Abstract
The present study investigated the comparative effect of anaerobic co-digestion of sewage sludge and wheat straw along with waste iron scraps on enhanced methane yield under mesophilic and thermophilic temperature. The co-digestion of sewage sludge with wheat straw enhances the cumulative biogas yield by 6.92 and 5.69 folds under mesophilic and thermophilic conditions respectively. The iron scraps of 250 mg/l as an additive were found to be more effective for methane enhancement as compared to 500 mg/l, under both conditions. The co-digestion of both substrates under mesophilic and thermophilic conditions could enhance 2.67 and 2.37 (approx.) folds methane yield, whereas waste iron scraps supplementation of 250 mg/l further improves it to 3.7 and 4.36 folds (approx.) methane yield respectively. The highest methane yield was observed in the thermophilic digester T-250 followed by M-250, M-500, T-500, M-CTRL, and T-CTRL. Among the tested models, the exponential model was best fitted for the methane production rate. The modified Gompertz model and logistic function model at mesophilic and thermophilic conditions respectively best evaluate the methane yield kinetics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Wastewater sludge is an inevitable by-product resulting from the processing of wastewater treatment that increases along with stringent discharge standards [1]. Every year, a huge quantity of sewage sludge has been generated from wastewater treatment plants (WWTPs), which should be efficiently disposed of to avoid its secondary pollution effects [2]. It has been considered one of the most hazardous categories among the different types of municipal wastes, the management of which must be the topmost priority in the whole world. The application of individual anaerobic fermentation of sewage sludge is often restricted due to the low methane yield and slow hydrolysis rate [3]. Mono-digestion of sewage sludge did not achieve efficient biogas generation due to low carbon and higher nitrogen contents, whereas agricultural waste is comprised of high carbon and low nitrogen contents [4]. The improved performance of co-digestion of waste activated sludge (WAS) with other organic substrates has become an efficient way to overcome the drawbacks of mono-digestion and accelerate the economic viability of anaerobic digestion (AD) for maximum methane production [5]. The space requirement and appropriate disposal of municipal solid waste (MSW) one of the challenges faced by many countries around the globe is becoming a huge concern [6]. The use of crop residuals, instead of crops, as a source of energy, contributes to food safety while creating an alternative solution to waste disposal problems [7]. Various studies of the co-digestion of energy crops (grass, maize, rice/wheat straw, algal biomass, etc.) with either sewage sludge or animal manure have been conducted for methane generation [8,9,10,11].
Under different operating conditions, some alternative techniques have also been carried out to enhance the biogas yield by stimulating microbial activity using different biological and chemical additives [1]. At present, different types of accelerants such as trace elements (Fe, Co, Ni, Ca, Mg, Zn), polymers, bio-additives (enzymes and microorganisms), macro-nutrients, and minerals are used to enhance the biogas production, shorten the digestion period, enhance the microbial and enzymatic activity, etc. [12]. Among all of these, supplementation of waste iron scraps as an accelerant seems to be a cost-effective alternative approach to boost up the methanogenic activity along with enhanced methane production. As different forms of iron are available but among these waste iron scraps (WIS), the most widely available and easily accessible from industry has the great potential to enhance biogas production. Based on previous studies, the most possible pathway that leads to the enhancement of biogas production includes (i) producing more acetate by reducing the oxidation–reduction potential (ORP); (ii) improving the biogas quality through hydrogen evolution corrosion; and (iii) stimulating the microbial as well as enzymatic activity [13]. Moreover, the rusty iron scraps (zero valent iron) having layers of iron oxides are more efficient than the cleaned surface iron scraps in enhancing the anaerobic digestion [4]. Some studies also suggested that conductive iron oxides (Fe3O4) are involved in promoting methane production through a direct interspecies electron transfer mechanism between the organic oxidizing bacteria and methanogens [4]. Additionally. the ZVI, an electron donor, also plays an important role in enhancing the anaerobic environment by increasing the working efficiency of anaerobic digesters for wastewater treatment [14]. Some studies reported that in anaerobic digesters, ZVI addition significantly improves the chemical oxygen demand (COD) removal efficiency by 25% for biological wastewater treatment [15,16,17,18]. In anaerobic digesters, the ZVI (zero valent iron) delivers electrons for methanogens due to its low oxidation–reduction potential (ORP) (Eo = − 440 mV), which aids in enhancing the methanogenic activity and AD performance [19]. Temperature one of the most important environmental factors directly affects the dynamic condition of microorganisms. Many earlier studies focused on promoting the efficiency of co-digestion at different operating temperatures, i.e., mesophilic, thermophilic, and even hyperthermophilic conditions [20]. As compared to the mesophilic anaerobic process, thermophilic fermentation possesses higher efficiency of waste stabilization through the destruction of pathogens, reduced retention time, and increased biogas yields along increased microbial activity [21]. Modeling represents an effective way to optimize anaerobic co-digestion (AcoD) process design and operation, monitor anaerobic digesters, and better formalize available knowledge [22].
