process which generates large amounts of MELs potentiates its industrialization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Since the industrial revolution, the chemical industry has undergone important developments, resulting in a variety of products that are used in a wide range of applications. Within these products, surfactants, molecules capable to stabilize oil–water interfaces and promote self-organized structures [1], are one of the most produced chemicals worldwide, with a market reaching US $40 billion in 2021 [2]. However, most of the surfactants are derived from petrochemicals, and thus contributing to greenhouse gas (GHGs). Moreover, surfactant production generates toxic waste and their use endangers the ecosystem, due to their toxicity and persistence in the environment [3].
In this regard, microbial biosurfactants (mBS) started to be envisioned as an alternative to chemical surfactants, providing a greener solution with faster biodegradability and lower eco-toxicity impacts. Their structural diversity and properties may offer higher efficiency (especially when used in extreme conditions), and new application opportunities, namely as antimicrobials [4], antiviral [5], anticancer [6] and anti-inflammatory agents [7]. Currently, the mBS market is evaluated at US $13 million (2012) and comprises rhamnolipids, sophorolipids and mannosylerythritol lipids (MELs). Nevertheless, the price of the cheapest mBs (US $34/kg of SL) is tenfold higher than chemical surfactants such as sodium lauryl sulphate (~ US $1–2/kg) [8]. This indicates that scalable fermentations and downstream processes need to be optimized and technological innovations are needed to make their production cost to be competitive with chemical surfactants.
Specifically, mannosylerythritol lipids (MELs), mainly produced by Ustilago and Moesziomyces (formerly known as Pseudozymas) spp. [9] belong to the class of glycolipids and contain a 4-O-β-D-mannopyranosyl-meso-erythritol as the hydrophilic group, and short- and medium-chain fatty acids as the hydrophobic group. MELs were the last ones to enter in the market of mBs, being one of most promising, especially because of the low CMC (0.0027 mM), at a value tenfold lower than the ones characteristic for sophorolipids and rhamnolipids (0.12–0.30 mM) [10,11,12,13], and of their potential use in different applications, such as hair repair [14], biopesticide [15] or even as a food preservative due to its potential inhibitory effect in Staphylococcus aureus [16]. The highest MELs productivity reported in literature (12 g/L/day of crude MELs) resulted from a long fed-batch fermentation using large amounts of soybean oil (SBO) (around 186 g/L), D-glucose (around 50 g/L), 14 g/L of yeast extract (YE) and mineral medium (including sodium nitrate) [17]. Such MELs productivity is far from the highest productivity values reported for sophorolipids (3.7 g/L/h) [18], demonstrating the existence of bottlenecks in MEL production that needs to addressed to attain economic viability of MELs applications, while respecting high sustainability standards.
In this regard, attention should be directed to, among others, the cultivation media and substrates fed to MELs production fermentations. The use of an enriched medium in different nutrients is a constraint for the total price of the process (around 21% of total OPEX, operational expenditure) [19]. Specifically, the use of SBO as a main substrate can constitute a threat for food availability and prices. Note that SBO is being directly produced from food crops, requiring large arable land area for its cultivation [20].
Substrate and media component selection is, therefore, of paramount importance to develop a successful bioprocess. Substrate composition determines carbon source type, nitrogen type, C/N ratio, content on other elements (salts, metals) and presence of eventual inhibitory products. Therefore, while substrate availability can affect process sustainability, substrate composition will greatly impact on microbial production. Several agro-industrial residues previously considered as substrates for MEL production, including crude glycerol [21], sugarcane juice [22], coconut water [23], soap stock [24], residual honey [25] and wheat straw [26]. However, the use of such substrates often requires an additional substrate pre-treatment step, which contributes to increases the total production cost. Additionally, these additional steps may lead to the presence of potentially inhibitors to cell growth and MELs production (e.g. polyphenols or acids) and/or results on low MELs final titres [27].
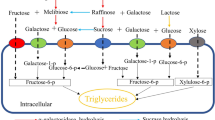
In a consolidated bioprocessing strategy (CBP), a single microorganism ensures enzyme production for substrate hydrolysis and bioconversion of released sugars into bio-based products. The natural or engineered microbial capacity of producing own cellulolytic and/or hemicellulolytic enzymes can improve the economy of the lignocellulose bioconversion processes by reducing and/or optimizing the use of commercial enzyme cocktails or even eliminating this significant operating cost in wastes/residues with complex carbon sources and its biorefining. In this regard, previous work has shown Moesziomyces spp. ability to produce MELs directly from xylan, due to the cellulase-free xylanases production, but not from the complete lignocellulosic material [26, 28, 29]. This ability of Moesziomyces spp. to produce a variety of enzymes to hydrolyse complex substrate structures makes them an interesting organism model to explore agro-industrial substrates with various compositions. Likewise, this study investigates the potential ability of Moesziomyces spp. to provide the tools to design CBPs based on D-lactose rich substrates, i.e. the production of enzymes able to hydrolyse lactose and ability to use not only D-Glucose but also D-galactose, the resulting sugar monomers, as carbon sources. Cheese whey (CW) is explored as a promising D-lactose rich alternative substrate. CW, a by-product of cheese production, is highly available as dairy industry, one of the major industrial effluents in Europe (40 million tons per year), and can be bioprocessed in different food applications [30, 31]. Moreover, CW discharge is restricted due to environmental concerns associated with high chemical oxygen demand (COD) and low biodegradability of the whey [32]. Along with a carbon source (D-lactose), CW composition includes minerals and other trace elements that may enrich a cultivation media for microbial production. This work reports the production of a class of promising biosurfactants, MELs, using two Moesziomyces yeasts, exploring their β-galactosidase production capacity. This brings new perspectives to the field of food by-products valorisation, while targeting biosurfactants production and their possible different biotechnological applications under circular economy concepts.
2 Materials and methods
2.1 Yeast strains, substrate, and cultivation conditions
Moesziomyces antarcticus PYCC 5048 T and M. aphidis PYCC 5535 T were obtained from the Portuguese Yeast Culture Collection (PYCC), CREM, FCT/UNL, Caparica, Portugal. Strains were plated in YM Agar (yeast extract 3 g/L, malt extract 3 g/L, peptone 5 g/L, D-glucose 10 g/L and agar 20 g/L) and incubated for 3 days at 25 °C. Stock cultures were prepared by propagation of yeast cells in liquid media described above for the inoculum and stored, in 20% (v/v) glycerol aliquots, at − 70 °C. An inoculum was prepared by transferring the stocks cultures of M. antarcticus and M. aphidis into an Erlenmeyer flask with 1/5 working volume (50 mL) of medium containing 0.3 g/L MgSO4, 3 g/L of NaNO3, 0.3 g/L KH2PO4, 1 g/L yeast extract (YE), 40 g/L D-glucose and incubating at 27 °C, 250 rpm, for 48 h. Then, 10% (v/v) of inoculum was added into an Erlenmeyer flask with 1/5 working volume (50 mL) of cultured medium. All cultures were carried out in biological duplicates and incubated at 27 °C, 250 rpm, for 10 days.
