Abstract
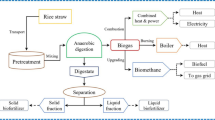
This study demonstrates a new biorefinery approach for efficient conversion of rice straw into biomethane and bioethanol. The main objective of this study was to investigate the production of these two bioenergy products from rice straw through sequential anaerobic digestion (AD) and fermentation. The mesophilic AD and thermophilic AD of the rice straw were investigated for biogas production and digestate characterization. Mesophilic and thermophilic AD generated biogas yield of 236.8 and 275.8 L/kgVS, respectively. The mesophilic digestate fiber is better for sequential production of biomethane and bioethanol. Different acid and alkali pretreatments were investigated for the bioethanol production. Both alkali and acidic pretreatments degraded the lignocellulose; the alkali pretreatment was more effective than the acidic pretreatment. The changes in the lignocellulose biomass of the pretreated digestate were investigated by Fourier transform infrared (FTIR) spectroscopy and X-ray diffraction (XRD). The pretreatment with 3% NaOH for 6 h significantly increased the glucose conversion and achieved the highest ethanol production of 87.13 g/kg dry fiber. The sequential route of AD and fermentation of rice straw enhanced the total gross energy output rate to 1.468 GJ ton−1 day−1. The current research illustrates a revolutionary rice straw-to-energy strategy that will have a massive effect on the biofuel industry progress.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
During the last 20 years, the rapid increase in urbanization and human population growth, which increasing the amounts of agri-wastes and greenhouse gases that have been released into the natural environment cause more emissions, pollution, and global warming [1]. In addition, the energy demand also increased dramatically and became one of the massive environmental challenges as the competition for the natural resources and the environmental degradation [2]. At the same time, the need to diversify energy sources to secure our energy supply has stepped up. Biofuel (i.e., biomethane and bioethanol) has been commonly accepted as a viable energy option among such renewable energy sources because of its general compatible with the existing electricity and heat applications in current distribution networks [3]. In addition, the carbon dioxide emitted by those biofuels can also be considered carbon neutral [4]. Therefore, the world needs inclusive and sustainable urbanization technologies as a milestone in the path towards socio-economic development [3]. The lignocellulose-based biorefinery has been adopted as a favorable option for enhancing the sustainability of these transport fuels [1, 4]. In this scenario, to achieve high energy recovery and zero-waste approach, it is crucial to consider a link between biomethane and bioethanol from the crop straw biorefinery.
Anaerobic digestion (AD) is considered a biorefinery technology that converts various biowaste streams into biogas energy and nutrient-rich digestate, while alleviating environmental pollution associated with the biowaste, such as odor and greenhouse gas (GHG) emissions [1, 6]. The sustainability of AD technology depends on the ability to deal with the excessive digestate [5]. That is because improper handling of digestate would lead to serious environmental problems. Therefore, it appears that proper recycling of digestate is a key factor to significantly reduce environmental problems as a result of direct application to agricultural soils [6].
Rice straw is the world’s largest cereal crop residue in many countries. Asia alone contributes for 90% of the world’s rice supply; China is the largest producer of rice straw accounting 180–270 million tons annually [7]. Noticeably, a large portion of rice straw is still burned in open fields causing environmental concerns. Moreover, this practice results in losses of nutrients and increases greenhouse gas emissions [4]. Therefore, there is a need to develop eco-friendly technologies for the integrated management of rice straw in many countries with rapidly growing economies.
Rice straw is a promising feedstock for bioenergy generation technologies, especially to produce biogas and bioethanol. However, due to its low degradability, a mild pretreatment is required such as mechanical grinding. In addition, the use of milled rice straw for biogas production without severe pretreatment was economically feasible [8]. In that regard, there are some studies that confirmed that AD process can be considered as a bio-treatment that converts hemicellulose (pentose sugars) into methane and concentrates the cellulose in the produced digestate fibers [4, 8]. Moreover, biomethane from AD of hemicellulose produces more energy yield than bioethanol from hemicellulose hydrolysis due to the low conversion rate (less than 80%) of pentose sugars to bioethanol [9].
Some researchers describe the solid digestate produced from various types of biowaste and under different conditions. Sambusiti et al. [10] found that the solid digestate produced from the mesophilic full-scale AD of mixed biomass contains 17.5% of cellulose, 20.3% of hemicellulose, and 24.1% of lignin after AD. Similarly, Wang et al. [11] found that the anaerobic digested corn stover contains 25.1% cellulose, 1.1% hemicellulose, and 21.7% lignin after 20 days. Previous studies showed that digestate contains large amounts of lignocelluloses, mainly cellulose, which can be further valorized by other appropriate technologies. In this context, it was recently reported that anaerobic treatment of lignocellulosic biomass and the quality of the produced digestate fibers have a great potential to be used as a feedstock for bioethanol production, which significantly enhances the economic benefits and sustainability of AD technology [12]. However, due to lignin being concentrated beside cellulose fiber in solid digestate, consequently, many studies have been focused on developing effective pretreatment for solid digestate and enzymatic hydrolysis to enhance the bioethanol yield [13].
