Abstract
Palm fatty acid distillate is a processing by-product resulting from physical refining of crude palm oil products. Producing renewable fuels and biodegradable ester-based biolubricants using saturated palm fatty acid distillate (SFA) is one of environmentally friendly and an efficient way to reuse such by-product for the production of feasible high-end products. This study was carried out to synthesize and determine the optimal conditions for the esterification of Malaysian SFA with high degree polyhydric alcohols (trimethylolpropane, TMP; di-trimethylolpropane, Di-TMP; pentaerythritol, PE; and di-pentaerythritol, Di-PE) in the presence of sulfuric acid as catalyst. The esterification reaction parameters involved such as amount of catalyst, effect of reaction temperature (°C) and time (h), and effect of different alcohols, and molar ratio used was investigated. The results showed that the ester yield percentages varied with the corresponding reaction parameters and chemical structure of the alcohol used. The resultant esters with small-branched hydrocarbon chain alcohol produced the highest yield percentage for the SFA-TMP tri-ester (89 ± 3%) followed by the SFA-Di-TMP tetra-ester (87 ± 4%), SFA-PE tetra-ester (83 ± 5%), and SFA-Di-PE hexa-ester (77 ± 4%), respectively. The results showed the reaction temperature has more significant effect on the esterification reaction yield as compared to other parameters. The lubrication properties of the synthesized polyol esters indicated their appropriateness to be used as grease and bearing lubricant application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The plant oil finds various industrial and technology applications to replace existing petroleum-based products such as in coatings and polymers, printing inks, lubricants, cosmetics/pharmaceuticals, leather processing, surfactants, solvents, hydraulic fluids, pesticide/herbicide adjuvants, glycerin (glycerol), and as fuels [1,2,3,4]. Plant oils are superior in terms of biodegradability, especially when compared to mineral oils. Attention has been focused on technologies that incorporate plant oils as biofuels and industrial lubricants, due to the fact that they are renewable and non‐toxic. Plant oils have different unique properties compared to mineral oils due to their unique and simple aliphatic chemical structure. Plant oils have a greater ability to lubricate and higher viscosity indices. Superior anti-corrosion properties are observed in plant oils and are induced by a greater affinity for metal surfaces [5]. High flash points over 300 °C classify plant oils as non‐flammable liquids. However, the applicability of plant oils in lubrication is partly limited, as these oils tend to show low oxidative stability and higher melting points. Chemical modification of plant oils is an attractive way of solving these problems [6,7,8].
The palm oil tree produces the highest yield of oil per unit area of cultivated land, an estimated 58.431 million metric tons per year [9, 10]. Palm oil is extracted from the fresh fruit bunches of palm oil, which have various applications in the oleochemical industry and food. There are two kinds of oil from the palm fruit flesh mesocarp, known as crude palm oil (CPO), while from the seed known as the crude palm kernel oil (CPKO) [11, 12]. The production of Malaysian CPO rose from 11.2 million tonnes in 2002 to 16.9 million tonnes in 2019, making Malaysia one of the world’s largest suppliers of palm oil. Acording global oils and fats export volume, even though Indonesia is the biggest producer of palm oil, its palm oil export is only 68% compared with 88% of Malaysia in 2011 [13, 14]. As of 2015, 5.642 million hectares of land in Malaysia is under oil palm cultivation with CPO and CPKO production reaching 19.961 and 2.276 million tons, respectively. Consequently, Malaysia is considered a leading producer and exporter of palm oil worldwide, with its production comprising 27% of fat and oil exports and contributing an estimated 12% of the world’s fat and oil production.
Palm fatty acid distillate (PFAD) is a by-product from refine palm oil product production. In the process of refining CPO, the free fatty acids (FFA) from the crude oil must be removed as PFAD. The amount of free fatty acids in the palm fruits is determined by how much of the oil in the fruit has been degraded by enzymes after harvest. The oil breakdown process is halted by sterilization of the fresh fruit bunches (FFB). PFAD has a lower market value than palm oil (currently ~ 85% lower); therefore, the palm oil producers attempt to minimize the accumulation of FFA in the FFB. Enzymes can degrade the oil in the fruit after harvest, so the percentage of FFA varies based on this factor. Fruit bunches can be sterilized to prevent the breakdown of oil into FFA [15]. The concentration of FFA in the crude palm oil is typically less than 10%. This is distilled out in the refining process, and thus the output of the refining process is usually containing less than 5% FFA and 95% refined, bleached and deodorized (RBD) palm oil. The RBD palm oil may then be further fractioned into RBD Olein (80%) and RBD Stearin (20%) [5, 9,10,11,12,13]. Compared to other refined oil, PFAD is much lower in price than other refined oils currently used as animal feedstock.
Although the PFAD is a cheap non-edible by-product from the palm oil production, PFAD has considerable value. It is used as feedstock for many different products for animal feeds, laundry soaps, the oleochemical industry, and combustion for local power/process heat. PFAD is also a source of vitamin E, squalene, and phytosterols—substances valuable for the nutraceutical (dietary supplements and functional foods) and cosmetic industries. These substances may be extracted and isolated from PFAD [13].