To evaluate the execution of anaerobic digestion (AD) process, different kinetic models, i.e., first-order model, Monod model, substrate mass balance model, Chen and Hashimoto model, logistic function model, modified Gompertz model, etc., have been used [23]. The anaerobic degradation of particulate matter in batch or continuous mode having a fixed kinetic structure can be modeled using appropriate kinetic models [24]. The modified Gompertz and Logistic models following the sigmoidal pattern can correlate methanogenic bacteria growth with produced methane [25]. A transference function model deliberates the stationary and exponential phase in biogas generation is used in case of near or zero lag phase [13, 26]. Predictive modeling not only plays important role in biogas production but also deeply elaborates the behavior of microorganisms at varying physical and chemical conditions like pH and temperature in food microbiology [27]. In addition to the above-discussed models, several other alternative kinetic models have also been proposed for anaerobic digestion of different types of substrates for biogas production.
The present study focused on co-digestion of sewage sludge and wheat straw on biogas production along with the feasibility of two different selected concentrations of waste iron scraps for biogas/methane production at mesophilic and thermophilic temperatures. The kinetic study of models for methane production rate and cumulative methane yield was also carried out. The best fit model was evaluated using the error functions (R2, AIC, and BIC) to describe the production rate and cumulative methane yield under mesophilic and thermophilic conditions.
2 Materials and methods
2.1 Culture preparation
The inoculum used in the present study was collected from a 5L anaerobic digester maintained at mesophilic conditions (35–40 °C). The microbial activity of the digester was retained by adding slurry (cow dung and water in the ratio 1:1) [28]. The microbial activity in the reactor was sustained by the addition of a small quantity of cow dung at an interval of 30–40 days. The volume of culture was maintained by adding the amount of slurry integrated into the digester and the same amount of slurry taken out from it [28]. The pH was maintained at 7.0 ± 0.5 using the 0.1 N NaOH/HCl, whenever required.
2.2 Experimental design for batch reactors
The primary sludge as nutrients source and wheat straw as substrate were collected from full-scale STP (Sewage Treatment Plant), and the agricultural field (Pethapur village), Gandhinagar, Gujarat (India) respectively. The granular iron scraps ranging from 0.12 to 2.0 mm were collected from the local iron welding shop, Gandhinagar, Gujarat (India). The experiments were conducted in triplicates in 130-ml serum bottles. Six sets of bottles with 50 ml working volume were used as digesters for anaerobic co-digestion. Three sets of bottles labeled as M-CTRL, M-250, and M-500 were operated under the mesophilic conditions and the remaining three sets labeled as T-CTRL, T-250, and T-500 were operated under thermophilic conditions. The required quantity of 1.25 g (5.0%) of air-dried pulverized wheat straw (size: ≤ 2 mm) mixed with 25 ml sludge was added to the digesters followed by the addition of 25 ml active inoculum. In the biogas production from wheat straw only, its initial and final digestate characterizations are presented in the earlier study [29]. In the digesters, M-250 and T-250, and M-500 and T-500 iron scraps granules of 250 mg/l and 500 mg/l respectively were amended. Anaerobic digesters M-CTRL and T-CTRL were operated without the addition of iron scraps granules to observe the effect of co-digestion of sewage sludge with wheat straw on biogas production. Furthermore, two more sets of digesters were designed to observe the biogas production potential of sewage sludge (SL) and inoculum (CUL) used only at mesophilic temperature. The reactors were shaken manually 3–4 times daily to mix the contents properly. The headspace of each digestor was purged with N2 (99.99% purity) for 5 min to create an anaerobic environment. The digestors were sealed using rubber septa and 20-mm aluminum caps. To avoid the excess pressure generated at the initial stage during biogas formation, 30 ml headspace gas was evacuated from each digester using an airtight syringe.
The digestors M-CTRL, M-250, and M-500 were incubated at 37 ± 1 °C in a BOD incubator, whereas the digestors T-CTRL, T-250, and T-500 were kept at 57 ± 1 °C in a hot air oven. The produced biogas volume was measured using a 60-ml glass syringe. At the initial stage, i.e., on the 1st day, the biogas was measured after 12 h followed by a 24-h interval. The dilution at the initial stage due to N2 purging was also corrected from the cumulative volume of biogas formed for the two initial measurements. Measurement of the methane contents in the biogas was measured by subtracting the CO2 contents from the total volume of gas produced using an airtight column based on the partial pressure change [28].
2.3 Kinetic modeling of methanogenesis
The methane production rate and cumulative methane produced were tested for various kinetic models. The model parameters were analyzed using the nonlinear fit function in Origin Pro-2018 software (learning edition). Curve Expert Professional 2.6.5 (Trial version) was used for the evaluation of the first-order kinetic model. The methane production rate was evaluated using the linear model [30, 31] and exponential model [12] as presented in Eqs. 1 and 2.
where y is methane production rate (ml/day); t is digestion time (days); a and b in (ml/day) are constants, c in (day−1) is also constant and is positive for the increasing order and negative for the decreasing order.
The substrate hydrolysis rate-limiting step was calculated using the first-order kinetic model by Eq. 3 [13].
where P (ml) is accumulated methane production; Po (ml) is maximum methane yield; k is methane production rate constant and t is incubation time (days).