Diverse pretreatment methods have been developed such as the alkali and acid pretreatments [14, 15]. Dilute alkali and acid pretreatment has been verified by previous studies as an effective way to pretreat lignocellulosic biomass, achieving increased accessibility area and improved conversion efficiency [16, 17]. Therefore, integrated co-production of biomethane and ethanol has become an attractive pathway as it could yield additional biofuels and enhance the energy output. In addition, this strategy provides integrated management of rice straw, which is consistent with the concept of circular economy [18, 19]. However, there is still a needs for research for the co-production of bioethanol- and biomethane-based biorefinery concept from different biomass materials. To our knowledge, there are few studies on mass balance and the structural properties of biofibers generated from sequential AD and yeast fermentation of alkali and acid pretreated rice straw. Such knowledge might aid in better understanding the biodegradation mechanism of structural biofibers produced from sequential AD and fermentation.
Therefore, the present study aimed to investigate the co-production of biogas and bioethanol from rice straw through sequential AD and yeast fermentation. In addition, the effects of alkali and acid pretreatment on digestate fiber and sugar contents were evaluated. Moreover, the effects of selected pretreatment and enzymatic hydrolysis on the structure and composition of biofibers were studied using a fiber analyzer, X-ray diffraction (XRD), and Fourier transform infrared (FTIR) spectroscopy. Finally, the gross energy output rate (GEOR) and mass balance using the sequential route of the AD and fermentation were estimated.
2 Materials and methods
2.1 Materials
Rice straw (RS) was collected from the agricultural fields of Huazhong Agricultural University. The collected RS was air-dried, ground, and passed through a 2-mm aperture standard screen. The processed RS was stored at room temperature in sealed plastic bags for further use and analysis. The anaerobic inoculum was provided from a biogas plant operated on the pig manure under mesophilic condition. Prior to thermophilic AD test, the inoculum reactor was slowly acclimatized in a water bath by gradually rising from 30 to 55 °C at a rate of 2 °C day−1, allowing to progressively enrich thermophilic bacteria [6]. The characteristics of the rice straw and the mesophilic inoculum are shown in Table 1.
2.2 Experimental methods
2.2.1 Anaerobic digestion
AD batch tests were conducted at conditions of mesophilic (37 °C) and thermophilic (55 °C). The batch experiment was performed in 1-L reactors. Each reactor contained 50 g (wet weight) of rice straw and inoculated with 200 g (wet weight) of inoculum sludge. 700 ml of deionized neutralized water (pH 7) was subsequently added to adjust the final TS concentration at 7.0%. The inoculum and straw were loaded in each reactor at a ratio of 2:3 (VS base). The start-up pH value was adjusted to be 7. The reactors running at the 20-day hydraulic retention period were considered [4]. The reactors were tightly closed with rubber septa and screw cap and then flushed with nitrogen to remove oxygen from the headspace for keeping anaerobic environment. Reactor containing the same inoculum ratio in water was used as a control. The biogas volume was measured by the drainage method, and the biogas was collected by a 5 mL plastic syringe for the gas composition analysis. After the end of AD tests, the raw digestate was centrifuged at 3000 × g to separate the liquid portion. The solid digestate was washed and dried, and then the changes in the composition of digestate fibers were analyzed.
2.2.2 Digestate pretreatment and enzymatic hydrolysis
Due to the low digestibility of fiber in digestates, the pretreatments by NaOH and HCl were selected for fiber in digestates because it is a cost-effective pretreatment, and it selectively removes lignin without degrading more carbohydrates, thereby enhancing enzymatic hydrolysis [4, 21, 44]. Also, the purpose of pretreatment at mild temperature was to avoid generating furfural or hydroxymethylfurfural (HMF) and organic acids, which were considered as inhibitory substances in most microorganisms for fermentation [4].
The digestate was pretreated by soaking in different concentrations of diluted alkali and acid (1% and 3%, v/v) for 6 h and 24 h at a liquid to solid ratio of 1/10 (v/w) at 60 ○C. After pretreatment of solid fiber was separated by filtration through 0.50-mm pore size sieve and then dried in an oven at 45 ○C until constant weight. The dried pretreated fiber was kept in a dry environment for further analysis and enzymatic hydrolysis test.