PFAD comprises palm free fatty acids mixture mainly with saturated palmitic and unsaturated oleic acids as the major fatty acid composition. The remaining components are partial glycerol and unsaponifiable matters, vitamin E, sterols, squalenes, and volatile substances [10]. Surveys on the characteristics and properties of PFAD from Malaysia refineries have been conducted by Ping and Yusof (2009) [16] who showed that PFAD consists of more than 80% free fatty acid and comprises saturated fatty acids (SFA) of C14:0 (1.2%), C16:0 (46.9%), C16:1 (0.15%), and C18:0 (4.3%); unsaturated fatty acids (USFA) of C18:1 (36.7%), C18:2 (9%), C18:3 (0.3%), and C20:0 (0.2%); and others (0.1%). The rest is 15% mono- and dicylglycerols, volatile components, 14.4% glycerols, and 5% unsaponifiable components, e.g., 0.5% vitamin E, 0.8% squalene, 0.4% sterols, and squalene and 2.2% others [16, 17]. Recent findings by Jumaah et al. [18] and Baharudin et al. [19] seem to be in agreement with previous report that palmitic acid (47.1%) was the dominant fatty acid in the Malaysian PFAD, followed by oleic acid (36.6%) and linoleic acid (9.6%). These cheap and non-edible fatty acids become increasing of many researchers’ interest to be used for the formulation of feasible high-end products with viable separation method. Several techniques have been used to separate saturated palm fatty acid distillate (SFA) and unsaturated palm fatty acid distillate (USFA) from PFAD [18, 20,21,22,23]. Green and low-cost separation methods are of researchers’ interest to separate SFA and USFA from PFAD. By using methanol solvent low temperature crystallization, Jumaah et al. reported that the composition of separated saturated palm fatty acid distillate (SFA) in the solid fraction contained 88.5% of palmitic acid (C16:0) as a major saturated fatty acid, 5.8% of stearic acid (C18:0), 1.6% of myristic acid (C14:0), and 3.6% Oleic acid (C18:1), respectively. These important SFA can be used as low-cost starting materials to produce high-end esters products such as biolubricants [18].
Saturated fatty acid-based biolubricants have shown good lubrication properties such as high oxidative stability and flash point [5, 12]. This is due to the properties of saturated fatty acids with long chain aliphatic carbon chain which have a well-structured arrangement in its crystal packing array that contributes high oxidative stability and flash point. Taking to the point, study on the used SFA separated from PFAD to produce polyol-based ester biolubricants is of researchers’ interest. Several possible types of esters (mono-, di-, tri-, tetra-, penta-, hexa-, poly-, and complex esters) might be classified as biodegradable lubricants [6, 15]. However, there is no comprehensive study on polyhydric alcohol-based esters from SFA. Many researchers have recognized the need to establish the role of polyhydric alcohol-based esters in the field of industrial application biolubricants. The work on the synthesis of saturated palm fatty acid distillate, SFA–based polyol esters, was carried out. The aims of this work are to discuss on the optimization of the esterification process between SFA and polyhydric alcohols such as trimethylolpropane (TMP), di-trimethylolpropane (Di-TMP), pentaerythritol (PE), and di-pentaerythritol (Di-PE). The effect of the esterification parameters such as the reaction temperature, reaction time, substrate molar ratio, amount of catalyst that produce the highest esters yield will be determined. The effect of alcohols chemical structures used on the resultant ester yield was also studied.
2 Materials and methods
2.1 Materials
In this study, PFAD was obtained from a local refinery, Sime Darby Plantation Berhad (647,766-V), located at Selangor, Malaysia. Saturated palm fatty acid distillate (SFA) is represented by palmitic acid; 88.5% was separated from PFAD using low-temperature methanol crystallization method [18]. Polyhydric alcohols, toluene, sulfuric acid (97%), sodium bicarbonate, ethyl acetate, sodium chloride, and anhydrous sodium sulfate were purchased from Sigma-Aldrich (Steinheim, Germany). All the chemicals used in this study were either analytical grade or high performance liquid chromatography (HPLC) grade and used directly without further purification.
2.2 Esterification reaction
Four saturated palm fatty acid distillate–based polyol esters were synthesized from esterification reaction between SFA with various high degree polyhydric alcohols according to Nor et al. [24]. Both reactants were overnight dried by using unhydrated sodium sulfate (Na2SO4) and filtered off. In a flask with a reflux condenser and three necks, SFA fatty acid (0.037 mol; 104.7 g) was mixed with 1 mol trimethylolpropane (0.037 mol; 5 g) at mole ratio of 3.5:1 in Dean-Stark distillation unit. The esterification was carried out at reaction temperature between 110 and 200 °C in oil bath equipped with stirrer magnetic heater. At required temperature, 1–5% concentrated H2SO4 (as a percentage of the weight of SFA) was added at specific reaction time. About 20–30 mL of toluene as azeotrope distillation agent for water removal improvement was then slowly added to the mixture during the esterification process. After the reaction end at 6 h, the flask was allowed to cool at room temperature, followed with the removal of toluene by using rotary evaporator at 100 °C. The reaction product was dissolved into 100 mL of ethyl acetate and transferred into a 150-mL separation funnel. About 30 ml of saturated sodium bicarbonate (NaHCO3) was added to the separation funnel and shaken for neutralization of the remaining SFA and acid catalyst. The funnel separator was left until two layers formed. The aqueous layer at the bottom was removed, leaving the organic layer. The organic layer was further washed three times with NaHCO3 solution. Subsequently, the organic layer was washed with 20 mL of 26% saturated sodium chloride (NaCl) and 20 mL of distilled water twice to avoid formation of emulsion. Once the two layers were formed, the bottom aqueous layer was removed. The washing process was repeated until the organic layer with pH 7 was obtained. Then, the sample was poured into a round flask and connected to a rotary evaporator apparatus (90–100 °C) to remove any excess toluene and unreacted alcohol. The remaining water in the sample was absorbed by sodium sulfate (Na2SO4) overnight and filtered off. The organic product was rotary-evaporated to remove ethyl acetate at 80 °C, giving a viscous semi-solid polyolester. The esterification was performed in triplicate.