The culture medium consisted for a first set of assays on mineral medium (0.3 g/L MgSO4, 0.3 g/L KH2PO4 at initial pH 6.0) supplemented with 1 g/L YE, in the presence or absence of further nitrogen source (3 g/L NaNO3), using 40 g/L of different carbon sources, i.e. D-glucose, D-galactose or D-lactose. The concentration of 3 g/L of NaNO3 and 40 g/L of a hydrophilic sugar follows the reference value of previous studies [28, 33]. A second set of assays explore the use of cheese whey (CW) (rich in D-lactose) alone (without any mineral media or other supplement) vs. CW in the aforementioned mineral medium with YE and NaNO3. A final set of assays is started with D-glucose, D-lactose or CW as carbon source and addition of 20 g/L of waste fried oil (WFO), a lipid-rich source, in the presence of absence or the aforementioned mineral media with YE and NaNO3. The selection of concentration of 20 g/L of WFO follows the results of preliminary assays performed with D-glucose [34]. CW was initially pre-treated as followed: after received, and it was heated until 90 °C and kept at such temperature for 15 min, then centrifuged at 10,000 rpm for 10 min, the supernatant collected, and the pellet discarded. CW is not only a source of carbon but also of nitrogen, phosphorous, other mineral and trace elements. Characterization of CW before and after pasteurization is presented in Table S1. Cultivations were performed at least in two replicates.
2.2 Growth and biomass determination
Yeast growth was determined by measuring the cell dry weight (CDW), periodically, during fermentation time. CDW was determined from 1 mL of culture broth by centrifugation at 10,000 rpm for 6 min, followed by cell pellet washing with deionized water (twice) and drying at 60 °C for 48 h. The supernatant collected was used to determine substrate consumption, extracellular protein and extracellular β-galactosidase activity.
2.3 Enzyme activity assays
Extracellular and intracellular β-galactosidase activities were determined in 1 mL of culture broth, after centrifugation at 10,000 rpm for 6 min, and separation of the supernatant (used for extracellular activity and protein determination) and pellet. The pellet was washed with deionized water (twice) and used for intracellular activity determination, as described below.
Extracellular β-galactosidase activity was determined by following an enzymatic assay adapted from the one described by Karasová et al. [35]. The substrate used for the enzymatic assays was o-nitrophenyl-β-D-galactopyranoside (ONPG), and the activity was determined by measuring the release of o-nitrophenol from ONPG. All enzymatic activities were carried out in a 96-well plate, and the reaction mixture was composed by: 20 mM of ONPG dissolved in 50 mM acetate buffer (pH 5.2). To initiate the enzymatic assay, 90 µL of ONPG, 20 mM solution and 10 µL of the supernatant were added to the 96-well plate. The reaction mixture was incubated at 37 °C for 15 min and stopped by adding 200 µL of 10% (w/w) Na2CO3. The o-nitrophenol was measured at 420 nm in a microplate spectrophotometer (MultiskanTM GO, ThermoFisher Scientific). One unit (U) of β- galactosidase activity is defined as the amount of enzyme releasing 1 µmol o-nitrophenol per min. Intracellular β-galactosidase was determined after the incubation of washed cells with Y-PER™ (Yeast Protein Extraction Reagent, Pierce, Thermo Scientific, USA). The cell crude extract was used in the enzymatic assay, as described before. The lipolytic enzymatic assays were performed as previously described [36]. The substrate used for the enzymatic assays was p-nitrophenyl butyrate. All enzymatic activities were carried out in triplicates, in a 96-well plate, and the reaction mixture composition was 2.63 mM of p-nitrophenol butyrate dissolved in 50 mM acetate buffer (pH 5.2) and 4% of triton-X-100.
To initiate the enzymatic assay, 90 µL of p-nitrophenol butyrate 2.63 mM solution and 10 µL of the supernatants diluted was added to the 96-well plate. Then the reaction mixture was incubated at 37 °C for 15 min, and after that, the reaction was stopped by adding 200 µL of acetone. The released p-nitrophenol was quantified in a microplate spectrophotometer (MultiskanTM GO, ThermoFisher Scientific), at 405 nm. One unit (U) of lipase activity is defined as the amount of enzyme releasing 1 µmol p-nitrophenol per min.
2.4 Substrate and product quantification
The quantification of D-glucose, D-lactose and D-galactose was performed using HPLC. Culture broth samples were centrifuged at 10,000 g for 6 min, and the supernatants were filtered through a 0.22 µm-pore size-filter and injected into a HPLC system (Merck Hitachi, Darmstadt, Germany) equipped with a refractive index detector (L-7490, Merck Hitachi, Darmstadt, Germany) and an Rezex ROA organic acid H + column (300 mm × 7.8 mm, Phenomenex, Torrance, CA, USA), at 65 °C. An aqueous solution of sulphuric acid (5 mM) was used as mobile phase at 0.5 mL/min.
The consumption of oil (triacylglyceride – TAG) in the culture samples was also analysed by HPLC, as described by Badenes et al. [37], and 500 µL of each sample was retrieved and mixture with 58.5 mM of 1 µL acetic acid and 499 µL n-hexane. Then, it was centrifuged at 10,000 rpm for 2 min, and the organic phase was extracted and injected into another HPLC system (Hitachi LaChrom Elite), equipped with a Chromolith Performance RP-18 endcapped (100 mm × 4.6 mm × 2 µm) column, an auto sampler (Hitachi LaChrom Elite L-2200), a pump (Hitachi LaChrom Elite L-2130) and a UV detector (Hitachi LaChrom Elite L-2400) set up at 205 nm. The flow rate was set up at 1 mL/min and the injection volume was 20 µL. Three mobile phases were employed: phase A consisted of 100% acetonitrile, phase B consisted of water 100% and phase C comprising a mixture of n-hexane and 2-propanol (4:5, v/v). Quantification was carried out using calibration curves of glyceryl trioleate and glyceryl trilinoleate (> 98%, Sigma-Aldrich GmbH) for TAG.