The enzymatic hydrolysis was performed in a 500-mL glass flask; 22.63 g of solid fiber fractions was mixed with 300 mL acetic acid-sodium acetate buffer (0.2 M, pH 4.8) and 10 mL mixed-enzymes were added; the final solid concentration was 5% (W/V). The concentration of hydrolysis enzymes was 6 × 104 mixed cellulases (ß-glucanase U, cellulose 600 U, and = 10 × 104xylanase U) and was obtained from Imperial Jade Biotechnology Co., Beijing, China. During the enzymatic hydrolysis, the samples were shaken under 150 rpm at 50 °C for 48 h. The amounts of hydrolyzed sugars were determined by high-performance liquid chromatography (1220 Series Agilent HPLC, USA) equipped with a Zorbax carbohydrate analytical column (4.6 × 150 mm) and a refractive index detector. The column temperature was 60 °C with a mobile phase acetonitrile to water (75:25) at a flow rate of 0.6 mL min−1. Fermentable glucose and xylose conversion of pretreated fiber were used as an indicator of the hydrolysis efficiency. The conversion of glucose and xylose was calculated according to [21] using the following equations:
where Cg and CX are the glucose and xylose concentrations, respectively, in the hydrolyzed slurry (g/L); Vh is the hydrolyzed slurry volume (L); Mg and Mx were the mass of cellulose and hemicellulose in the sample (g); and 1.11 and 1.14 were the conversion factor of cellulose and hemicellulose to equivalent glucose and xylose, respectively.
2.2.3 Ethanol fermentation
The fermentation test was conducted by inoculation with Saccharomyces cerevisiae (obtained from the Angel Biotechnology Group in Hubei, China) in a ratio of 1:20 (V/V) at 37 ℃ for 48 h [4]. At the beginning and end of this process, samples were taken for the analysis of ethanol and sugars. The ethanol produced was distilled from the fermentation broth at 80 °C using a rotary evaporator. The rest of the fiber after distillation was separated by filtration through a 0.50-mm pore size sieve and then dried in an oven at 45 °C until constant weight. The fiber was kept in a dry environment for further fiber composition analysis.
2.3 Analytical methods
Total solids (TS) and volatile solids (VS) were measured according to standard methods [22]. The samples were dried over night at 105 ℃ to determine TS and burned at 550 ℃ to determine VS. The biogas composition was determined using GC9790II gas chromatography with a thermal conductivity detector (TCD), and 1.5-m stainless steel packed column with 5A molecular sieve and Hayesep Q packed column (Lanzhou Atech Technologies Co., China). The temperatures of the injector, detector, and oven were maintained at 55, 100, and 50 °C, respectively. Argon was used as a carrier gas at a flow rate of 30 mL min−1.
Fiber composition was determined in the studied substrates using a fiber analyzer (ANKOM A2000i, USA) by measuring the neutral detergent fibers (NDF), acid detergent lignin (ADL), and acid detergent fibers (ADF) according to Soest et al. (1991) [23]. This technique allows to divide fiber components into cellulose “CEL” (ADF-ADL), hemicelluloses “HEM” (NDF-ADF), lignin (ADL), and neutral detergent soluble fraction “NDS” (1-NDF). Detailed protocols can be found at the website of ANKOM Technology (http://ANKOM.com/09_procedures/procedures.shtml). FTIR was examined by Nicolet Avatar 330FT-IR (Thermo Electron Corporation, Boston, MA, USA). The recorded spectra were in the range from 4000 to 500 cm−1. Crystallinity, a physical property expressed as crystallinity index (CrI), was examined by measuring X-ray diffraction (XRD) using a powder X-ray diffractometer (Bruker D8 Advance, Germany) with Cu Ka radiation at 40 kV and 40 mA [24]. The samples were scanned in the 2θ range of 0–80° at a rate of 10°/min, and the CrI was calculated according to Eq. (3):
where I002 is the crystalline diffraction intensity of the 002 peak at 2θ = 22.5° and Iam is the amorphous zone diffraction intensity of 2θ = 18°.
Ethanol concentrations in the fermentation process were also determined by the gas chromatograph (GC9790II, equipped with a KB-Wax column, oven temperature at 130 ℃, FID (flame ionized detector) at 250 ℃, injector temperature at 250 ℃, and nitrogen as the carrier gas).
2.4 Statistical analysis
Samples from each replicate were collected randomly; all experiments were carried out in three replicates; and the results were represented as the mean ± standard deviation (SD). SPSS 19.0 statistically evaluated the experimental data using the one-way variance analysis (ANOVA). The Fisher least significant difference (LSD) was estimated at probability level (P) ≤ 0.05 to perform pair comparisons of the means of each parameter.