2.3 Instrumentation
The analysis of synthesized ester compound was performed to confirm and determine the optimized ester structure and determine the composition of ester. In the first step, Nuclear Magnetic Resonance (NMR) and Fourier Transform infrared spectroscopy (FTIR) were employed; FT-NMR (1H and 13C NMR) analysis was recorded on JEOL-ECP 400 spectrometer using CDCl3 as the solvent. The FTIR spectra were recorded on Perkin Elmer Infrared Spectrophotometer within the range 500 to 4000 cm−1. GC instrument model Shimadzu GC-17A was used for ester composition determination. Gas Chromatography (DB-5HT (30 m × 0.25 mm × 0.25 µm) equipped with a Flame Ionization Detector (GC-FID) was used in the second step. The sample preparation involved the addition of exactly 0.5 mL ester to a vial of 10 mL capacity, after which the mixture was diluted using GC grade ethyl acetate (5 mL). An initial temperature of 100 °C was set for the oven, and then kept constant for 1 min. Then, the temperature was increased to 380 °C by a step increment of 5 °C/min and then kept constant for 25 min. The column temperature was adjusted at 100 °C and increased to 380 °C with a temperature rate increase of 5 °C/min and step increase of about 1 °C. The temperature was then kept constant for 20 min upon reaching 380 °C. Temperatures of 380 and 400 °C were set as the injector and detector temperatures, respectively. The carrier gas for the GC system Helium was used with a flow rate of 0.3 ml/min. The parameters of GC were carried out according to Nowicki et al. [25]. The peaks were identified by comparing the retention times to authentic standards. Fatty acid composition in ester was determined by using GC-FID with nonpolar DB-5 column according to Jumaah et al. [18]. All the measurements were performed in triplicate and the data was reported as a mean ± SD of triplicate determinations.
3 Results and discussion
3.1 Esterification reaction of SFA
The initial free fatty acid of PFAD raw material consists of 52.8% saturated fatty acids and 47.2% unsaturated fatty acids. The saturated fatty acid portion comprises mainly myristic acid (1.18%), palmitic acid (48.91%), and stearic acid (2.7%), whereas oleic acid (37.4%) and linoleic acid (9.7%) are the main components of the unsaturated fatty acid portion [18]. The saturated palm fatty acid (SFA) fraction was separated from PFAD by using low-temperature methanol crystallization method. The SFA is best represented by dominated composition of palmitic acid (88.5%). Other components were 1.6% of myristic, 5.8% of stearic, 3.6% of oleic, and 0.5% of linoleic, respectively. The water content of SFA and polyhydric alcohols was in the range of 0.01 to 0.03 wt.%. Saturated palm fatty acid distillate (SFA) was mixed separately with four high degree polyhydric alcohols at different molar ratios such as SFA: TMP (3:1), SFA: Di-TMP (4:1), SFA: PE (4:1), and SFA: Di-PE (6:1) during the esterification process. Four SFA polyol-based esters known as SFA-TMP ester, SFA-Di-TMP ester, SFA-PE ester, and SFA-Di-PE ester were produced.
3.1.1 SFA-trimethylolpropane ester
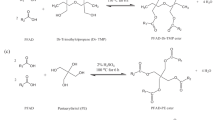
Figure 1 shows the esterification reaction between 3 mol of SFA and 1 mol of trimethylolpropane (TMP) in the presence of H2SO4 (2%) as a catalyst at 150 °C for 6 h. The palmitic was chosen on the representative of SFA PFAD fatty acids due to its dominant composition, palmitic: stearic: myristic acids (88.5:5.8:1.6). The reaction product was TMP tri-palmitate (SFA-TMP ester) and water molecules (by-products). The water was removed during reaction steps. It was observed that esterification proceeded stepwise. Initially, SFA-TMP mono-ester (ME), which was a single branch polyol ester, was formed as an intermediate during the reaction. The increasing amount of ME, however, would immediately undergo conversion to form SFA-TMP diester (DE), which would further react with SFA to produce SFA-TMP tri-ester (TE). The concentration of SFA-TMP tri-ester would rise with the decrease of DE and ME concentrations. The yield percentage of pure TMP tri-palmitate was 86 ± 3% and tri-ester composition was 90 ± 4%. The ester conversion profile graph is shown in Fig. 2.
3.1.2 SFA-di-trimethylolpropane ester
Figure 3 shows the esterification reaction between 4 mol of SFA and 1 mol of di-trimethylolpropane (Di-TMP) in the presence of H2SO4 (2%) as a catalyst at 150 °C for 6 h. The resultant ester, Di-TMP tetra-palmitate (Di-TMP tetra-ester), and water molecules (by-product) were produced. The water was removed during the reaction. The yield percentage of pure Di-TMP tetra-palmitate was recorded as 82 ± 4% and tetra-ester composition was 84 ± 5%. The ester conversion profile graph is quite similar as in Fig. 2 except the reaction response values are different and the final product was SFA-Di-TMP tetra-ester.
3.1.3 SFA-pentaerythritol ester
Figure 4 shows the esterification reaction between 4 mol of SFA and 1 mol of pentaerythritol (PE) in the presence of H2SO4 (2%) as a catalyst at 200 °C for 6 h. The reaction products were Di-TMP tetra-palmitate (SFA-PE tetra-ester) and water molecules (by-products). The water was removed during the reaction steps. The obtained yield percentage of pure Di-TMP tetra-palmitate was 78 ± 5% and tetra-ester composition was 81 ± 5%. The ester conversion profile graph is quite similar as for SFA-Di-TMP with difference in the reaction response values.
3.1.4 SFA-di-pentaerythritol ester
Figure 5 shows the esterification reaction between 6 mol of SFA and 1 mol of di-pentaerythritol (Di-PE) in the presence of H2SO4 (2%) as a catalyst at 200 °C for 6 h. The reaction products were of Di-PE hexa-palmitate (SFA-Di-PE hexa-ester) and water molecules (by-products). The water was removed during reaction step. The yield percentage of pure Di-PE hexa-palmitate was 70 ± 4% and hexa-ester composition was 77 ± 5%. The ester conversion profile graph is shown in Fig. 6.