MELs were quantified as previously described, through GC analysis of methyl esters generated by methanolysis of freeze-dried biological samples (1 mL) [26]. For the cases where beads enriched in MELs were observed and no representative sample could be retrieved, the total fermentation broth was extracted, using equal volumes of ethyl acetate to the broth volume in three successive extractions, the solvent evaporated in a rotavapor (Buchi R3, Switzerland) at 40 °C and 240 mbar, the bottom phase collected and weighted and 50 mg of it retrieved and submitted to methanolysis and GC analysis as previously described [26].
2.5 Statistical analysis
Statistics were performed, using graph-pad, by analysis of variance (two-way ANOVA) and p-values of the differences between groups were corrected for simultaneous hypothesis testing according to Tukey’s method. The level of significance was set at p ≤ 0.05.
3 Results and discussion
3.1 D-Lactose as a sole carbon and energy source for MELs production — the role of endogenous β-galactosidase
The ability of Moesziomyces spp. to grow and produce MELs using D-lactose (40 g/L) as a sole carbon source was evaluated for M. antarcticus PYCC 5048 T and M. aphidis 5535 T. In this regard, yeast cells were directly inoculated in media containing D-lactose. Mineral media with YE was used for these set of experiments. In addition, nitrogen source (3 g/L of NaNO3) supplementation was investigated (Fig. 1), since it is known that the absence of nitrogen promotes MELs production; however, its presence is crucial for amino acid synthesis and consequently enzyme production.

source profiles (lactose, galactose and glucose represented with inverted triangles, triangles and squares, respectively) (C, D); extracellular β-galactosidase profile activities (circles) (E, F). Standard deviations values lower than 1 g/L and 1 IU/mL are not represented
Cultivation of M. antarcticus PYCC 5048 T (A, C, E) and M. aphidis PYCC 5535.T (B, D, F) in D-lactose (40 g/L), mineral medium with YE and in the presence or absence of NaNO3 (dashed line and filled line, respectively), during 10 days at 27 °C. Yeast biomass (circles) and MELs production (squares) (A, B); carbon
Cell growth was monitored by the determination of the CDW. Both strains were able to grow using D-lactose as carbon source, regardless the nitrogen source supplementation (Fig. 1A and B). After 10 days of cultivation, cell biomass values of 18.0 ± 4.0 and 13.5 ± 1.5 g/L were obtained for M. antarcticus and M. aphidis, respectively, in the presence of nitrogen source. Interestingly, for both strains, there is not significant differences in the final cell biomass with or without nitrogen supplementation.
Comparing D-lactose consumption rate in both strains, M. aphidis cultivation stands out, with no differences regardless the nitrogen supplementation (0.38 ± 0.02 and 0.39 ± 0.04 g/L/h, without and with nitrogen supplementation, respectively) (Fig. 1D). In M. antarcticus, an increase on sugar consumption rate was observed with nitrogen supplementation, from 0.24 ± 0.00 to 0.32 ± 0.02 g/L/h (Fig. 1C), but still lower than the ones observed in M. aphidis cultivations and with an adaptation to the substrate (D-lactose) observed in the first day of cultivation, where only the residual D-glucose present in the cultivation media seems to be consumed. It is important to notice that although the relatively high values reported for the D-lactose consumption rate, D-galactose slightly accumulates in the broth. In M. aphidis cultivation without nitrogen supplementation, more than 10 g/L of D-galactose was accumulated at day 4, which were consumed until the end of the cultivation (10 days). In the case of M. antarcticus, a maximum of 6 g/L of D-galactose was accumulated at day 7, but not fully consumed, with 4 g/L of D-glucose and 4 g/L of D-galactose left in the end of the cultivation. With nitrogen supplementation, there was no D-galactose accumulated at day 7 and 10 of M. antarcticus cultivation, after an accumulation of 10.6 g/L at day 4.
Direct MEL production from D-lactose was observed in all conditions tested. Interestingly, although a lower substrate consumption rate reported above, the cultivation conditions using no supplementation of inorganic nitrate source (NaNO3) rendered higher MELs titres, and analogous effects of addition of NaNO3 on MEL production by these yeasts have been previously reported for D-glucose and D-xylose [29]. Similar MEL titres were achieved for the two yeasts after 4 days of culture. However, after 10 days cultivation, at 27 °C and 250 rpm, M. antarcticus reached 4.93 ± 0.53 g/L and M. aphidis, 2.20 ± 0.10 g/L. With NaNO3 supplementation, the final MELs titre was 2.93 ± 0.53 and 0.15 ± 0.02 g/L for M. antarcticus and M. aphidis, respectively (where maximum MELs titre on M. aphidis cultivation was reached at day 4, a maximum of 1.91 ± 0.01 g/L) (Fig. 1A and B). This discrepancy on MEL production for these two particular Moesziomyces strains was previously observed, while M. antarcticus seems to be a more efficient MEL producer from D-glucose and D-xylose, and M. aphidis was reported to accumulate more storage lipids and be slower on their use for MEL production [29]. Again, when these yeasts were fed with xylan and the xylanolytic capability of these yeasts was required for hydrolyse of the xylan into D-xylose, M. antarcticus shows to be a more efficient MEL producer [38]. In this study, both strains are able to produce β-galactosidase (Fig. 1E and F), with higher β-galactosidase extracellular activity obtained when additional nitrogen source is added. Still, even without the addition of NaNO3, the enzymatic activity seems to be high enough to promote D-lactose hydrolysis into its monomers D-galactose and D-glucose, which are consumed to support cell growth and MEL production. The high standard deviations found on the β-galactosidase extracellular activity profiles for M. aphidis, (40 g/L of lactose, without NaNO3) are indicative of culture behaviour variability as enzyme production was halt after 1 day in culture for one of the replicates (Fig. S7).
The maximum MEL titres using D-lactose were about 1.8-fold lower, for both strains, when compared to previous studies using the same strains cultivated in D-glucose; 4.9 and 3.4 g/L of MELs were reported for M. antarcticus and M. aphidis, respectively, in similar C/N ratio [29]. Nevertheless, higher biomass values were observed when D-lactose was used (1.7- and 1.2-fold higher, respectively). These results may indicate that the cell is dispending more energy to hydrolyse lactose, which is reflected in the use of more carbon source for production of building blocks, enzymes and biomass, rather than MEL.
3.2 Characterization of β-galactosidase profiles using different substrates
The hydrolytic potential of M. antarcticus and M. aphidis, regarding extracellular β-galactosidase activity, was evaluated. Following the trends observed for D-lactose consumption (Fig. 1C and D), supplementation of nitrogen source resulted in higher β-galactosidase activity, 2.6- and 4.2-fold higher than for cultures without NaNO3 supplementation, for a maximum of 44.5 ± 3.3 and 37.5 ± 10 IU/mL, obtained with M. antarcticus and M. aphids, respectively.