3 Results and discussion
3.1 Biogas production
Figure 1 illustrates cumulative (1a) and daily (1b) biogas production during mesophilic (37 ○C, 6%TS) and thermophilic (55 ○C, 6%TS) anaerobic digestion. The thermophilic AD showed a relative increase in daily and cumulative biogas production as compared to the mesophilic AD. The daily biogas yields were decreased to less than 5 L kg−1VS after 20 days of digestion. After 20 days of digestion, the cumulative biogas yields of mesophilic and thermophilic AD were 236.8 and 275.8 L kg−1VS, respectively, lower than that recorded by Elsayed et al. [4] who indicated biogas yield of 286.9 L kg−1 VS from NaOH pretreated rice straw under mesophilic digestion. However, this result agreed with Riggio et al. [25] who reported that the cumulative biogas production of thermophilic digestion was higher than that of mesophilic by 20%. The technical digestion time (T80) is the time required to produce 80% of total biogas yield. T80 can be used to indicate the rate of biogas yield and the biodigestibility of the substrate [4]. The AD in the present work lasted up to 20 days; the T80 values for AD of thermophilic and mesophilic were 13 days and 12 days, respectively. These results indicate that the both AD of thermophilic and mesophilic of rice straw were digested in a shorter time. In addition, the curves of biogas yield of mesophilic and thermophilic AD showed a mono-phasic shape with no diauxic growth, which indicates that there was no further degradation of slowly biodegradable organic fractions. The highest recorded biogas yield by thermophilic AD is attributed to improve holocellulosic fiber digestion. Despite the relative increase in biogas from thermophilic AD, it does not usually compensate the amount of energy inputs and operating costs, in addition to the fact that performance of a thermophilic anaerobic reactor at industrial scale is unstable due to inhibition occur [25]. In addition, the mesophilic digestate showed a high cellulose retention (28.62), while in lignin content (3.44%), it would be better to reserve cellulose fiber for next ethanol production. Therefore, mesophilic AD of rice straw is better in terms of economic biogas production and cellulose retention; thus, the choice of mesophilic AD was adopted as the first step in biorefining route of rice straw.
3.2 Effects of the pretreatment and enzymatic hydrolysis on the digestate fiber
3.2.1 Fiber composition
For the purpose of ethanol fermentation, the pretreatment and enzymatic hydrolysis were conducted to the digestate fibers. The main aim of the pretreatment and the enzymatic hydrolysis processes is increasing the soluble sugar contents that can be converted into bioethanol biochemically [26, 44]. Pretreatment can be performed with dilute acid or alkali at low temperatures, but it takes a relatively long time. The pretreatment conditions were dilute acid or alkali, at mild temperature 60 ℃, which can effectively avoid the extreme corrosion and less dangerous compared to the concentrated acid/base at high temperature [27]. Diluted concentrations (1% and 3%) of NaOH and HCl were used with different soaking times (6 h and 24 h) in the pretreatment process.
Regarding the composition, the main objective of the pretreatment process is separating of structural linkages between lignin and holocellulose fractions, which increases the surface fiber and decreases the degree of crystallinity. Figure 2 illustrates the lignocellulose contents after pretreatment and enzymatic hydrolysis. Both alkali and acidic pretreatment degraded the lignocelluloses; however, the alkali pretreatment was more effective than the acidic pretreatment. The alkali pretreatment solubilized lignin and hemicellulose fractions; however, the average alkali effect was less than that of acidic pretreatment on the cellulose solubilization. These results agreed with Sarbishei et al. [28], who found that alkali pretreatments are more effective for lignin solubilization, but have minor cellulose and hemicellulose solubilization than acidic pretreatment. The 3%NaOH/24 h showed the best solubilization in cellulose, hemicellulose, and lignin fractions. Although dilute acid pretreatment is possible to remove almost 100% hemicelluloses, it is not effective in dissolving lignin, just disrupt lignin and increase the susceptibility to enzyme [29]. It can be noticed that NaOH treatment separates the structural linkages between lignin and holocellulose, making cellulose and hemicellulose easily utilized by enzymatic attack leading to improved sugar production [30, 31]. Reduction in holocellulose ratio results in increase in the relative proportion of lignin (not increase in lignin content). Holocellulose (cellulose and hemicellulose) content from alkaline pretreatment at 3% for 6 h represented 37.91% and 22.23%, respectively, which was significantly decreased after enzymatic hydrolysis to 26.35% and 11.15%. However, the optimum conditions for pretreatment do not necessarily mean the most effective conditions for enzymatic hydrolysis.
3.2.2 X-ray diffraction
The XRD is used to study the crystalline structure of rice straw and to determine the crystalline cellulose phase. The cellulose crystallization is a significant factor during the lignocellulosic digestion [4, 32, 33]. Table 2 summarizes the crystalline index calculated from X-ray diffraction profiles showing the peaks in both crystalline and amorphous zones. Although the intensities of both zones decreased during pretreatment and enzymatic hydrolysis, the crystalline zone was clearer than the amorphous zone. We could see that the effects of acid and alkali pretreatment on the intensities of diffraction in both zones are different. In pretreatment, the effect in the crystalline zone is more distinct than that in the amorphous zone. As shown in Fig. 3a, the amorphous zone is near to 18○(2Ɵ), while the crystalline zone is near to 22.5○(2Ɵ). The CrI value of the rice straw was 32.6%. Both alkali and acid pretreatments for 24 h increased CrI to be 44.6% and 67.7%, respectively. This may be due to the removal of hemicellulose and lignin in the amorphous region. Boukir et al. (2019) [33] referred that the cellulose crystallinity is affected by the percentage of the lignin and hemicellulose in the amorphous region, and the reduction in the crystalline fraction is directly correlated with the reduction in the crystallite size parameter. The enzymatic hydrolysis decreased the CrI to be around 30%; this indicated that lignocellulosic material was hydrolyzed and changing in the crystal structure of cellulose.