3.2 Ester structural characterization
The chemical structure of synthesized SFA-polyol esters after optimization was verified using FTIR and NMR (1H and 13C) spectroscopy and high temperature column GC-FID. FTIR analyses were carried out to verify the ester functional group and confirm the success of the esterification reaction. The FTIR spectra of SFA polyol esters (SFA-TMP, SFA-Di-TMP, SFA-PE, and SFA-Di-PE) were similar due to their chemical functional groups. Strong band intensities were observed corresponding to stretching vibration of the ester carbonyl group (C = O) and C-O stretching bands for aliphatic esters are present in the wave number range from 1732 to 1740 cm−1 and 1172–1240 cm−1, respectively [26, 27]. On the other hand, the carboxylic acid carbonyl group C = O stretching vibration presented in wave numbers ranges from 1690 to 1725 cm−1. From the FTIR spectra, the stretching vibration of the carbonyl group (C = O) for aliphatic esters is in wave number 1732, 1738, 1737, and 1740 cm−1 for SFA-TMP ester, SFA-Di-TMP ester, SFA-PE ester, and SFA-Di-PE ester, respectively. It can be observed that the unreacted alcohol and SFA were not detected in the final ester products. The FTIR spectra showed no stretching vibrations of both functionals alcohol (OH) and band stretching of carboxylic acid (− COOH) as shown in Fig. 7.
The 1H spectrum of SFA-TMP ester is shown in Fig. 8 and corresponding band assignments for SFA and SFA-TMP esters are tabulated in Table 1. The disappearance of the 1H chemical shift of the proton (H) of the carboxylic acid group (− COOH) at 11.0 ppm, and for the alcohol group (− OH) at 4.7 ppm, confirmed the success of the esterification reaction [26]. 1H chemical shift ranges for aliphatic protons (− CH2) were detected, normally at about 1.25–1.56 ppm. However, the two protons of the − CH2-O-C = O ester group shift appeared at about 3.80–4.01 ppm [26]. The saturated fatty acid mixture of palmitic acid, stearic acid, and myristic acid composes the SFA-TMP ester. Alkene functional group was used to identify the presence of unsaturated fatty acids in the mixture. The spectrum shows no methylene proton signal (− CH = CH-) which was observed at 5.32–5.38 ppm, signifying that the unsaturated fatty acids were absent in SFA-TMP ester [26]. Other SFA ester compounds of SFA-Di-TMP, SFA-PE, and SFA-Di-PE have shown nearly the same band assignment position due to their same palmitate saturated acyl groups present as indicated in Table 1.
The 13C NMR spectrum of SFA-TMP ester is shown in Fig. 9 and corresponding band assignments for SFA and SFA-TMP esters are tabulated in Table 2. The signal at 171.09–174.07 ppm indicates the carbon ester carbonyl (C = O) in SFA-TMP ester and followed by a peak at 60.32–64.14 ppm attributed to − C-O- group of ester quaternary carbon [26]. These confirm that the ester product has been formed. A signal at 40.57–42.44 ppm was found to correspond to the carbon atom in TMP (− CH2-COOR). The methyl carbon atoms of − CH2- were identified from the peaks at 22.16–36.46 ppm, denoting saturated alkyl chain, a common indication of the presence of these carbon long chain compounds [28, 29]. The terminal methyl (− CH3) peak appeared at 14.04–14.08 ppm. In general, other SFA ester compounds SFA-Di-TMP, SFA-PE, and SFA-Di-PE have shown nearly the same band assignment position due to their same palmitate saturated acyl groups present as indicated in Table 2.
3.3 Optimization process
Four SFA esters known as SFA-TMP ester, SFA-Di-TMP ester, SFA-PE ester, and SFA-Di-PE ester were produced. The optimal value of a particular parameter in the esterification process was obtained by conducting a series of experiments with different values of that particular parameter and keeping the other parameters constant at any random value. Once the optimal value was attained for that particular parameter, that value was fixed constant for the optimization of the other parameters [30, 31]. Five foremost reaction parameters such as molar ratio of SFA to alcohol, quantity of catalyst, reaction temperature, reaction time, and type of alcohol used were considered for optimization in the esterification process. The optimization of the esterification process was first investigated by the amount of H2SO4 catalyst used in the range of 1 to 3% weight of the reactants. The other reaction parameters were fixed at specific reactants molar ratio used, 150 °C, for 4-h reaction time. Figure 10 exhibits the effect of the catalyst used on the esterification process to produce these esters. The results show that the yields of the esters increase as the amount of the catalyst increases and reached a maximum of 83 ± 3% of SFA TMP at 2% catalyst. This fact can be attributed to a higher number of molecules of substrate activated by the polarization of carbonyl, in presence of catalyst. Thus, the nucleophilic attack by alcohol becomes more favorable and, consequently, an increase on the formation of ester was observed [32]. However, the yield % did not increase with the further increasing amount of catalyst. This was due to water produced from the esterification reaction could show the negative effects. In a strong acid catalyst, where a fast interaction between the water is produced during the esterification, catalyst dilution occurred and thus reduces the rate of reaction. The same yields trend for other resultant esters.
Figures 11, 13, and 14 exhibit the effect of other optimization parameters of the esterification reaction yields. For the yield of SFA esters for SFA esterification reaction with different temperatures used at 4 h, 2% of acid catalyst and fix reactants molar ratio were studied (Fig. 11). As can be seen, temperatures have a large effect on the esterification reaction. The maximum yield of ester TMP and Di-TMP of esterified SFA appeared in the temperature of 150 °C. A reaction temperature of 150 °C gave approximately 83 ± 3% conversion to ester TMP and 78 ± 4% of ester Di-TMP. The maximum yield of ester PE and Di-PE of 72 ± 5% and 63 ± 4% appeared in the temperature at 200 °C, respectively. As the reaction temperature increases, the yield percentage of product increases. Increasing the temperature of a reaction generally speeds up the process (increases the rate) because the rate constant increases according to the Arrhenius reaction rate theory. The esterification of SFA was further performed in different reaction time of 4, 5, 6, 7, and 8 h to determine the reaction time effect at fix reaction conditions.