The above model (Eq. 3) does not estimate the specific methane yield rate and lag phase hence modified Gompertz (Eq. 4) and logistic function (Eq. 5) models were applied to predict the cumulative methane production. These two sigmoidal functions help in correlating the biogas produced in the anaerobic digester by methanogenic archaea activity ƛ [31, 32].
where y is cumulative methane production (ml); A is the maximum specific methane production potential (ml); Um is maximum specific methane production rate (days); λ is a lag phase (days); t is the incubation time (days); e is exponential (1). Model parameters A, Um, and λ of the models (Gompertz model and logistic model) were analyzed using the Solver function (MS Excel- 2010).
2.4 Analytical methods and methane contents measurement
Total solids and volatile solids contents were analyzed as per APHA (2012) [33]. The digestate was centrifuged for 15 min at 7000 rpm. The obtained supernatant was further processed for the analysis of COD (chemical oxygen demand), ammonia (NH3), total alkalinity, and VFAs (volatile fatty acids) as per standard methods [33]. The Double Beam Spectrophotometer (Model-Dynamica HOLO DB-20 UV–VIS) was used for components analysis. The methane content present in biogas was measured based on the partial pressure method [28]. MS Excel (Version 2010) was used for statistical analysis.
3 Results and discussion
3.1 Substrate and nutrients source characteristics
The general physicochemical characteristics of feedstock (wheat straw), sewage sludge, and inoculum are summarized in Table 1. The analysis shows that wheat straw comprised of 97.87 ± 0.71% total solids on a wet weight basis and 75.6 ± 0.77% volatile solids on a dry weight basis, whereas total solids and volatile solids content present in sludge were 4.72 ± 0.36% and 25.15 ± 0.99% respectively. The pH and alkalinity level of the sewage sludge has the potential to support microbial growth by maintaining the buffering capacity during biogas production and neutralizing the accumulation of volatile fatty acids (VFAs) during the acidification process.
3.2 Effect of co-digestion on biogas and methane content
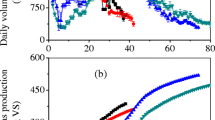
The cumulative biogas and methane production along with methane content (%) at mesophilic and thermophilic experimental groups are plotted in Fig. 1. The cumulative biogas yield of 566.0 ± 4.24 ml and 465.0 ± 7.07 ml was observed under the mesophilic and thermophilic conditions respectively. The biogas production in anaerobic digesters M-CTRL and T-CTRL lasted for 27 days, whereas in iron scraps, amended digesters M-250, M-500, T-250, and T-500 biogas formation lasted for 31 days and 30 days respectively. As shown in Table 2, the cumulative biogas and methane yield of mono-digested sewage sludge was found to be 39.16 ± 2.12 ml and 28.45 ± 1.56 ml and that of inoculum 42.66 ± 4.48 ml and 31.0 ± 3.23 ml respectively. The mono-digestion of primary sludge having sufficient buffering capacity produces less volume of biogas due to the presence of low content of volatile solids. The higher volatile content addition of pulverized wheat straw (< 2 mm) generates a cumulative biogas yield of 484.18 ml at mesophilic and 383.18 ml at thermophilic processes. Co-digestion of sewage sludge with wheat straw enhances the biogas and methane yield by 6.9 folds (approx.) and 2.64 folds (approx.) under the mesophilic condition and 5.69 folds (approx.) and 2.4 folds (approx.) at the thermophilic condition respectively. In terms of ml/g VS, the maximum biogas production of 68.082 ± 1.035 ml/gVS was observed in the digester T-CTRL followed by digesters of M-CTRL (47.804 ± 0.358 ml/g VS), M-500 (38.153 ± 0.462 ml/g VS), T-500 (36.2944 ± 0.41 ml/g VS), M-250 (33.424 ± 0.213 ml/g Vs), and T-250 (30.534 ± 0.611 ml/g VS). The co-digestion generally has a positive synergistic effect on biogas and methane production as the case relies mostly on the composition of the substrate being used [34,35,36]. The co-digestion not only increases the cumulative biogas and methane yield but also improve the biogas quality in the anaerobic digesters (T-CTRL), producing 13.23 ± 0.41 ml/g VS (30.26 ± 0.09%) followed by M-CTRL which produces 20.60 ± 0.37 ml/g VS (27.68 ± 1.07%) of methane. The higher biogas production rate and cumulative yield from faster disintegration of feedstock and hydrolysis at thermophilic conditions mostly were due to the higher enzymatic activity at higher temperatures [37].
As shown in (Fig. 1B and C), the methane production rate was improved after co-digesting the sludge (SL) with wheat straw at mesophilic and thermophilic operation. As the mono-digestion of sewage sludge (SL) and inoculum (CUL) alone does not produce too much biogas, therefore, co-digesting the waste aids in the improvement of the quality and quantity of biogas produced. Not only does this increased temperature also make the methane production rate faster and better co-digestion of the wastes. Temperature plays a vital role in the anaerobic digestion of substrate; the higher the temperature, the more is the conversion of product from a complex to a simple form [38], whereas effective conversion of substrate to methane needs a temperature-dependent microbial community structure having unique properties [39].