X-ray diffraction profiles and FTIR spectra of rice straw in different stages (a X-ray diffraction profiles, b FTIR spectra): 1, rice straw; 2, biogas digestate; 3, pretreatment: 1% HCl for 24 h; 4, pretreatment: 3% HCl for 24 h; 5, pretreatment: 1% NaOH for 24 h; 6, pretreatment: 3% NaOH for 24 h; 7, enzymatic hydrolysis: 1% HCl for 24 h; 8, enzymatic hydrolysis: 3% HCl for 24 h; 9, enzymatic hydrolysis: 1% NaOH for 24 h; 10, enzymatic hydrolysis: 3% NaOH for 24 h
3.2.3 Fourier transform infrared spectroscopy
FTIR spectra can illustrate the structural changes and component decomposition of lignocellulosic materials and can support the XRD results. A shown in Fig. 3b, the transmittance spectra of the FTIR are studied in range from 4000 to 400 cm−1. The peak at 3400 cm−1 indicated to the –OH stretching band of hydroxyl that was associated to the cellulose. It is reported that the –OH peak was slightly increased and broadened as a result of pretreatment and enzymatic hydrolysis, indicating weaker intra- and intermolecular hydrogen bonding which leads to lower crystallinity as a result of partial degradation of hemicellulose and lignin. The peak at 2900 cm−1 was detected in the digestate sample, and it indicated the absorption peak of C–H stretching vibrations, confirming the breaking down of intermolecular hydrogen bonding between cellulose and hemicellulose and the generation of CH4 [34]. The peak at 1637 cm−1 represented the stretching vibrations of C = O bonds, which is the characteristic functional group of ortho-substituted aryl skeleton. This indicated the acetic acid and aldehyde generation during the pretreatment and enzymatic hydrolysis. The pretreatment and enzymatic hydrolysis changed the transmittance peak at 1565 cm−1which is an indication of the change in the aromatic skeletal vibration of lignin [34]. The change in peak 1415 cm−1 which is associated to the –CH2 bending vibration indicated the change in the aromatic ring vibration of lignin [35]. The hydrolysis of lignocellulosic compounds causes changes in the peak of 1063 cm−1 that indicated C–O–C asymmetrical stretching of cellulose and hemicellulose [36].
3.3 Mono-sugar conversion
To evaluate the pretreatment of digestate fibers with dilute HCl and NaOH, the rare enzymatic hydrolysis of fiber and the glucose and xylose conversion are measured and calculated. As shown in Fig. 4, the pretreatment with 3% NaOH for 6 h achieved the highest glucose conversion of 58.66%, while the glucose conversion of 17.12% with acid pretreatment at 1% HCl for 24 h. The glucose conversion was increased with increasing alkali concentrations and pretreatment times; however, increasing the pretreatment time of 3% NaOH from 6 to 24 h, the glucose conversion decreased to 48.56%. This may be attributed to the formation of furfural and 5-hydroxymethyl furfural (HMF) compounds during the hexose degradation [4, 44]. These compounds inhibit the yeast growth, and hence, the bioethanol yield decreases [37]. In addition, diluted HCl pretreatment under mild temperature was not able to hydrolyze the lignocellulose efficiently; therefore, the lignocellulose fractions cannot be hydrolyzed easily by the enzyme [21].
3.4 Ethanol production
The diluted alkali and acid pretreatment methods were adopted to enhance the bioethanol production from mesophilic digestate of rice straw. Figure 5 shows the ethanol production from the different pretreatments of digestate. The ethanol production from alkali pretreatment was significantly higher than that with acid pretreatment. The highest ethanol production was 87.13 g/kg dry fiber at pretreatment: 3% NaOH with 60 °C for 6 h. The second best yield was achieved at pretreatment: 3% NaOH for 24 h that accounted 72.1 g ethanol/kg dry fiber. The possible reason for this could be that alkaline reagent degrades the lignin and hemicellulose, giving access to the cellulose fiber [4, 21]. In addition, NaOH reagent is more helpful for cellulose hydrolysis than HCl reagent. This could explain the higher glucose conversion at alkali pretreatment compared with acid pretreatment. In this context, Saccharomyces cerevisiae prefer glucose as carbon source than xylose [38–40]. Therefore, the glucose conversions are the key factor of the bioethanol production from lignocellulose. The recorded ethanol yield from digestate fiber in the present study was similar with that reported by others [4, 13]. This demonstrated that cellulose was effectively recovered from mesophilic digestate of rice straw using dilute alkali pretreatment at 3% NaOH with 60 °C for 6 h, which is a significant finding in sequential AD and fermentation.