As seen in Fig. 12, by increasing the reaction time, ester yield % will steadily increase until 6 h time. The SFA-TMP ester was achieved at highest yield 86 ± 3%, while 82 ± 4% for SFA-Di-TMP ester, 78 ± 5% of SFA-PE ester, and 70 ± 4% of SFA-Di-PE ester, respectively. Further increase reaction time after 6 h, the ester yields were slowly decreased. As the reaction further proceeds, the substrate concentration slowly decreases, which leads to a fall in the degree of saturation of the catalyst with the substrates. As a result, the optimum time for the esterification process to obtain higher conversion SFA to polyol esters was established at 6 h.
Figure 13 shows the yield percentages vary according to the chemical structure of alcohol used. Among the branched alcohols used in this study, alcohols with small-branched hydrocarbon chains such as TMP produced higher yields of SFA-TMP ester compared to the high-branched alcohols of Di-TMP, PE, and Di-PE. These results may be due to the fact that the small-branched hydrocarbon chain in TMP exhibits less steric hindrance and thus enhanced the rate of esterification. The higher rate of the reactions produced higher ester yield percentages [33]. Other reasons to have a lower percentage yield when more branches of alcohol are used may be due to the physical properties of those alcohols. A less molar number of OH functional group amount in TMP molecule (3 mol) as compared to other polyhydric alcohols used in this study (more than 3 mol) increases the higher chance of the OH to be protanated by acid catalyst. Therefore, increase in the rate of the esterification process thus increases the yield percentage.
The effect of different polyhydric alcohols on SFA PFAD esters yield (%). Note: SFA-TMP, molar ratio of 4:1, H2SO4 (2%), 150 °C for 6 h; SFA-Di-TMP, molar ratio of 5:1, H2SO4 (2%), 150 °C for 6 h; SFA-PE, molar ratio of 5:1, H2SO4 (2%), 200 °C for 6 h; and SFA-Di-PE, molar ratio of 7:1, H2SO4 (2%), 200 °C for 6 h
The effect of the reactant’s molar ratio of SFA: alcohols were also studied. Figure 14 shows the effect of the molar ratio of SFA, alcohol used on the reaction yields. When the reactant molar ratio was varied in three different molar ratios for each all alcohols, the amounts of esters formed from each slightly varied from 85 to 89% (SFA-TMP), 82 to 87% (SFA-Di-TMP), 78 to 83% (SFA-PE), and 70 to 78% (SFA-Di-PE), respectively. It also indicated that ratio of 3.5:1 (SFA:TMP), 4.5:1(SFA:Di-TMP and SFA:PE), and 6.5:1 (SFA:Di-PE) are the optimum molar ratios since the amounts of esters decrease when molar ratio further increased to 4:1 (TMP), 5:1 (Di-TMP and PE), and 7:1 (Di-PE), respectively.
The esterification processes were then repeated at least for three times at their optimal conditions to verify the ester yield percentages. The optimal conditions for SFA:TMP ester were at mole ratio of 3.5:1, H2SO4 (2%), 150 °C for 6 h, and mole ratio of 4.5:1 for SFA:Di-TMP ester and SFA:PE ester was at mole ratio of 4.5:1, H2SO4 (2%), 200 °C for 6 h; SFA:Di-PE ester was at mole ratio of 6.5:1, H2SO4 (2%), 200 °C for 6 h, respectively. The results showed that the resultant products were yielded 89 ± 3% for SFA-TMP, 87 ± 4% for SFA-Di-TMP, 83 ± 5% for SFA-PE, and 78 ± 4% for SFA-Di-PE esters at their esterification optimal condition, respectively.
The esterification between SFA and TMP was producing the SFA-TMP tri-ester. The final resultant tri-ester was then determined by using high temperature column GC-FID (DB-5HT). The terminology used in fat and oil analysis was referred to identify the tri-ester peaks by carbon number of attached alkyl group [33]. The TMP skeleton-backbone was replacing the oil glycerol backbone. The tripalmitate TMP (PPP TMP, TE54) with peak retention time (Rt) at 51.8 min has been used as authentic standard compound for the identification of TMP tri-ester product. Figure 15 shows GC chromatogram of SFA-TMP tri-ester product where the peaks starting to appear at Rt of 51.20 min for TE52 tri-ester are represented by di-palmitoyl-myristate TMP (PPM TMP), followed by TE54 (Rt = 51.8 min) of tripalmitate TMP (PPP TMP), TE56 (Rt = 52.4 min) of di-palmitoyl-stearate TMP (PPS TMP), and TE56 (Rt = 53.8 min) of di-palmitoyl-oleate TMP (PPO TMP). The results showed the resultant product composed about 99.0 ± 3% tri-ester as a major composition. This indicates the successful conversion of SFA and TMP to TMP tri-ester product. For further confirm which fatty acid composition was successfully converted to SFA-TMP tri-ester, the resultant product was analyzed for its fatty acid methyl ester (FAME) composition by GC-FID with nonpolar DB-5 column.
Figure 16 shows GC chromatogram of FAME composition in SFA-TMP tri-ester. The result showed that SFA-TMP tri-ester was composed by 88.6 ± 3% of palmitic acid (C16:0) as a major saturated fatty acid, 3.9% of stearic acid (C18:0), 4.6% oleic acid (C18:1), and 1.7% of myristic acid (C14:0). The result shows that the same dominated and composition saturated fatty acid of palmitic fatty acid composing the SFA-TMP tri-ester as compared to other saturated fatty acid composition in SFA [18]. This confirms the correct selection of palmitic acid as a theoretical representative of SFA in the esterification chemical reaction involved. The yield percentage of resultant SFA-TMP tri-ester was expected to increase after the optimization employed.
3.4 Lubrication property
The lubrication property summary of the synthesized SFA-PFAD esters is shown in Table 3. These results appeared to show that the lubrication properties of the SFA-PFAD esters depend on the chemical structure and weight of alcohols used. It is pronounced that SFA-PFAD esters with high molecular weight have the most promising criteria to be used as a biolubricant with high kinematic viscosity and VI and high FP, OST, and PP values as compared to other synthesized ester dodecanedioate ester (DHD) and commercial lubricant PAO6 [33]. SFA-PFAD esters with high branched carbon chain also showed high VI and FP and inversely reduce the PP and OST values [34].