Previous studies with M. antarcticus and M. aphidis have shown that the detection of enzymatic activities is highly dependent on the substrate used. Lipase activity can be detected when M. antarcticus was grown on D-glucose [39], while xylanase activity was not detected, but only when cultured in pentose-based sugars [28]. In this regard, the induction of β-galactosidase activity by using the D-lactose monomers, D-glucose and D-galactose (separated and mixed) was evaluated, supplementing the medium with nitrogen source (3 g/L of NaNO3) (Fig. 2 and Table S3). The results of D-galactose cultivation for both strains, without addition of NaNO3, are presented as supplementary data (Fig. S1 and Table S2). Analysing the values obtained, we observed that when D-galactose and D-glucose were mixed, the volumetric activity values were similar to the ones obtained with D-lactose (around 30–50 IU/mL) (Fig. 2 and Table S3). Surprisingly, when D-galactose was used as a sole carbon source, the highest volumetric activity was obtained, 12.8- and 8.3-fold higher than the ones obtain for cultures using D-lactose as substrate, reaching a maximum of 505.2 ± 3.1 and 127 ± 31.2 IU/mL, for M. antarcticus and M. aphidis cultures, respectively. Even though a positive effect of D-galactose was observed for β-galactosidase activity, it seems that this substrate does not promote extensive yeast growth, as the maximum biomass reached to such cultures was 1.8-fold lower when than the one obtained for D-lactose based cultures (Tables S3 and S4). In the presence of both monomers (D-galactose and D-glucose), the values of biomass were comparable with D-lactose cultivations.

source profiles (D-glucose and D-galactose consumption represented with squares and triangles, respectively) and β-galactosidase production (circles) are shown. Standard deviation lower values than 1 g/L and 10 IU/mL (or 1 IU/mL for inserted figures) are not represented
Cultivation of M. antarcticus PYCC 5048 T (A, C) and M. aphidis PYCC 5535.T (B, D) in D-galactose (40 g/L) (A, B) and a mixture of D-glucose (20 g/L) with D-galactose (20 g/L) in mineral medium with YE and NaNO3 (C, D), during 10 days at 27 °C. For all graphics the patterns of yeast biomass (circles), carbon
In fact, both strains had shown different patterns regarding sugar consumption, when D-glucose and D-galactose were used as carbon source. Sugar consumption trends in M. aphidis cultivations were similar regardless the substrate used. In fact, both D-glucose and D-galactose were fully consumed at day 4 of cultivation. M. antarcticus had shown a preference for D-glucose, and at day 4 of fermentation, while most of D-glucose is consumed, only 5 g/L of D-galactose was consumed.
In this regard, the fast consumption rate of D-lactose and limited D-galactose accumulation in the media contrasts with the lower consumption rate of D-galactose, when used as sole carbon source (Figs. 1 and 2). Therefore, the presence of additional β-galactosidase mechanisms (other than extracellular) was investigated. Namely, intracellular β-galactosidase activity was estimated in samples collected at days 2 and 4 of cultivation of M. antarcticus and M. aphidis using D-lactose, D-galactose and mixtures of D-glucose and D-galactose (Fig. 3, Table S5). Notable, all the conditions showed the presence of significant intracellular β-galactosidase activity, but at day 4, its values were lower than the extracellular β-galactosidase activity, with exception for M. antarcticus cultures on D-galactose (Fig. 3C). When D-galactose was used as substrate, initially at day 2 of fermentation (Fig. 3A and B, Table S5), the intracellular and extracellular volumetric activity were, respectively, 8- and 18-fold lower for M. antarcticus (Fig. 3A) than for M. aphidis cultivations (Fig. 3B), but at day 4 of fermentation (Fig. 3C and D, Table S5), the intracellular specific activity for M. antarcticus became fivefold higher than the values obtained with M. aphidis. The volumetric activities values obtained for cultures using D-lactose as carbon source or its monomers mixture were similar among them and significantly lower than the ones obtained when using D-galactose as sole carbon source. However, for day 2 in M. antarcticus, the intracellular volumetric activity was virtually 0 (0.03 ± 0.02 IU/mL) for mixture of monomers cultures, and, although still low, it was two orders of magnitude higher (1.2 ± 0.2 IU/mL) in D-lactose cultures. These results may indicate that the induction of β-galactosidase is promoted by the metabolites driven from metabolization of D-galactose in both strains, which explains the low activities when it is used D-lactose and the monomers.
Intracellular (grey) and extracellular (black) β-galactosidase activity determined at days 2 (A, B) and 4 (C, D) for M. antarcticus PYCC 5048 T (A, C) and M. aphidis PYCC 5535.T (B, D) cultivated in 40 g/L of D-galactose, 40 g/L of D-lactose and 20 g/L of D-galactose and 20 g/L of D-glucose as carbon sources in mineral medium with YE and NaNO3. *p ≤ 0.05; **p ≤ 0.003; ****p < 0.0001
From our literature search, the only species, more closely related with the strains used in this work, that is able to produce β-galactosidase in presence of galactose was Hypocrea jecoina (Basidiomycota). Fekete et al. [40] have shown that the ability of this strain to grow in D-lactose is strongly dependent on the formation of an extracellular glycoside hydrolase (6H) family 35 β-galactosidase encoded by Baga1 gene. Additionally, the authors also had shown the importance of D-galactose degradation into galactitol, to induce β-galactosidase expression. In this regard, it was performed a Blast-P (computational tool used to compare different proteins, provided by NCBI – National Centre for Biotechnology Information) of this protein from H. jacoina (glycoside hydrolase (6H) family 35 β-galactosidase) against M. antarcticus and M. aphidis sequences. A glycosidase hydrolase, family GH35 and a hypothetical protein PaG_04781 were found (93% of coverage for both), for M. antarcticus and M. aphidis, respectively. The protein found for M. antarcticus belongs to a family of hydrolases (GH1, GH2, GH35 and GH42) [41]. From those hydrolases, GHx and GHy have β-galactosidase activity. The discovery that this type of proteins can also be expressed in M. aphidis has the potential to identify a new β-galactosidase or β-galactosidase producer.
Until date, the highest specific activity reported in literature for β-galactosidase is 4.2 IU/mgbiomass (obtained after cell permeabilization with isoamyl alcohol), using Kluvyromyces lactis [42], one of the yeast industrially used for the production of the enzyme. Using D-lactose, the same substrate used in the study of K. lactis, a specific activity of 5.1 ± 0.8 IU/mgbiomass, with M. antarcticus, is achieved, representing 44.5 ± 3.3 IU/mL of volumetric activity. Remarkably, using M. antarcticus and D-galactose as carbon source, it was achieved the value for β-galactosidase specific activity, at day 4, considering intracellular activity (57.2 ± 15.5 IU/mgbiomass, and 525.9 ± 16.3 IU/ml), and the highest β-galactosidase activity reported in this work, extracellular, at day 7, reaching 63.1 ± 6.1 IU/mg (505.2 ± 3.1 IU/ml), which is 15-fold higher than the reported value (4.2 IU/mgbiomass).