3.3 Effect of waste iron scraps amendment on methane production
Table 2 shows the cumulative biogas and methane production amended with iron scraps (ISs) at 250 mg/l and 500 mg/l under mesophilic and thermophilic conditions. Cumulative biogas yield of 566.0 ± 4.24 ml, 555.5 ± 3.54 ml, and 584.5 ± 7.07 ml were produced in mesophilic systems M-CTRL, M-250, and M-500 respectively, whereas thermophilic digesters produced 465.0 ± 7.07 ml (T-CTRL); 442.74 ± 8.86 ml (T-250), and 500.50 ± 5.66 ml (T-500) of biogas. Despite the lesser volumetric biogas production, the thermophilic incubation enhances the disintegration of the substrate and thereby the conversion rates to enhanced methane contents in the digestors. The biogas quality at thermophilic conditions was also improved as compared to mesophilic conditions, that is why more biomethane was produced. The digestion at a higher temperature (57 °C) catalyzes the reaction rate which leads to enhanced biogas production as compared to a lower temperature (mesophilic). The addition of the sewage sludge may be providing micronutrients to the microbes to improve the bio-gasification under thermophilic conditions. The enhancement in methanogenesis with an increase in the critical biological diversity involved in denitrification and the increased redundancy in the utilization of substrate and primary metabolites like acetate for stable production of biogas utilizing food waste in two-phase thermophilic AD by supplementation by nutrients [40]. Cecchi et al., in a study, also reported that the digestion of municipal solid waste results in increased gas production at thermophilic incubation up to 2–3 times than mesophilic incubation [41].
The cumulative methane yield of 13.19 ± 0.28 ml/g VS and 17.89 ± 0.83 ml/g VS were observed in M-250 and T-250 anaerobic digesters, whereas digesters M-500 and T-500 having double the iron scraps produce 11.60 ± 0.76 ml/g VS and 12.73 ± 0.71 ml/g VS of methane respectively. Under thermophilic operation anaerobic digesters, T-250 and T-500 produce 84.34% and 27.76% more methane as compared to the control T-CTRL. In a study, Logan observed the thermophilic degradation of fat, oil, and grease (FOG) could improve process performance [42]. Maximum methane production achieved in anaerobic digester M-250 under mesophilic operation was 39.96% higher followed by 13.44% (M-500) as compared to the M-CTRL. The corroded rusty waste iron scraps, as a small electrochemical cell under anaerobic conditions, generate hydrogen which along with CO2 was oxidized by hydrogenotrophic methanogens producing methane. The addition of the blast furnace slag (BFS) dramatically affects COD removal rates and releases much more Fe in the aquatic environment compared to the other slags like steelmaking converter slag (SCS), and steelmaking ladle slag (SLS); therefore, higher biogas production is obtained in reactors where BFS with higher Fe content [43]. Methanogenesis was improved by hydrogen evolution through corrosion [44], whereas the addition of iron reduces the ORP thereby increasing the production of more acetate resulting in more methane formation [45]. Generally, it has been conceivable that waste iron scraps generated from industries are the rusty iron after the consumption of the rusty layer, the inner metal iron (Fe°) starts functioning under anaerobic conditions. The other impurities of heavy metals which at trace levels associated with iron scraps may also result in an increase in methane production by enhancing anaerobic digestion. Moreover, Lovely [46] in their study reported that Iron (Fe3+) was reduced into soluble iron (Fe2+) and the complex organic matter was gradually oxidized to its simple form and even mineralized. The highest enriched methane production was observed in T-250, indicating the synergistic effect of waste iron scraps under thermophilic incubation conditions. Maintaining optimal iron concentration in anaerobic digesters is a critical step to improve bacterial activity [47]. The anaerobic reactor T-250 (58.62 ± 3.90%) produces highest enriched methane followed by M-250 (39.48 ± 1.08%); T-500 (35.05 ± 1.57%); and M-500 (30.4 ± 1.63%) respectively. Instead of working as an electron donor, zero valent iron also helps in accelerating the anaerobic environment which improves the working efficiency of the digester [14].
As compared to mesophilic reactors (M-CTRL, M-250, and M-500), the methane production rate was higher in thermophilic digesters (T-CTRL, T-250, and T-500) (Fig. 1B and C). A continuous decreasing trend was observed in M-250 and M-500 in iron scraps amended digester as well as in M-CTRL beyond the 4th day of the incubation except for a sudden increase in production rate in the M-250 digester which starts from the 6th day and continues till 13th day. The production rate observed was also a little bit higher for the digester M-250 as compared to M-CTRL and M-500 till the end of the experiment, whereas under thermophilic, a decreasing trend was observed till the 6th day and after that production rate again rises to the 9th day and then again starts decreasing in the T-250 and T-500 digesters along with T-CTRL. In comparison to the digester T-CTRL and T-500, the methane production rate was higher in the T-250 digester amended with iron scraps. This probably may be due to the discrepancy in the growth period required by the microbes under mesophilic and thermophilic conditions. The early methane production peak begins on the 1st day of incubation which may be due to the available soluble components for methanogens under both mesophilic and thermophilic conditions. The early peak of the methane may be due to the formation of hydrogen, which was not estimated in the present study. Though later on this, hydrogen produced is consumed in the methanogenesis process. The maximum methane production rate of 32.87 ml/day and 28.25 ml/day was observed in the digester T-250 and T-500 under thermophilic conditions while it was 30.62 ml/day and 33.49 ml/day in the anaerobic system under mesophilic process respectively. After 24 h of the incubation period, a continuous decrease in the mesophilic digester and thermophilic digester was observed, whereas the production rate increased when supplemented with 250 mg/l and 500 mg/l of ISs. This might be due to the increase in the solubility of Fe in the system. A similar finding on an elevated level of the Fe in bio-electrolysis was also reported [28].