The efficiency of the biolubricant in reducing friction and wear is greatly influenced by its viscosity. Biolubricants must reduce friction between moving components regardless of whether during application either during cold or hot season at temperatures of up to 200 °C [35]. The viscosity index (VI) highlights how the biolubricant viscosity changes with the variation in temperature [33]. A low VI represents relatively large changes of kinematic viscosity induced by changes in temperature. On the other hand, a high VI indicates that kinematic viscosity is largely constant over a wide temperature range [36]. As the synthesized SFA-PFAD esters have shown high VI values in the range of 115 ± 3 to 135 ± 5, they can be classified as high VI semi-solid base esters. In general, the VI values of the SFA esters are high as compared to the commercial lubricants (PAO6) which VI of 132 ± 3.
The minimum temperature at which the biolubricant oil will pour or flow when it is cooled is defined by pour point (PP). The PP should be low enough to ensure that the biolubricant is pump-able when the equipment is initiated at extremely low temperatures. At the same time, industries are using moderate PP esters to formulate grease, as gear and bearing lubrication [6]. Most of saturated branched esters with relatively poor flow characteristics at low temperatures particularly blend well with additive and thickener for grease lubricating applications. The PP of SFA-PFAD esters in this work, SFA-TMP, SFA-Di-TMP, SFA-PE, and SFA-Di-PE shows PP of 35 ± 2, 30 ± 2, 33 ± 2, and 25 ± 2 °C, respectively. The SFA-Di-PE ester has a high degree of branched carbon chain which shows lower PP among the synthesized esters. Increasing carbon chain branched at a constant carbon chain number will decrease the PP of the ester [37]. Cermak et al. found that linear carbon chained esters have higher PP compared to branched esters [38]. This is because crystallization is prevented by a steric barrier around the individual molecule, owing to the presence of a large branching point in the SFA esters, thus decreasing its PP. Most liquid lubricants require pour point depressant (PPD) molecules to reduce its PP at their low attempted lubrication temperature application. The existing PPD in the market such as chlorinated paraffins and naphthalene polyacrylates, copolymers of ethylene and vinyl esters, copolymers of a-olefins and maleates, poly-α-olefin and polymethacrylate are petroleum based [39]. However, due to the environmental concern, PPD derived from plants and animals is of interest of concern for many researchers. PPDs such as diisopropyl azelate (DIAZ) are among of them [40]. However, the synthesized esters in this study are semi-solid and plausible to be used in formulate grease lubricant for any gear and bearing operating application.
The lowest temperature at which a biolubricant will form a vapor in the air near its surface that will briefly ignite, on exposure to an open flame, is describe by its flash point (FP). Biolubricant should have high FP to allow safe operation and minimum volatilization upon maximum operating temperature [36, 41]. From this study, it was known that the values of FP increase with the molecular weight of the esters. SFA-Di-PE (793.1 g/mol) and SFA-Di-TMP (789.2 g/mol) have the highest FP value at 310 ± 4 and 290 ± 5 °C, respectively, among other SFA-PFAD esters, while SFA-TMP (673 g/mol) and SFA-PE (675 g/mol) have FP of 270 ± 3 and 275 ± 5 °C, respectively. The high FP of SFA-esters makes it suitable to be used for aviation jet engine gear and bearing system [21]. All the prepared esters have higher FP than the commercial PAO6 lubricant with FP of 226 ± 3 °C. These esters still maintained good lubrication properties even at high temperatures.
Biolubricant oxidative stability is another important property which determines the degree of the resistance of the biolubricant to oxidative degradation. Biolubricant must have long lifespan, especially if used in heat transfer units, transformers, turbines, and hydraulics application. The potential esters that are used as biolubricants must be resistant to oxidition during the operational. The higher its oxidative stability temperature (OST) value shows the more stable biolubricant. A rather unexpected outcome was discovered, when SFA-TMP and SFA-PE appeared with the highest OST values among the synthesized esters with 322 ± 6 °C and 300 ± 4 °C, respectively, while OST values of SFA-Di-TMP and SFA-Di-PE were 260 ± 2 °C and 251 ± 3 °C, respectively. Numerous possible aspects could be explained from its hydrocarbon chain chemical composition [8]. SFA-TMP with less branching and uniform structure is thermodynamically stable than others [42]. Despite having a more carbon-hydrogen bond with higher molecular weight, the OST value of SFA-Di-PE is insignificant compared to other SFA-esters. This finding is somewhat counterintuitive and might be due to a negative effect from the increase of branching in SFA-Di-PE against the OST value. This finding trend was in agreement with report claiming that the OST decreases with the increasing in branching of the esters [43]. In general, all the synthesized product of SFA-esters showed high OST values at the range of 251–322 °C, which is higher than the commercialized lubricants. This indicated their suitability to be used as a commercial grease and bearing lubricant application.
4 Conclusion
The esterification reaction of SFA with four different types of alcohols TMP, Di-TMP, PE, and Di-PE was successfully carried out to produce in high yield polyol esters. The optimization process has successfully increased the polyol ester yield and ester selectivity. Reaction temperature has significant effect on the esterification reaction yield. The optimal yield of SFA-TMP and SFA-Di-TMP esters appeared at temperature of 150 °C while the optimal yield of SFA-PE and SFA-Di-PE esters appeared at 200 °C. Polyhydric alcohol with low-branched hydrocarbon chains endorses the esterification process as compared to high-branched alcohols. The study concludes that polyol SFA-TMP ester shows the highest ester yield and ester selectivity. Whereas, polyol SFA-Di-PE was produced at lower yield and hexa-ester selectivity. The results showed that the high end SFA–based polyol esters can be produced through the esterification method, which is cost-effective polyol ester production from palm oil processing by-product. The lubrication properties of the synthesized polyol esters indicated their appropriateness to be used as grease and bearing lubricant application.Acknowledgements.