3.3 Exploring Moesziomyces spp. as MELs producer from renewable substrates
Moesziomyces antarcticus and M. aphidis are described as MEL producers. Both strains can use a variety of substrates, including sugar-based carbon sources, such as D-glucose [38] or D-xylose [29]. Interestingly, in this work, MEL production efficiency from D-lactose (4 g/L, Fig. 1) was similar to the one observed when D-glucose was used as carbon source, highlighting the efficient hydrolytic capacity of both M. antarcticus and M. aphidis. Cheese whey, a D-lactose-rich by-product of dairy products (see Table S1 for detailed composition of CW), was explored as a complex cultivation media (mineral medium, YE and NaNO3) for M. antarcticus and M. aphidis cultivations, first alone, and then in combination with WFO, a lipidic rich carbon source.
3.3.1 Direct conversion of cheese-whey into MELs
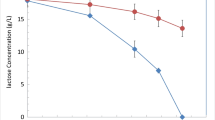
The use of CW as sole nutrient source was first assessed against conditions where CW was used in mineral media with YE (Fig. 4 and Table S6). The experiments were carried out during 10 days at 27 °C and 250 rpm, using CW (30% v/v), corresponding to a 40 g/L of D-lactose in the cultivation media. Both yeasts were able to grow in CW (Fig. 4A and B), but slower when compared with cultures on refined D-lactose in mineral media with YE (Fig. 1). However, only M. antarcticus produced lower biomass when grown in CW if compared with D-lactose (11.5 and 11.0 g/L, Table S6, using CW as sole media component or in mineral media with YE, respectively, compared to 18.0 g/L, Table S4, of biomass when using D-lactose in mineral media with YE).
D-lactose consumption rate was higher in M. antarcticus than M. aphidis when cultured in CW (0.41 and 0.32 g/L/h, respectively) (Fig. 4C and D). However, the direct use of CW alone (without mineral media nor YE supplementation) reduced D-lactose consumption rate in M. antarcticus cultivations (from 0.41 to 0.25 g/L/h) (Table S6). Also, an accumulation of D-galactose was observed, reaching 10 g/L of D-galactose at day 4. In opposition, in M. aphidis cultivations, the accumulation of D-glucose and D-galactose was not observed (Fig. S4). The emphasized efficient mechanisms for lactose assimilation were verified, especially in M. aphidis with CW, even on the absence of addition of mineral media and YE addition. The hydrolytic potential of M. antarcticus and M. aphidis was evaluated regarding the extracellular β-galactosidase activity (Fig. 4C and D). Interestingly, in spite of CW do not present measurable D-galactose, β-galactosidase activity using this substrate was estimate to be tenfold higher than the one for D-lactose cultures. Therefore, enzyme production by the yeast and activity may benefit from other compounds that not D-galactose, such as additional nitrogen, trace elements and ions potentially provided by the CW (Table S1). Also, the use of mineral media induces higher extracellular β-galactosidase activity, for both strains, but more pronounced in M. antarcticus, at day 4 (305.2 IU/mL).
Cultivation of M. antarcticus PYCC 5048 T (A, C) and M. aphidis PYCC 5535.T (B, D) in CW (40 g/L), during 10 days at 27 °C in the presence and absence of mineral medium with YE (dashed line and filled line, respectively), on both cases with addition of NaNO3. Yeast biomass (circles) and MELs production (squares) (A, B); Carbon source (inverted triangles) and extracellular β-galactosidase activities profiles (circles) (C, D). The D-glucose and D-galactose monomers profiles are represented in Fig. S4, in supplementary data. Standard deviations values lower 1 g/L are not represented
The direct MEL production from CW was observed (Fig. 4A and B). Although the lower D-lactose consumption rate in cultivation of M. antarticus using CW without addition of mineral media and YE, MEL production was higher in such conditions (1.94 g/L), while the use of M. aphidis rendered the lowest MELs value, 0.77 g/L. Nevertheless, MEL obtained from CW cultures (without mineral media nor YE) was consistently lower if compared with the use of D-lactose as carbon source in mineral medium with YE (Table S4). The carbon chain length of MELs acyl groups obtained was similar to the ones found both in D-lactose and D-glucose, mainly composed of C10:n and C12:n (data not shown).
Considering the fact that 65% of world population are lactose intolerants, β-galactosidase plays a crucial role in dairy industry by producing lactose-free products, a market in expansion with a CAGR (compound annual growth rate) of 11% and evaluated at US $4.69 billion in 2015 (pre-COVID-19) [43, 44]. In this regard, these results achieved with M. antarcticus, especially the values achieved when using CW as sole carbon source and medium component (137.54 ± 10.42 IU/mL), can open new perspectives and studies for this type of industry.
3.3.2 Towards sustainable MELs production — combination of CW with waste frying oil (WFO)
CW was successfully assessed as sole media component for MEL production. Nevertheless, as observed for other sugar-based carbon sources, MEL production was rather limited. MEL productivities can be enhanced through addition of a relatively small amount of a lipid rich carbon source; however, such strategy relies on the lipolytic activity of Moesziomyces spp. Therefore, the enzymatic activity of lipases for D-glucose, D-galactose, D-lactose and CW (Fig. S2), in the absence or presence of mineral media supplemented with YE and NaNO3, as well a mixture of D-galactose and D-glucose in mineral media with YE and NaNO3 (Fig. S3) was determined. The results obtained showed lipase activity profiles for the cultures on CW and on D-glucose to be very close.
Three carbon sources D-glucose (40 g/L), D-lactose (40 g/L) or CW (40 g/L in D-lactose) were assessed in combination with WFO (20 g/L), with or without mineral media, YE nor NaNO3 supplementation, for both M. antarcticus and M. aphidis. The results are resumed on Table 1. Biomass, MEL production, sugar, and residual lipids profiles are represented for cultures using WFO combined with CW (Fig. 5) or refined sugars (Figs. S5 and S6), either in the presence (Fig. 5A and B) or in the absence (Fig. 5C and D) of mineral medium, YE and NaNO3.