3.4 Changes in liquid phase components
The pH observed at the end of the experiment was higher under thermophilic conditions as compared to the mesophilic incubation. For all the experimental groups, the pH value ranges from 7.45 ± 0.07 to 7.793 ± 0.015 indicating the normal microbial activities in the anaerobic digester. The optimum pH range for methanogenic activity ranges from 6.5 to 7.5 and below 6.5, and bacterial growth and methane yield both were suppressed [48,49,50]. Total alkalinity (TA) and NH4+-N in digesters having waste iron scraps were found lower in comparison to respective mesophilic/thermophilic-control digester except T-250 having equivalent to T-CTRL. The increase in total alkalinity may be due to the release of ammonia that binds with CO2 forming ammonium bicarbonate thereby neutralizing the effect of excess CO2 released. The anaerobic digestion of N-rich feedstock leads to the degradation of proteinaceous material resulting in ammonia accumulation [51]. An optimum concentration of ammonical nitrogen (NH4+-N) ensures sufficient buffering capacity to the anaerobic digesters, while a higher concentration is reported as a strong inhibiting factor of biogas production [52, 53].
VFAs play an important role in anaerobic digestion for biogas formation. When the concentration of VFAs (as acetic acid) exceeds 800 mg/l in an anaerobic digester, it leads to its failure [54] and stops when the concentration reaches above 13,000 mg/l [55]. VFA concentration during anaerobic digestion of agriculture waste co-digested with sewage sludge under mesophilic and thermophilic conditions in the presence of waste iron scraps is shown in Table 2. The lesser VFAs in M-CTRL and T-CTRL digester with lesser methane generation indicated poor acidogenesis in absence of iron scraps. Additionally, no significant accumulation of the VFAs, an advantage for methanogenesis was observed in the digesters supplemented with waste iron scraps. So, iron scraps aid in enhancing the transformation of VFAs to methane. The s-COD and VS contents reported were also found higher in M-CTRL and T-CTRL in comparison to the digesters supplemented with iron scrap, indicating the lesser efficiency of methane production. The VS destruction of 50.34 ± 0.12%, 51.64 ± 1.72%, 52.46 ± 0.65%, and 53.17 ± 2.7% in M-250, M-500, T-250, and T-500 respectively was higher under the thermophilic conditions. Interestingly, digesters M-CTRL and T-CTRL show much higher VS destruction 55.12 ± 1.03% and 60.13 ± 2.97% respectively in comparison to digesters operated in absence of iron scraps. This is indicating the more susceptibility of the microbes involved in the hydrolysis of the biomass towards the presence of Fe generated from the leaching of ISs.
3.5 Methanogenesis kinetic modeling
The hydrolysis constants (k) evaluated from the first-order kinetic model are shown in Table 2. The digester M-CTRL shows the maximum k-value of 0.007 followed by 0.005 (M-500) and 0.003 (M-250) under mesophilic operation. A higher k-value for M-CTRL justifies the maximum degradability but the substrate is not wholly converted to products of interest, maybe due to diversion of the electrons flux to products other than the methane, whereas in M-250, maximum methane potential (Po) was observed with a lower k-value in comparison to M-500 with maximum k (0.005) and Po (175.46). The lower Po value in the presence of 500 mg/l iron scraps causes iron toxicity and thereby inhibiting the specific microbial growth.
Under thermophilic operation, maximum k-value for substrate hydrolysis rate constant was found in the digester T-500 (0.003) followed by T-CTRL (0.002) and T-250 (0.002) but the methane production potential (Po) for these digesters follows the order of T-250 (350.374) > T-500 (207.382) > T-CTRL (191.622). It can be easily inferred that the addition of optimum waste iron scraps concentrations enhances the methane yield by efficient conversion rather than increasing the degradation rate. As reported in the earlier study, the mechanism behind the increased methane production using ZVI addition hypothesized the enrichment of major enzyme activities linked to hydrolysis and acidification [45].
Figure 2 shows the linear and exponential plots of methane production rate from sewage sludge co-digested with wheat straw along with waste iron scraps at mesophilic (37 ± 1 °C) and thermophilic (57 ± 1 °C) conditions. The coefficient of determination (R2) values of the Linear model vary from 0.435 to 0.804 and the exponential model ranges from 0.742 to 0.992 (Table 3) for all the anaerobic digesters under different conditions (mesophilic and thermophilic). Higher R2 values of the exponential model show better simulation as compared to the Linear model, as well as closer values of R2 and adjusted R2 of methane production rate analyzed by linear and exponential models and the goodness of fit of experimental data, fit of the model. The F-test value also confirms the suitability of models. High kinetic constant values (a), 0.349 (M-250) and 0.476 (T-250), simulated by the exponential model also ratifies the optimum 250 mg/l concentration of waste iron scraps suitable for methanogenesis at mesophilic and thermophilic conditions whereas the higher value (a = 0.476) also confirms the maximum methane production potential at thermophilic conditions.