The authors would like to thank the Universiti Kebangsaan Malaysia for providing laboratory facilities and Sime Darby Plantation Berhad for providing the PFAD samples.
References
Tulashie SK, Kotoka F (2020) The potential of castor, palm kernel, and coconut oils as biolubricant base oil via chemical modification and formulation. Therm Sci Eng Prog 16:100480. https://doi.org/10.1016/j.tsep.2020.100480
Ahmed WA, Salih N, Salimon J (2021) Lubricity, tribological and rheological properties of green ester oil prepared from bio-based azelaic acid. Asian J Chem 33:1363–1369. https://doi.org/10.14233/ajchem.2021.23209
Salih N, Salimon J (2022) A review on new trends, challenges and prospects of ecofriendly friendly green food-grade biolubricants. Biointerface Res Appl Chem 12:1185–1207. https://doi.org/10.33263/BRIAC121.11851207
Afifah AN, Syahrullail S, Azlee NIW, Sidik NAC, Yahya WJ, Abd Rahim E (2019) Biolubricant production from palm stearin through enzymatic transesterification method. Biochem Eng J 148:178–184. https://doi.org/10.1016/j.bej.2019.05.009
Sapawe N, Hanafi MF, Samion S (2019) The use of palm oil as new alternative biolubricant for improving anti-friction and anti-wear properties. Mater today: proceed 19:1126–1135. https://doi.org/10.1016/j.matpr.2019.11.005
Salih N, Salimon J (2021) A Review on eco-friendly green biolubricants from renewable and sustainable plant oil sources. Biointerface Res Appl Chem 11:13303–13327. https://doi.org/10.33263/BRIAC115.1330313327
Calero J, Luna D, Sancho ED, Luna C, Bautista FM, Romero AA, Posadillo A, Berbel J, Verdugo-Escamilla C (2015) An overview on glycerol-free processes for the production of renewable liquid biofuels, applicable in diesel engines. Renew Sustain Energy Rev 42:1437–1452. https://doi.org/10.1016/j.rser.2014.11.007
Nor NM, Salih N, Salimon J (2021) Optimization of the ring opening of epoxidized palm oil using D-optimal design. Asian J Chem 33:67–75. https://doi.org/10.14233/ajchem.2021.22938
Mba OI, Dumont MJ, Ngadi M (2015) Palm oil: processing, characterization and utilization in the food industry - a review. Food Biosci 10:26–41. https://doi.org/10.1016/j.fbio.2015.01.003
Teng S, Khong KW, Ha NC (2020) Palm oil and its environmental impacts: a big data analytics study. J Clean Prod 274:122901. https://doi.org/10.1016/j.jclepro.2020.122901
Japir AA, Salih N, Salimon J (2021) Synthesis and characterization of biodegradable palm palmitic acid based bioplastic. Turkish J Chem 45:585–599. https://doi.org/10.3906/kim-2011-31
Koushki M, Nahidi M, Cheraghali F (2015) Physico-chemical properties, fatty acid profile and nutrition in palm oil. Arch Adv Biosci 6:117–134. https://doi.org/10.22037/jps.v6i3.9772
Ludin NA, Bakri MAM, Kamaruddin N, Sopian K, Deraman MS, Hamid NH, Asim N, Othman MY (2014) Malaysian oil palm plantation sector: exploiting renewable energy toward sustainability production. J Clean Prod 65:9–15. https://doi.org/10.1016/j.jclepro.2013.11.063
Mannu A, Ferro M, Dugoni GC, Garroni S, Taras A, Mele A (2020) Response surface analysis of density and flash point in recycled waste cooking oils. Chem Data Collect 25:100329. https://doi.org/10.1016/j.cdc.2019.100329
Zulkifli NWM, Azman SSN, Kalam MA, Masjuki HH, Yunus R, Gulzar M (2016) Lubricity of bio-based lubricant derived from different chemically modified fatty acid methyl ester. Tribol Int 93:555–562. https://doi.org/10.1016/j.triboint.2015.03.024
Ping BTY, Yusof M (2009) Characteristics and properties of fatty acid distillates from palm oil. Oil palm bull 59:5–11
Metre AV, Nath K (2015) Super phosphoric acid catalyzed esterification of palm fatty acid distillate for biodiesel production: physicochemical parameters and kinetics. Polish J Chem Technol 17:88–96. https://doi.org/10.1515/pjct-2015-0013
Jumaah MA, Yousif MFM, Salimon J, Murad B (2019) Separation of saturated and unsaturated fatty acids of palm fatty acid distilled via low-temperature methanol crystallization. Malaysian J Chem 21:8–16
Baharudin KB, Taufiq-Yap YH, Hunns J, Isaacs M, Wilson K, Derawi D (2019) Mesoporous NiO/Al-SBA-15 catalysts for solvent-free deoxygenation of palm fatty acid distillate. Micropor Mesopor Mat 276:13–22. https://doi.org/10.1016/j.micromeso.2018.09.014
Haraldsson G (1984) Separation of saturated/unsaturated fatty acids. J Am Oil Chem Soc 61:219–222. https://doi.org/10.1007/BF02678772
Bahadi M, Salih N, Salimon J (2021) D-Optimal design optimization for the separation of oleic acid from Malaysian high free fatty acid crude palm oil fatty acids mixture using urea complex fractionation. Appl Sci Eng Prog 14:175–186. https://doi.org/10.14416/j.asep.2021.03.004
Machado NT, Brunner G (1997) Separation of saturated and unsaturated fatty acids from palm fatty acids distillates in continuous multistage counter current columns with supercritical carbon dioxide as solvent: a process design methodology. Ciênc Tecnol Aliment 17:361–370
Qiwen Y, Guoming Y, Haijun L (2021) Extraction and separation of unsaturated fatty acids from sunflower oil. IOP Conf Series: Earth Environ Sci 680:012063. https://doi.org/10.1088/1755-1315/680/1/012063