Cultivation of M. antarcticus PYCC 5048 T (A, C) and M. aphidis PYCC 5535 T (B, D) in CW (40 g/L) and WFO (20 g/L), during 10 days at 27 °C, in the absence (A, B) and presence of mineral medium with YE and NaNO3 (C, D). For all graphics the patterns of yeast biomass (circles) and MELs production, D-lactose (inverted triangles) and residual lipids (driven from WFO, crosses) consumption are shown. The red point indicates the appearance of beads enriched in MEL and residual lipids. Standard deviation values lower 1 g/L are not represented
As expected, when following the strategy to combine WFO and D-glucose or D-lactate, the use of mineral medium with YE supplementation was crucial to obtain high MELs productivity (Fig. S5). Indeed, when the culture medium was not supplemented (Fig. S6), D-lactose and D-glucose are not consumed, while a fraction of WFO lipids are consumed and MELs were produced, but at low concentrations (5.41 ± 0.15 and 2.53 ± 0.02 for M. antarcticus and M. aphidis).
Remarkable, high MEL productivities were reached using CW and WFO alone, without using mineral media nor YE. Such values definitively represent a significant increase in the product yield and productivities, when compared with the sole CW utilization (Table S6). Impressively, the level of MELs production using only CW and WFO (titres of 13.98 ± 0.06 g/L and 13.10 ± 1.77 g/L and productivities of 1.40 ± 0.01 and 1.31 ± 0.18 g/L/h for M. antarcticus PYCC 5048 T and M. aphidis PYCC 5535 T), close to the ones obtained using D-glucose in mineral media with YE supplementation (titres of 19.21 ± 0.02 g/L and 15.02 ± 0.99 g/L and productivities of 1.92 ± 0.24 and 1.50 ± 0.10 g/L/h for M. antarcticus PYCC 5048 T and M. aphidis PYCC 5535 T), representing a potential route to circumvent the use of refined or expensive substrates, including D-glucose, NaNO3, MgSO4, KH2PO4 or YE. The co-utilization of WFO seems to positively impact on D-lactose consumption for cultures with CW (Fig. 5 vs. Figure 4) or D-lactose (Fig. 5A and B vs Fig. 1.), emphasizing the role of CW as a media substitute.
Although M. antarcticus cultivations using CW and WFO reach similar maximum MELs titres (13–14 g/L) regardless the conditions tested (Table 1), very different cultivation profiles were observed (Fig. 5). Noteworthy, the cultivations in CW and WFO with mineral media and YE supplementation reached the highest MELs at day 7 (12.63 g/L), with a decrease after that time point (Fig. 5C) while in cultivations with no supplementation, the highest MELs value (13.98 g/L) is obtained only at day 10 (Fig. 5A). The decrease observed for MELs after day 7, in conditions using the culture medium, may result from the depletion of carbon sources, and consequent starvation of M. antarcticus and M. aphidis, suggesting energy storage as a possible biological function for MEL.
The values obtained for biomass at day 1 were higher for cultures of CW and WFO alone, i.e. without mineral media and YE supplementation, but no significant further increases in cell biomass over time (except between days 7 and 10, Fig. 5A) were observed. When mineral media with YE was added, biomass increased until day 4, where it reached its highest value (31.5 g/L). Another interesting achievement was the faster consumption of D-lactose and lipids observed for M. antarcticus cultivations supplemented with mineral media and YE, where after 1 day of cultivation, less than 10 g/L of D-lactose was present (Fig. 5C vs. Figure 5A). Moesziomyces aphidis cultivations followed the same trends observed to M. antarcticus for the same conditions, but with lower sugar and lipids consumption. Importantly, when M. aphidis is cultivated in CW and WFO in mineral media and YE, the maximum MELs is produced early, at day 4, but at a lower value of 8.93 g/L, while cultures without mineral media or YE supplementation reached a MELs titre of 13.1 g/L MELs, but only at day 10.
Until now, most of the studies performed to optimize MELs production required a complex mixture of nutrients that increase the final cost of the process. For example, Beck A and Zibek S [45] have performed a study testing different medium components, aiming to achieve fast biomass growth (around 0.16–25 h−1), without affecting MEL production. However, medium formulation continues to be a bottleneck in the fermentation process, as the use of cofactors and vitamins increase the process complexity and final manufacture cost. Furthermore, in most of the studies using renewable substrates, there is the need to continuous supplement together the carbon source and medium components (such as YE, mineral salts), representing additional cost increases.
Production of biosurfactants from CW by probiotic bacteria was previously reported [46, 47]. However, so far, there is only one study in literature, reporting the production of MELs using CW, although, in such study is reported the pre-treated of CW with a commercial β-galactosidase [24]. Here, for the first time, it is observed the capacity of Moesziomyces spp. to produce β-galactosidase, and consequently, it is reported the production of MELs using only CW or using two industrial residues (CW and WFO), without using any pretreatment, mineral medium nor YE. These results are very promising, since they show the capacity of CW to replace the whole medium, allowing to overcome one of the major bottlenecks in industrial MELs production. In this regard, more studies are being conducted, especially using fed-batch fermentation in bioreactors to increase MEL titres.
4 Conclusions
Remarkably, it was observed the capacity of M. antarcticus 5048 T and M. aphidis 5535 T to produce β-galactosidase from D-lactose and D-galactose. The highest extracellular β-galactosidase activity was observed when D-galactose was used as sole carbon source, 60.3 and 11.2 IU/mgbiomass, using M. antarcticus 5048 T and M. aphidis 5535 T, respectively.
Cheese whey, one the major residues produced in Europe, is rich in lactose. Considering the discover that the efficient MEL producers, Moesziomyces spp., are also able to produce β-galactosidase, CW was assessed as carbon source. CW was used as sole media component, directly used with no further addition of any other organic nor inorganic media component. Furthermore, when CW was used alone with supplementation of 20 g/L of WFO, a lipidic-rich substrate to boost MELs production, productivities of 1.3 and 1.4 g/L.day−1 for M. aphidis 5535 T and M. antarcticus 5048 T were obtained, respectively. These productivity values are only 13–26% lower than the ones previously obtained for an optimized medium using refined D-glucose, WFO, YE and inorganic sources of nitrogen and phosphate. Those are promising results on further bioprocess development due to the potential to perform cultivations using low-cost and renewable raw materials instead of complex media with refined components, solving one of the current bottlenecks for sustainable microbial surfactants production.
References
Seymour KG (1979) Surfactants and interfacial phenomena. J AOAC Int 62:700–700. https://doi.org/10.1093/jaoac/62.3.700
MarketsandMarketsTM, Surfactants market by application & type - global forecast 2021, 2020. https://www.marketsandmarkets.com/Market-Reports/biosurfactants-market-493.html (accessed May 15, 2020).