Table 4 shows the simulated kinetic parameters obtained from modified Gompertz model (MGM) and logistic function model (LFM). The predicted cumulative methane data obtained from nonlinear regression analysis were plotted against the experimental methane to ascertain a visual fit, to begin with (Fig. 3). The experimental values with a very little deviation of cumulative methane yield (y) were found nearly equivalent to the predicted values as analyzed by Gompertz and the Logistic models. The closer values show the degree of goodness of fit of the experimental data and the proposed models.
Under mesophilic temperature, the cumulative methane production from different anaerobic digesters (M-Control, M-250, and M-500) forms a reverse L-shape curve (Fig. 3A, C, and E) which may be due to the breakdown of easily available soluble organic matter into the products. An elongated S-shaped or stepped curve has been observed from the cumulative product formed under thermophilic conditions in digesters T-CTRL, T-250, and T-500 (Fig. 3B, D, and F). As in the present study, mesophilic inoculum is used for product formation while using the same mesophilic inoculum under thermophilic temperature and the anaerobes need to acclimatize themselves according to the thermophilic conditions. The simple substrate or easily degradable organic matter adopts the L-shape curve while the degradation of the complex substrate or potential of acute inhibition results in an elongated S-shape or stepped curve [56].
While using the modified Gompertz model (MGM), maximum methane production rate (Um) for digesters T-250 and T-500 was 16.59 ml/day and 10.71 ml/day respectively. Similarly, for the logistic function model, the maximum methane production rate was 17.28 ml/day and 10.31 ml/day for digesters T-500 and T-250 respectively at thermophilic conditions. The value of Um shows that the addition of waste iron scraps of 250 mg/l and 500 mg/l to the anaerobic digesters had improved the rate of methane production under the anaerobic thermophilic process (Table 4). As compared to the mesophilic process, an increase in the lag phase (λ) time is observed in the thermophilic process in the digester T-250 (1.61 days MGM and 2.28 days LFM) followed by T-CTRL (8.16 days MGM and 8.60 days LFM); however, the lag phase is absent in the digester T-500. An increase in lag phase was observed due to the acclimatized mesophilic inoculum and therefore under the thermophilic process, the bacteria need to adapt themselves at the higher temperature.
The higher R2 values of the tested models assured the suitability of methane production data (Table 4). The coefficient of determination R2 values of all the executed models under mesophilic and thermophilic conditions at different iron scraps concentrations range from 0.925 to 0.993. The higher R2 values were observed by the modified Gompertz model (MGM) under thermophilic conditions and the logistic function model at mesophilic conditions. The R2 values of the logistic function model (LFM) at thermophilic conditions for T-CTRL, T-250, and M-T-500 were 0.988, 0.993, and 0.991 for modified Gompertz model (MGM) under mesophilic conditions for M-CTRL, M-250, and M-500 were 0.944, 0.984 and 0.954 respectively.
4 Conclusion
The mesophilic co-digestion of primary sewage sludge with wheat straw enhances the methane yield by 2.63 (approx.) while at thermophilic by 2.37 (approx.) folds. The addition of waste iron scraps to the co-digested waste not only enhanced the biogas yield quantitatively but also enriched it qualitatively. The addition of waste iron scraps of 250 mg/l to the co-digestion of sewage sludge with wheat straw enhances the methane yield by 3.7 folds (approx.) and 4.36 folds (approx.) under mesophilic and thermophilic conditions respectively, whereas the addition of 500 mg/l of iron scraps inhibits the methanogenic activity. The improved performance ascribed that the supplementation of waste iron scraps reduces the oxidation–reduction potential to enhance the anaerobic conditions and the evolved hydrogen also leads to enhanced methane production. The simulated kinetic model also supports the goodness of fit. Higher values of the coefficient of determination (R2) and lower AIC and BIC error function values also support the exponential model as the best fit for methane production rate and modified Gompertz model and logistic function model for cumulative methanogenesis. The modified Gompertz model was satisfied with the experimental data at the mesophilic process whereas the logistic function model fitted with the thermophilic data. The present study can be used to evaluate the kinetics of biogas production using waste iron scraps more reasonably.
Data availability
The data is submitted to the Central University of Gujarat, Gandhinagar-382030 (Gujarat) India, as the Ph.D. thesis.