3.5 Energy recovery and mass balance analysis
The mass balance is shown in Fig. 6; the 50 g of rice straw contained 45.88 g of total solid (TS) and was composed of 17.21 g cellulose, 14.41 g hemicellulose, 2.06 g lignin, 0.61 g ash, and 11.59 g other components. The rice straw was used as substrate with 17.58 g sludge for the AD process. The cellulose content was decreased; 48.16 g solid digestate was produced from the AD and was composed of 16.75 g cellulose, 11.75 g hemicellulose, 1.95 g lignin, 2.56 g ash, and 15.65 g other components, respectively. The alkali pretreatment solubilized 45.12% of the solid digestate TS; this may be due to formation of gases such as CO2 [41]. The 3% NaOH/6 h hydrolyzed the solid digestate into pretreated fibers that were composed of 12.93 g cellulose, 4.18 g hemicellulose, 0.77 g lignin, 1.35 g ash, and 7.20 g other components, respectively. Dilute alkali facilitate the enzymatic hydrolysis action by disrupting the ester bonds cross-linked in the cell wall matrix and removing acetyl groups in the digested fiber [27, 42]. The enzymatic hydrolysis resulted in the conversion of the fiber content, cellulose, and hemicellulose into mono-sugars (9.54 g glucose and 2.68 g xylose). MacLellan et al. (2013) [12] assumed that the carbohydrate contents of the pretreated fiber were converted efficiently by the enzymatic hydrolysis, and the stover-to-manure ratio of 40:60 was able to produced 17.3 g L−1 and 6.2 g L−1 of glucose and xylose, respectively, and the fiber quality among different stover-to-manure ratios was indicated by carbohydrate conversion [12]. The mono-sugars were converted into bioethanol through the fermentation process. The pretreated digestate (26.34 g) was used to produce 4.19 g ethanol. Therefore, the ethanol yield in this study is 87.1 g/kg dry fibers.
The gross energy output rate (GEOR, as GJ ton−1 day−1) of the mesophilic anaerobic digestion of rice straw and bioethanol production from the digestate after different treatments is shown in Table 3. GEOR of biomethane and bioethanol from each treatment was determined based on the corresponding higher heating value (HHV) multiplied by bioethanol or biomethane productivity. The HHV of biomethane and bioethanol were 35.9 kJ L−1and 26.7 MJ kg−1, respectively [43]. The biomethane generated from the mesophilic anaerobic digestion of rice straw was 11.84 L kg−1dw day−1. The GEOR of the generated biomethane was 0.4251 GJ ton−1 day−1. The GEOR of bioethanol (GEORETH) was higher than that of biomethane (GEORMEH) due to the shorter time of bioethanol production compared to the anaerobic digestion. The alkali pretreatment groups had higher GEORETH than the acid pretreatment groups. These results supported the higher bioethanol productivity of alkali pretreated that merits the advantage of alkali over the acid pretreatment. The highest bioethanol produced was 0.03907 kg kg−1dw day−1 and the highest GOER ethanol of 1.043GJ ton−1 day−1 achieved with pretreated digestate at 3%NaOH for 6 h. The bioethanol production increased the net energy output. Therefore, the sequential route of AD and fermentation of rice straw enhanced the total GEOR to 1.468GJ ton−1 day−1. These results emphasized the feasibility of the recycling of the anaerobic digestate of rice straw for bioethanol production and positive gross energy rate. In addition, sequential route of AD and fermentation is compared to data in previous literature shown in Table 4, suggesting that similar methane and ethanol production was achieved. This is beneficial to have such information for practical application of the two-stage conversion of rice straw biomass to biomethane and bioethanol.
4 Conclusion
The present study demonstrated that mesophilic digestate is better in sequential production of biomethane and bioethanol. Most of the cellulose and hemicellulose in digestate fiber can be retained for bioethanol production after alkali pretreatment at 3% NaOH for 6 h. The alkali pretreatment of digestate fiber achieved the glucose conversion of 58.66% and increased the bioethanol yield of 87.1 g/kg dry fiber. The sequential route of AD and fermentation of rice straw enhanced the total GEOR to 1.468 GJ ton−1 day−1. Therefore, the present study suggested application of sequential production of biomethane and bioethanol as an eco-friendly route for the integrated management of rice straw for effective utilization of organic carbon in the second-generation lignocellulosic biofuel feedstocks.