Ying G-G (2006) Fate, behavior and effects of surfactants and their degradationproducts in the environment. Environ Int 32:417–431
Ceresa C, Hutton S, Lajarin-Cuesta M, Heaton R, Hargreaves I, Fracchia L, De Rienzo MAD (2020) Production of mannosylerythritol lipids (MELs) to be used as antimicrobial agents against S. aureus ATCC 6538. Curr. Microbiol. 77:1373–1380. https://doi.org/10.1007/s00284-020-01927-2
Gross RA, Shah V. WO2007130738A1 - Anti-herpes virus properties of various forms of sophorolipids - Google Patents, 2006. https://patents.google.com/patent/WO2007130738A1/en (accessed July 13, 2020)
Chen J, Song X, Zhang H, Qu YB, Miao JY (2006) Sophorolipid produced from the new yeast strain Wickerhamiella domercqiae induces apoptosis in H7402 human liver cancer cells. Appl Microbiol Biotechnol 72:52–59. https://doi.org/10.1007/s00253-005-0243-z
Morita Y, Tadokoro S, Sasai M, Kitamoto D, Hirashima N (1810) Biosurfactant mannosyl-erythritol lipid inhibits secretion of inflammatory mediators from RBL-2H3 cells. Biochim Biophys Acta - Gen Subj 2011:1302–1308. https://doi.org/10.1016/j.bbagen.2011.07.002
Dolman BM, Wang F, Winterburn JB (2019) Integrated production and separation of biosurfactants. Process Biochem 83:1–8. https://doi.org/10.1016/j.procbio.2019.05.002
Marchant R, Banat IM (2012) Biosurfactants: a sustainable replacement for chemical surfactants? Biotechnol Lett. https://doi.org/10.1007/s10529-012-0956-x
Lang S, Wullbrandt D (1999) Rhamnose lipids - biosynthesis, microbial production and application potential. Appl Microbiol Biotechnol 51:22–32. https://doi.org/10.1007/s002530051358
Konoshi M, Fukuoka T, Morita T, Imura T, Kitamoto D (2008) Production of new types of sophorolipids by Candida batistae. J Oleo Sci 57:359–369. https://doi.org/10.5650/jos.57.359
Imura T, Ohta N, Inoue K, Yagi N, Negishi H, Yanagishita H, Kitamoto D (2006) Naturally engineered glycolipid biosurfactants leading to distinctive self-assembled structures. Chem - A Eur J 12:2434–2440. https://doi.org/10.1002/chem.200501199
Kitamoto D, Isoda H, Nakahara T (2002) Functions and potential applications of glycolipid biosurfactants–from energy-saving materials to gene delivery carriers. J Biosci Bioeng 94:187–201. https://doi.org/10.1263/jbb.94.187
Morita T, Kitagawa M, Yamamoto S, Sogabe A, Imura T, Fukuoka T, Kitamoto D (2010) Glycolipid biosurfactants, mannosylerythritol lipids, repair the damaged hair. J Oleo Sci 59:267–272. https://doi.org/10.5650/jos.59.267
Fukuoka T, Yoshida S, Nakamura J, Koitabashi M, Sakai H, Abe M, Kitamoto D, Kitamoto H (2015) Application of yeast glycolipid biosurfactant, mannosylerythritol lipid, as agrospreaders. J Oleo Sci 64:689–695. https://doi.org/10.5650/jos.ess15017
Shu Q, Wei T, Lu H, Niu Y, Chen Q (2020) Mannosylerythritol lipids: dual inhibitory modes against Staphylococcus aureus through membrane-mediated apoptosis and biofilm disruption, Appl Microbiol Biotechnol Lipids Dual Inhib Modes against Staphylococcus Aureus through Membr. Apoptosis Biofilm Disrupt. 104:5053–5064. https://doi.org/10.1007/s00253-020-10561-8
Rau U, Nguyen LA, Roeper H, Koch H, Lang S (2005) Fed-batch bioreactor production of mannosylerythritol lipids secreted by Pseudozyma aphidis. Appl Microbiol Biotechnol 68:607–613. https://doi.org/10.1007/s00253-005-1906-5
Gao R, Falkeborg M, Xu X, Guo Z (2013) Production of sophorolipids with enhanced volumetric productivity by means of high cell density fermentation. Appl Microbiol Biotechnol 97:1103–1111. https://doi.org/10.1007/s00253-012-4399-z
Dhanarajan G, Ramkrishna S (2014) Cost analysis of biosurfactant production from a scientist’s perspective. In: Biosurfactants Prod. Util. Technol. Econ., pp. 153–160
Anto S, Mukherjee SS, Muthappa R, Mathimani T, Deviram G, Kumar SS, Verma TN, Pugazhendhi A (2020) algae as green energy reserve: technological outlook on biofuel production. Chemosphere 242:125079. https://doi.org/10.1016/j.chemosphere.2019.125079
Liu Y, Koh CMJ, Ji L (2011) Bioconversion of crude glycerol to glycolipids in Ustilago maydis. Bioresour Technol 102:3927–3933. https://doi.org/10.1016/j.biortech.2010.11.115
Morita T, Ishibashi Y, Hirose N, Wada K, Takahashi M, Fukuoka T, Imura T, Sakai H, Abe M, Kitamoto D (2011) Production and characterization of a glycolipid biosurfactant, mannosylerythritol lipid B, from sugarcane juice by Ustilago Scitaminea NBRC 32730. Biosci Biotechnol Biochem 75:1371–1376. https://doi.org/10.1271/bbb.110221
Madihalli C, Sudhakar H (2020) · Mukesh Doble, Production and investigation of the physico-chemical properties of MEL-A from glycerol and coconut water. World J Microbiol Biotechnol 36:88. https://doi.org/10.1007/s11274-020-02857-8
Dzięgielewska E, Adamczak M (2013) Evaluation of waste products in the synthesis of surfactants by yeasts. Chem Pap 67:1113–1122. https://doi.org/10.2478/s11696-013-0349-1
Bhangale A, Wadekar S, Kale S, Pratap A (2013) Optimization and monitoring of water soluble substrate for synthesis of mannosylerythritol lipids by Pseudozyma antarctica (ATCC 32657), Biotechnol. Bioprocess Eng 18:679–685. https://doi.org/10.1007/s12257-012-0647-4
Faria N, Santos M, Ferreira C, Marques S, Ferreira F, Fonseca C (2014) Conversion of cellulosic materials into glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma spp. under SHF and SSF processes. Microb. Cell Fact. 13:155. https://doi.org/10.1186/s12934-014-0155-7
Santos MV, Faria NT, Fonseca C, Ferreira FC (2019) Production of mannosylerythritol lipids from lignocellulose hydrolysates: tolerance thresholds of Moesziomyces antarcticus to inhibitors. J Chem Technol Biotechnol 94:1064–1072. https://doi.org/10.1002/jctb.5853