References
Abdelsalam E, Samer M, Attia YA, Abdel-Hadi MA, Hassan HE, Badr Y (2012) Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 120:842–853
Dai X, Li X, Zhang D, Chen Y, Dai L (2016) Simultaneous enhancement of methane production and methane content in biogas from waste activated sludge and perennial ryegrass anaerobic co-digestion: the effects of pH and C/N ratio. Biores Technol 216:323–330
Zhang Y, Feng Y, Yu Q, Xu Z, Quan X (2014) Enhanced high-solids anaerobic digestion of waste activated sludge by the addition of scrap iron. Biores Technol 159:297–304
Elsayed M, Andres Y, Blel W, Gad A, Ahmed A (2016) Effect of VS organic loads and buckwheat husk on methane production by anaerobic co-digestion of primary sludge and wheat straw. Energy Convers Manage 117:538–547
Mata-Alvarez J, Mace S, Llabres P (2000) Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Biores Technol 74:3–16
Al-Ghouti MA, Khan M, Nasser MS, Al-Saad K, Heng E (2021) Recent advances and applications of municipal solid wastes bottom and fly ashes: insights into sustainable management and conservation of resources. Environ Technol Innov 21:101267
Alagöz AB, Yenigün O, Erdinçler A (2018) Ultrasound assisted biogas production from co-digestion of wastewater sludges and agricultural wastes: comparison with microwave pre-treatment. Ultrason Sonochem 40:193–200
Ahn HK, Smith MC, Kondrad SL, White JW (2010) Evaluation of biogas production potential by dry anaerobic digestion of switchgrass–animal manure mixtures. Biotechnol Appl Biochem 160:965–975
Amon T, Amon B, Kryvoruchko V, Zollitsch W, Mayer K, Gruber L (2007) Biogas production from maize and dairy cattle manure—influence of biomass composition on the methane yield. Agr Ecosyst Environ 118:173–182
Kim J, Kang CM (2015) Increased anaerobic production of methane by co-digestion of sludge with microalgal biomass and food waste leachate. Biores Technol 189:409–412
Asam ZUZ, Poulsen GT, Nizami AS, Rafique R, Kiely G, Murphy JD (2011) How can we improve biomethane production per unit of feedstock in biogas plants? Appl Energy 88:2013–2018
De Gioannis G, Muntoni A, Cappai G, Milia S (2009) Landfill gas generation after mechanical biological treatment of municipal solid waste. Estimation of gas generation rate constants. Waste Manag 29:1026–1034
Donoso-Bravo A, Pérez-Elvira SI, Fdz-Polanco F (2010) Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chem Eng J 160:607–614
Zhang Y, Jing Y, Zhang J, Sun L, Quan X (2011) Performance of a ZVI-UASB reactor for azo dye wastewater treatment. J Chem Technol Biotechnol 86:199–204
Zhang J, Zhang Y, Quan X, Liu Y, An X, Chen S et al (2011) Bioaugmentation and functional partitioning in a zero valent iron-anaerobic reactor for sulfate-containing wastewater treatment. Chem Eng J 174:159–165
Zhang Y, Jing Y, Quan X, Liu Y, Onu P (2011) A built-in zero valent iron anaerobic reactor to enhance treatment of azo dye wastewater. Water Sci Technol 63:741–746
Liu Y, Zhang Y, Quan X, Zhang J, Zhao H, Chen S (2011) Effects of an electric field and zero valent iron on anaerobic treatment of azo dye wastewater and microbial community structures. Biores Technol 102:2578–2584
Zhang Y, Liu Y, Jing Y, Zhao Z, Quan X (2012) Steady performance of a zero valent iron packed anaerobic reactor for azo dye wastewater treatment under variable influent quality. J Environ Sci 24:720–727
Xiao X, Sheng GP, Mu Y, Yu HQ (2013) A modeling approach to describe ZVI-based anaerobic system. Water Res 47:6007–6013
Gou C, Yang Z, Huang J, Wang H, Xu H, Wang L (2014) Effects of temperature and organic loading rate on the performance and microbial community of anaerobic co-digestion of waste activated sludge and food waste. Chemosphere 105:146–151
Liu C, Wang W, Anwar N, Ma Z, Liu G, Zhang R (2017) Effect of organic loading rate on anaerobic digestion of food waste under mesophilic and thermophilic conditions. Energy Fuels 31:2976–2984
Kouas M, Torrijos M, Schmitz S, Sousbie P, Sami S, Harmand J (2018) Co-digestion of solid waste: towards a simple model to predict methane production. Biores Technol 254:40–49
Rajput AA, Visvanathan C (2018) Effect of thermal pretreatment on chemical composition, physical structure and biogas production kinetics of wheat straw. J Environ Manage 221:45–52
Strömberg S, Nistor M, Liu J (2015) Early prediction of Biochemical Methane Potential through statistical and kinetic modelling of initial gas production. Biores Technol 176:233–241
Veluchamy C, Kalamdhad AS (2017) Enhanced methane production and its kinetics model of thermally pretreated lignocellulose waste material. Biores Technol 241:1–9
Li L, Kong X, Yang F, Li D, Yuan Z, Sun Y (2012) Biogas production potential and kinetics of microwave and conventional thermal pretreatment of grass. Appl Biochem Biotechnol 166(5):1183–1191
Zwietering MH, Jongenburger I, Rombouts FM, Van’t Riet K (1990) Modeling of the bacterial growth curve. Appl Environ Microbiol 56(6):1875–1881
Prajapati B, Singh R (2018) Kinetic modelling of methane production during bio-electrolysis from anaerobic co-digestion of sewage sludge and food waste. Biores Technol 263:491–498
Prajapati KB, Singh R (2020) Enhancement of biogas production in bio-electrochemical digester from agricultural waste mixed with wastewater. Renew Energy 146:460–468