Nor NM, Derawi D, Salimon J (2019) Esterification and evaluation of palm oil as biolubricant base stock. Malaysian J Chem 21:28–35
Nowicki J, Stańczyk D, Drabik J, Mosio-Mosiewski J, Woszczyński P, Warzała M (2016) Synthesis of fatty acid esters of selected higher polyols over homogeneous metallic catalysts. J Am Oil Chem Soc 93:973–981. https://doi.org/10.1007/s11746-016-2840-7
Pavia DL, Lampman GM, Kriz GS, Vyvyan JR (2015) Introduction to spectroscopy, 5th edn. Cengage Learning Inc, USA
Polansky R, Prosr P, Vik R, Moravcova D, Pihera J (2017) Comparison of the mineral oil lifetime estimates obtained by differential scanning calorimetry, infrared spectroscopy, and dielectric dissipation factor measurements. Thermochim Acta 647:86–93. https://doi.org/10.1016/j.tca.2016.12.002
Alexandri E, Ahmed R, Siddiqui H, Choudhary MI, Tsiafoulis CG, Gerothanassis IP (2017) High resolution NMR spectroscopy as a structural and analytical tool for unsaturated lipids in solution. Molecules 22:1663. https://doi.org/10.3390/molecules22101663
Mortier RM, Fox MF, Orszulik S (2010) Chemistry and technology of lubricants, 3rd edn. Springer, New York
Lye YN, Salih N, Salimon J (2021) Optimization of partial epoxidation on Jatropha curcas oil based methyl linoleate using urea-hydrogen peroxide and methyltrioxorhenium catalyst. Appl Sci Eng Prog 14:89–99. https://doi.org/10.14416/j.asep.2020.12.006
Jumaah MA, Salih N, Salimon J (2021) Optimization for esterification of palm fatty acid distillate based biolubricant using D-optimal design. Iran J Chem Chem Eng. https://doi.org/10.30492/IJCCE.2021.521586.4481
Samidin S, Salih N, Salimon J (2021) Synthesis and characterization of trimethylolpropane based esters as green biolubricant basestock. Biointerface Res Appl Chem 11:13638–13651. https://doi.org/10.33263/BRIAC115.1363813651
Nor NM, Salih N, Salimon J (2021) Chemically modified Jatropha curcas oil for biolubricant applications. Hem Ind 75:117–128. https://doi.org/10.2298/HEMIND200809009N
Jumaah MA, Salih N, Salimon J (2022) Optimization process for the synthesis of polyol-oleates from Malaysian unsaturated palm fatty acid distillate. Iran J Chem Chem Eng. https://doi.org/10.30492/IJCCE.2022.526491.4617
Ocholi O, Menkiti M, Auta M, Ezemagu I (2018) Optimization of the operating parameters for the extractive synthesis of biolubricant from sesame seed oil via response surface methodology. Egypt J Pet 27:265–275. https://doi.org/10.1016/j.ejpe.2017.04.001
Owuna FJ, Dabai MU, Sokoto MA, Dangoggo SM, Bagudo BU, Birnin-Yauri UA, Hassan LG, Sada I, Abubakar AL, Jibrin MS (2020) Chemical modification of vegetable oils for the production of biolubricants using trimethylolpropane: a review. Egypt J Pet 29:75–82. https://doi.org/10.1016/j.ejpe.2019.11.004
Totten GE, Westbrook SR, Shah RJ (2003) Fuels and lubricants handbook: technology, properties, performance, and testing New York, USA.
Cermak SC, Brandon KB, Isbell TA (2006) Synthesis and physical properties of estolides from lesquerella and castor fatty acid esters. Ind Crops Prod 23:54–64. https://doi.org/10.1016/j.indcrop.2005.04.001
Verma P, Dwivedi G, Behura AK, Patel DK, Verma TN, Pugazhendhi A (2020) Experimental investigation of diesel engine fuelled with different alkyl esters of karanja oil. Fuel 275:117920. https://doi.org/10.1016/j.fuel.2020.117920
Warasanam S, Pengprecha S (2012) Synthesis of pour point depressant from dicarboxylic acid for biodiesel. Proceed Inter Conf Chem Process Environ Issues, Singapore 15–16:183–186
Encinar JM, Nogales-Delgado S, Sánchez N, González JF (2020) Biolubricants from rapeseed and castor oil transesterification by using titanium isopropoxide as a catalyst: production and characterization. Catalysts 10:366. https://doi.org/10.3390/catal10040366
Chan CH, Tang SW, Mohd NK, Lim WH, Yeong SK, Idris Z (2018) Tribological behavior of biolubricant base stocks and additives. Renew Sust Energy Rev 93:145–157. https://doi.org/10.1016/j.rser.2018.05.024
Arbain NH, Salimon J, Salih N, Ahmed WA (2022) Optimization for epoxidation of Malaysian Jatropha curcas oil based trimethylolpropane ester biolubricant. Appl Sci Eng Prog. https://doi.org/10.14416/j.asep.2021.10.009
Funding
This project received funding from Universiti Kebangsaan Malaysia under the research grant no. GUP-2016–058 and Sime Darby Plantation Berhad through grant no. ST-2014–019.
Author information
Authors and Affiliations
Contributions
Majd Ahmed Jumaah: investigation and methodology. Firas Layth Khaleel: formal data analysis and data curation. Nadia Salih: visualization, writing—original draft, and writing—review and editing. Jumat Salimon: conceptualization, funding acquisition, resources, project administration, supervision, validation, and writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jumaah, M.A., Khaleel, F.L., Salih, N. et al. Synthesis of tri, tetra, and hexa-palmitate polyol esters from Malaysian saturated palm fatty acid distillate for biolubricant production. Biomass Conv. Bioref. 14, 1919–1937 (2024). https://doi.org/10.1007/s13399-022-02517-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02517-x