Faria NT, Marques S, Ferreira FC, Fonseca C (2019) Production of xylanolytic enzymes by Moesziomyces spp. using xylose, xylan and brewery’s spent grain as substrates. N. Biotechnol. 49:137–143. https://doi.org/10.1016/j.nbt.2018.11.001
Faria NT, Santos MV, Fernandes P, Fonseca LL, Fonseca C, Ferreira FC (2014) Production of glycolipid biosurfactants, mannosylerythritol lipids, from pentoses and d-glucose/d-xylose mixtures by Pseudozyma yeast strains. Process Biochem 49:1790–1799. https://doi.org/10.1016/j.procbio.2014.08.004
Zotta T, Solieri L, Iacumin L, Picozzi C, Gullo M (2020) Valorization of cheese whey using microbial fermentations. Appl Microbiol Biotechnol 104:2749–2764. https://doi.org/10.1007/s00253-020-10408-2
Lappa IK, Papadaki A, Kachrimanidou V, Terpou A, Koulougliotis D, Eriotou E, Kopsahelis N et al (2019) Cheese whey processing: integrated biorefinery concepts and emerging food applications. Foods 8:8–15. https://doi.org/10.3390/foods8080347
Smithers GW (2008) Whey and whey proteins-from “gutter-to-gold.” Int Dairy J 18:695–704. https://doi.org/10.1016/j.idairyj.2008.03.008
Morita T, Konishi M, Fukuoka T, Imura T, Kitamoto D (2007) Physiological differences in the formation of the glycolipid biosurfactants, mannosylerythritol lipids, between Pseudozyma antarctica and Pseudozyma aphidis. Appl Microbiol Biotechnol 74(2):307–315
Nascimento M (2017) Mannosylerythritol lipids bioproduction by Moesziomyces spp.: assessing alternative culture strategies and nanofiltration downstream purification. MSc Thesis, Instituto Superior Técnico
Karasová P, Spiwok V, Malá Š, Králová B, Russell NJ (2018) Beta-galactosidase activity in psychrotrophic microorganisms and their potential use in food industry. Czech J Food Sci 20:43–47. https://doi.org/10.17221/3508-cjfs.
Gomes N, Gonçalves C, García-Román M, Teixeira JA, Belo I (2011) Optimization of a colorimetric assay for yeast lipase activity in complex systems. Anal Methods. 3:1008. https://doi.org/10.1039/c0ay00680g
Badenes SM, Lemos F, Cabral JMS (2010) Assessing the use of cutinase reversed micellar catalytic system for the production of biodiesel from triglycerides. J Chem Technol Biotechnol 85:993–998. https://doi.org/10.1002/jctb.2393
Faria NT, Marques S, Ferreira FC, Fonseca C (2015) Direct xylan conversion into glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma antarctica PYCC 5048T. Enzyme and Microb Technol 71:58–65. https://doi.org/10.1016/j.enzmictec.2014.10.008
Morita T, Konishi M, Fukuoka T, Imura T, Kitamoto D (2007) Microbial conversion of glycerol into glycolipid biosurfactants, mannosylerythritol lipids, by a basidiomycete yeast, Pseudozyma antarctica JCM 10317T. J Biosci Bioeng 104:78–81. https://doi.org/10.1263/jbb.104.78
Fekete E, Karaffa L, Kubicek CP, Szentirmai A, Seiboth B (2007) Induction of extracellular β-galactosidase (Bga1) formation by D-galactose in Hypocrea jecorina is mediated by galactitol. Microbiology 153:507–512. https://doi.org/10.1099/mic.0.2006/001602-0
Lombard V, GolacondaRamulu H, Drula E, Coutinho PM, Henrissat B (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42:D490–D495. https://doi.org/10.1093/nar/gkt1178
Dagbagli S, Goksungur Y (2008) Optimization of β-galactosidase production using Kluyveromyces lactis NRRL Y-8279 by response surface methodology. Electron J Biotechnol 11. https://doi.org/10.2225/vol11-issue4-fulltext-12
Saqib S, Akram A, Halim SA, Tassaduq R (2017) Sources of β-galactosidase and its applications in food industry. 3 Biotech 7. https://doi.org/10.1007/s13205-017-0645-5
J. (Technavio) Maida, Rising Levels of lactose intolerance predicted to propel the global lactose free food market until 2020, says Technavio | Business Wire, Businesswire. (2016) 1. https://www.businesswire.com/news/home/20160504005106/en/Rising-Levels-Lactose-Intolerance-Predicted-Propel-Global (accessed May 5, 2020).
Beck A, Zibek S (2020) Growth behavior of selected ustilaginaceae fungi used for mannosylerythritol lipid (MEL) biosurfactant production – evaluation of a defined culture medium. Front Bioeng Biotechnol 8. https://doi.org/10.3389/fbioe.2020.555280.
Rodrigues LR, Teixeira JA, Oliveira R (2006) Low-cost fermentative medium for biosurfactant production by probiotic bacteria. Biochem Eng J 32:135–142. https://doi.org/10.1016/j.bej.2006.09.012
Kachrimanidou V, Alimpoumpa D, Papadaki A, Lappa I, Alexopoulos K (2022) Cheese whey utilization for biosurfactant production: evaluation of bioprocessing strategies using novel Lactobacillus strains. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02767-9
Acknowledgements
This work is financed by national funds from FCT—Fundação para a Ciência e a Tecnologia, I.P., through iBB (UIDB/04565/2020 and UIDP/04565/2020), i4HB (LA/P/0140/2020) and the PhD grant (SFRH/BD/137007/2018). The authors also acknowledge the Portuguese Yeast Culture Collection (PYCC), UCIBIO/Requimte, FCT/UNL and Lacticínios Paiva SA for providing the yeast strains and the CW, respectively.
Author information
Authors and Affiliations
Contributions
MFN — conceptualization, methodology, validation, formal analysis, investigation; data curation, writing — original draft; RB — investigation; ACO — investigation; NTF — conceptualization, methodology, validation, data curation, writing — review and editing; FCF — conceptualization, writing — review and editing, funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nascimento, M.F., Barreiros, R., Oliveira, A.C. et al. Moesziomyces spp. cultivation using cheese whey: new yeast extract-free media, β-galactosidase biosynthesis and mannosylerythritol lipids production. Biomass Conv. Bioref. 14, 6783–6796 (2024). https://doi.org/10.1007/s13399-022-02837-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02837-y