References
Elsayed M, Ran Y, Ai P, et al (2020) Innovative integrated approach of biofuel production from agricultural wastes by anaerobic digestion and black soldier fly larvae. J Clean Prod 263:121495. https://doi.org/10.1016/j.jclepro.2020.121495
Elsayed M, Li W, Abdalla N, et al (2022) Innovative approach for rapeseed straw recycling using black solider fly larvae: Towards enhanced energy recovery. Renew Energy 188:211–222. https://doi.org/10.1016/j.renene.2022.02.029
Abomohra AE, Elsayed M, Esakkimuthu S et al (2020) Potential of fat, oil and grease ( FOG ) for biodiesel production : a critical review on the recent progress and future perspectives. Prog Energy Combust Sci 81:100868. https://doi.org/10.1016/j.pecs.2020.100868
Elsayed M, Abomohra AE-FF, Ai P et al (2018) Biorefining of rice straw by sequential fermentation and anaerobic digestion for bioethanol and/or biomethane production: comparison of structural properties and energy output. Bioresour Technol 268:183–189. https://doi.org/10.1016/j.biortech.2018.07.130
Dahlin J, Herbes C, Nelles M (2015) Biogas digestate marketing: qualitative insights into the supply side. Resour Conserv Recycl 104:152–161. https://doi.org/10.1016/j.resconrec.2015.08.013
Elsayed M, Abomohra AEF, Ai P et al (2019) Acetogenesis and methanogenesis liquid digestates for pretreatment of rice straw: a holistic approach for efficient biomethane production and nutrient recycling. Energy Convers Manag 195:447–456. https://doi.org/10.1016/j.enconman.2019.05.011
Xia T, Huang H, Wu G et al (2018) The characteristic changes of rice straw fibers in anaerobic digestion and its effect on rice straw-reinforced composites. Ind Crops Prod 121:73–79. https://doi.org/10.1016/j.indcrop.2018.04.004
Bahmani MA, Shafiei M, Karimi K (2016) Anaerobic digestion as a pretreatment to enhance ethanol yield from lignocelluloses. Process Biochem 51:1256–1263
Yue Z, Teater C, Liu Y et al (2010) A sustainable pathway of cellulosic ethanol production integrating anaerobic digestion with biorefining. Biotechnol Bioeng 105:1031–1039
Sambusiti C, Monlau F, Ficara E et al (2015) Comparison of various post-treatments for recovering methane from agricultural digestate. Fuel Process Technol 137:359–365. https://doi.org/10.1016/j.fuproc.2015.04.028
Wang J, Peng S, Wan Z et al (2013) Feasibility of anaerobic digested corn stover as biosorbent for heavy metal. Bioresour Technol 132:453–456. https://doi.org/10.1016/j.biortech.2013.01.160
MacLellan J, Chen R, Kraemer R et al (2013) Anaerobic treatment of lignocellulosic material to co-produce methane and digested fiber for ethanol biorefining. Bioresour Technol 130:418–423
Jarunglumlert T, Prommuak C (2021) Net energy analysis and techno-economic assessment of co-production of bioethanol and biogas from cellulosic biomass. Fermentation 7:229
Bensah EC, Mensah M (2013) Chemical pretreatment methods for the production of cellulosic ethanol: technologies and innovations. Int J Chem Eng 2013. https://doi.org/10.1155/2013/719607
Amin FR, Khalid H, Zhang H et al (2017) Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 7:72
Teater C, Yue Z, MacLellan J et al (2011) Assessing solid digestate from anaerobic digestion as feedstock for ethanol production. Bioresour Technol 102:1856–1862
Kainthola J, Kalamdhad AS, Goud VV (2019) A review on enhanced biogas production from anaerobic digestion of lignocellulosic biomass by different enhancement techniques. Process Biochem 84:81–90
Parajuli R, Dalgaard T, Jørgensen U et al (2015) Biorefining in the prevailing energy and materials crisis: a review of sustainable pathways for biorefinery value chains and sustainability assessment methodologies. Renew Sustain Energy Rev 43:244–263
Martin M, Svensson N, Fonseca J, Eklund M (2014) Quantifying the environmental performance of integrated bioethanol and biogas production. Renew energy 61:109–116
Eraky M, Jin K, Zhang Q et al (2021) Acidogenic biorefinery of rice straw for volatile fatty acids production via sequential two-stage fermentation: effects of pre-treatments. Environ Technol Innov 23:101686. https://doi.org/10.1016/J.ETI.2021.101686
Wang D, Ai P, Yu L et al (2015) Comparing the hydrolysis and biogas production performance of alkali and acid pretreatments of rice straw using two-stage anaerobic fermentation. Biosyst Eng 132:47–55
APHA A (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597
Fu S-F, Wang F, Yuan X-Z et al (2015) The thermophilic (55 C) microaerobic pretreatment of corn straw for anaerobic digestion. Bioresour Technol 175:203–208
Riggio S, Hernandéz-Shek MA, Torrijos M et al (2017) Comparison of the mesophilic and thermophilic anaerobic digestion of spent cow bedding in leach-bed reactors. Bioresour Technol 234:466–471