Lo HM, Kurniawan TA, Sillanpää MET, Pai TY, Chiang CF, Chao KP et al (2010) Modeling biogas production from organic fraction of MSW co-digested with MSWI ashes in anaerobic bioreactors. Biores Technol 101:6329–6335
Kumar S, Mondal AN, Gaikwad SA, Devotta S, Singh RN (2004) Qualitative assessment of methane emission inventory from municipal solid waste disposal sites: a case study. Atmos Environ 38:4921–4929
Huiliñir C, Quintriqueo A, Antileo C, Montalvo S (2014) Methane production from secondary paper and pulp sludge: effect of natural zeolite and modeling. Chem Eng J 257:131–137
American Public Health Association (2012) American Public Health Association - Standard methods for examination of water and wastewater method 9221B. APHA, Washington DC
Koch K, Helmreich B, Drewes JE (2015) Co-digestion of food waste in municipal wastewater treatment plants: effect of different mixtures on methane yield and hydrolysis rate constant. Appl Energy 137:250–255
Wang X, Yang G, Feng Y, Ren G, Han X (2012) Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Biores Technol 120:78–83
Wang X, Yang G, Li F, Feng Y, Ren G, Han X (2013) Evaluation of two statistical methods for optimizing the feeding composition in anaerobic co-digestion: mixture design and central composite design. Biores Technol 131:172–178
Kim HW, Nam JY, Kang ST, Kim DH, Jung KW, Shin HS (2012) Hydrolytic activities of extracellular enzymes in thermophilic and mesophilic anaerobic sequencing-batch reactors treating organic fractions of municipal solid wastes. Biores Technol 110:130–134
Luostarinen S, Sanders W, Kujawa-Roeleveld K, Zeeman G (2007) EVect of temperature on anaerobic treatment of black water in UASB-septic tank systems. Biores Technol 98:980–986
Raskin L, Poulsen LK, Noguera DR, Rittmann BE, Stahl DA (1994) Quantification of methanogenic groups in anaerobic biological reactors by oligonucleotide probe hybridization. Appl Environ Microbiol 60:1241–1248
Menon A, Lyng J, Giannis A (2021) Higher bacterial diversity in two-phase thermophilic anaerobic digestion of food waste after micronutrient supplementation. Biomass Conv Bioref 11:1–9
Cecchi F, Pavan P, Alvarez JM, Bassetti A, Cozzolino C (1991) Anaerobic digestion of municipal solid waste: thermophilic vs. mesophilic performance at high solids. Waste Manag Res 9:305–315
Logan M, Ravishankar H, Tan LC, Lawrence J, Fitzgerald D, Lens PNL (2021) Anaerobic digestion of dissolved air floatation slurries: effect of substrate concentration and pH. Environ Technol Innov 21:101352
Canan A, Calhan R, Ozkaymak M (2021) Investigation of the effects of different slags as accelerant on anaerobic digestion and methane yield. Biomass Conv Bioref 11:1395–1406
Hu Y, Hao X, Zhao D, Fu K (2015) Enhancing the CH4 yield of anaerobic digestion via endogenous CO2 fixation by exogenous H2. Chemosphere 140:34–39
Feng Y, Zhang Y, Quan X, Chen S (2014) Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res 52:242–250
Lovley DR, Phillips EJP (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54:1472–1480
Casals E, Barrena R, García A, González E, Delgado L, Busquets Fité M et al (2014) Programmed iron oxide nanoparticles disintegration in anaerobic digesters boosts biogas production. Small 14:2801–2808
Kim M, Gomec CY, Ahn Y, Speece RE (2003) Hydrolysis and acidogenesis of particulate organic material in mesophilic and thermophilic anaerobic digestion. Environ Technol 24:1183–1190
Zhai N, Zhang T, Yin D, Yang G, Wang X, Ren G et al (2015) Effect of initial pH on anaerobic co-digestion of kitchen waste and cow manure. Waste Manag 38:126–131
Zhang T, Mao C, Zhai N, Wang X, Yang G (2015) Influence of initial pH on thermophilic anaerobic co-digestion of swine manure and maize stalk. Waste Manag 35:119–126
Sung S, Liu T (2003) Ammonia inhibition on thermophilic anaerobic digestion. Chemosphere 53:43–52
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Biores Technol 99:4044–4064
Rajagopal R, Massé DI, Singh G (2013) A critical review on inhibition of anaerobic digestion process by excess ammonia. Biores Technol 143:632–641
Hill DT, Cobb AS, Bolte JP (1987) Using volatile fatty acid relationships to predict anaerobic digester failure. Trans ASAE 30:496–501
VieAitez ER, Ghosh S (1999) Biogasi®cation of solid wastes by two-phase anaerobic fermentation. Biomass Bioenerg 16:299–309
Ware A, Power N (2017) Modelling methane production kinetics of complex poultry slaughterhouse wastes using sigmoidal growth functions. Renew Energy 104:50–59
Funding
One of the authors, Prajapati, K.B, is grateful to the University Grants Commission (UGC), New Delhi for providing a Non-NET Fellowship.
Author information
Authors and Affiliations
Contributions
Kalp Bhusan Prajapati: conceptualize and design of work, formal analysis, interpretation of data, and writing draft the manuscript; Rajesh Singh: revised the manuscript for intellectual content and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prajapati, K.B., Singh, R. Co-digestion of sewage sludge and wheat straw in presence of iron scraps in mesophilic and thermophilic conditions for generating methane. Biomass Conv. Bioref. 14, 3319–3330 (2024). https://doi.org/10.1007/s13399-022-02417-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02417-0