Öner K, Nazan M (2018) Comparison of acid and alkaline pretreatment methods for the bioethanol production from kitchen waste. In: The Role of Exergy in Energy and the Environment. Springer, p 363–372
Taherzadeh MJ, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9:1621–1651
Sarbishei S, Goshadrou A, Hatamipour MS (2021) Mild sodium hydroxide pretreatment of tobacco product waste to enable efficient bioethanol production by separate hydrolysis and fermentation. Biomass Convers Biorefinery 11:2963–2973
Yang B, Wyman CE (2004) Effect of xylan and lignin removal by batch and flow through pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol Bioeng 86:88–98
Khan MU, Usman M, Ashraf MA, et al (2022) A review of recent advancements in pretreatment techniques of lignocellulosic materials for biogas production: Opportunities and Limitations. Chem Eng J Adv 10:100263. https://doi.org/10.1016/j.ceja.2022.100263
Gáspár M, Kálmán G, Réczey K (2007) Corn fiber as a raw material for hemicellulose and ethanol production. Process Biochem 42:1135–1139
Ai P, Zhang X, Dinamarca C et al (2019) Different effects of ozone and aqueous ammonia in a combined pretreatment method on rice straw and dairy manure fiber for enhancing biomethane production. Bioresour Technol 282:275–284. https://doi.org/10.1016/j.biortech.2019.03.021
Boukir A, Fellak S, Doumenq P (2019) Structural characterization of Argania spinosa Moroccan wooden artifacts during natural degradation progress using infrared spectroscopy (ATR-FTIR) and X-Ray diffraction (XRD). Heliyon 5:e02477. https://doi.org/10.1016/j.heliyon.2019.e02477
Malik K, Salama E-S, Kim TH, Li X (2020) Enhanced ethanol production by Saccharomyces cerevisiae fermentation post acidic and alkali chemical pretreatments of cotton stalk lignocellulose. Int Biodeterior Biodegradation 147:104869. https://doi.org/10.1016/j.ibiod.2019.104869
Zhao C, Shao Q, Ma Z et al (2016) Physical and chemical characterizations of corn stalk resulting from hydrogen peroxide presoaking prior to ammonia fiber expansion pretreatment. Ind Crops Prod 83:86–93
Hassan SS, Tiwari BK, Williams GA, Jaiswal AK (2020) Bioprocessing of brewers’ spent grain for production of xylanopectinolytic enzymes by Mucor sp. Bioresour Technol Rep 9:100371. https://doi.org/10.1016/j.biteb.2019.100371
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33. https://doi.org/10.1016/S0960-8524(99)00161-3
Katahira S, Mizuike A, Fukuda H, Kondo A (2006) Ethanol fermentation from lignocellulosic hydrolysate by a recombinant xylose-and cellooligosaccharide-assimilating yeast strain. Appl Microbiol Biotechnol 72:1136–1143
Tajmirriahi M, Momayez F, Karimi K (2021) The critical impact of rice straw extractives on biogas and bioethanol production. Bioresour Technol 319:124167. https://doi.org/10.1016/j.biortech.2020.124167
Germec M, Turhan I (2018) Ethanol production from acid-pretreated and detoxified rice straw as sole renewable resource. Biomass Convers Biorefinery 8:607–619. https://doi.org/10.1007/s13399-018-0310-1
Kaparaju P, Serrano M, Thomsen AB et al (2009) Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour Technol 100:2562–2568
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729
Meng L, Jin K, Yi R, et al (2020) Enhancement of bioenergy recovery from agricultural wastes through recycling of cellulosic alcoholic fermentation vinasse for anaerobic co-digestion. Bioresour Technol 311:123511. https://doi.org/10.1016/J.BIORTECH.2020.123511
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199:49–58
Funding
This work was financially supported by the National Natural Science Foundation Program of China (No. 31972611), the Central Universities Fundamental Research Funds (No. 2662020GXPY006), Agricultural Science and Technology Innovation Program (ASTIP) of Chinese Academy of Agricultural Sciences (No. 1610012020004_01900, CAAS-ASTIP-2016-BIOMA), and Key Laboratory on Development and Application of Rural Renewable Energy. The authors gratefully acknowledge their supports.
Author information
Authors and Affiliations
Contributions
Ran Yi: resources, project administration, methodology, formal analysis. Mahdy Elsayed: conceptualization, formal analysis, writing—review and editing. Mohamed Eraky: writing—original draft preparation, formal analysis. Dianlong Wang: investigation, data curation, formal analysis, methodology. Ping Ai: conceptualization, supervision, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yi Ran and Mahdy Elsayed are considered as co-first author.
Rights and permissions
About this article
Cite this article
Ran, Y., Elsayed, M., Eraky, M. et al. Sequential production of biomethane and bioethanol through the whole biorefining of rice straw: Analysis of structural properties and mass balance. Biomass Conv. Bioref. 14, 2693–2705 (2024). https://doi.org/10.1007/s13399-022-02548-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02548-4