Abstract
The synthesis of polyhydroxyalkanoates (PHA) using mixed microbial cultures (MMC) has been studied in three stages: a sequencing batch reactor, a PHA accumulation reactor, and a pyruvate effect. Tainan pig farm, Chiayi pig farm, and Feng Chia University’s sludge were the three seed sludges tested. Two distinct substrates are used, such as effluent from sucrose acidified fermenter and molasses waste acidified fermenter. Both substrates showed significant COD and VFA, but the effluent from molasses waste acidified fermenter had more significant acetic acid and butyric acid levels, with 4,087 ± 128 and 8,151 ± 152 mL/L, respectively. The propionic acid content in effluent from sucrose acidified fermenter is more significant (4,789 ± 36 mg/L). The highest PHA production yield in the three seed sludges was 26.88%, 22.88%, and 21.90%. Tainan pig farm sludge had the highest PHA accumulation of 26.88%. This study validated MMC’s ability to produce PHA in 8 h using VFA efficiency. The present work employed the addition of pyruvate to enhance the MMC. The 1 g addition of pyruvate in a sequencing batch reactor (SBR) can increase the productivity of PHAs yielded 53.58 g PHAs/g VSS (%), respectively. Therefore, MMC boosted with pyruvate used in high organic wastewater to produce PHA could potentially further commercial activities.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent days, worldwide plastic waste generation and consumption increased to about 280 million tonnes and 4%, respectively, in 2011, increasing annually until 2020 [1, 2]. Totally, 4.8 to 12.7 million ton plastics made of fossil fuel enter the ocean from land, which increased twice in 2025 compared to 2015 [3]. Plastic has become a primary concern towards the environment because of the lower rate of biodegradation. These plastics require about 100 years for complete degradation. Plastics make human life more accessible but also encourage environmental pollution. The pollutions of plastics are garbage waste and the end product of the industrial process. This process releases many kinds of toxic pollutants, which affect the ecosystem and human health [4]. Therefore, to overcome these plastic wastes, alternative environmentally friendly discovery needs to be followed. The community role is essential to replace petrochemical plastic wastes. Many kinds of bioplastic are available, mainly polythene (Bio-PE), polythene terephthalate (Bio-PET), and polyester and polylactic acid. Among them, Bio-PE and Bio-PET are non-biodegradable bioplastics [5, 6]. Polylactic acid is a biodegradable plastic that takes 90 days to biodegrade but degrades under simulated landfills with limited oxygen [7]. Most potential biodegradable plastic is polyhydroxyalkanoates (PHAs) [8].
Polyhydroxyalkanoates (PHAs) are ideal bioplastic to replace conventional oil-based plastics [9]. Polyhydroxyalkanoates are produced by microorganisms’ intracellular metabolism (PHAs). Most PHA has been composed of polyhydroxybutyrate (PHB) and polyhydroxyvalerate (PHA/PHV). Because of a lack of long-chain structures, hydroxybutyrate (HB) is rigid and heat intolerant. 3-Hydroxybutyrate-co-3-hydroxyvalerate (PHBV) is a polymer of 3-HB and 3-HV with a high molecular weight. PHBV is an ideal bioplastic material due to its sufficient mechanical strength and resilience [10, 11]. PHAs belong similar to fossil fuel-based plastics, but PHAs are more environmentally friendly [12]. PHAs are obtained by a simple processing method and can be used as UV-resistant material as PHAs take a long time to dissolve in water [13]. PHAs have thermoplastic qualities that vary depending on the substrate and the methods of fermentation. Numerous chemical molecules can serve as the substrate for component PHAs. These organic chemicals are commonly found in industrial, agricultural, and even municipal wastewaters [14].
The majority of the substrates used to create PHA are organic acids. Several organic wastewater by-products are high in organic acid and carbon [15], olive oil factory wastewater [16], molasses wastewater [17], and pulp wastewater [18]. The utilization of these wastewaters as a substrate effectively reduces the production cost of PHAs. There are numerous methods for increasing PHA production by controlling phosphorus, nitrogen, and carbon concentrations. The sequencing batch reactor is the most common method to use MMC with wastewater to produce PHA [19]. SBR is usually operated under aerobic conditions with repeating feast/famine. Feast–famine (FF) was often utilized in selecting train MMC to have more ability to produce PHA [20].
Dissolved oxygen [12] and repeating feast/famine phases in sequencing batch reactor are also important factors, and numerous studies show that the level of dissolved oxygen is associated with PHA synthesis. Several studies have proved different DO levels during feast and famine [21]. Dissolved oxygen acts as a crucial indicator when using a sequencing batch reactor to produce PHAs. The oxygen intake and carbon evolution rates from the bioreactor off-gas control PHA synthesis [22]. The supernatant replacement cycle is determined by the change in dissolved oxygen [23,24,25]. To effectively produce PHAs from mixed microbial cultures (MMC), various researchers used a sequencing batch reactor to induce MMC to synthesize additional PHA [16, 21, 26].
As of now, most investigations have focused on how to collect PHAs using pure cultures of pure substrates such as acetate and glucose [20]. PHA manufacture on a considerable scale is currently prohibitively expensive, ranging from 2.2 to 5.0 €/kg [23]. However, the prices of conventional oil-based plastic have always been less than 1 €/kg. PHA, on the other hand, costs more than three times as much as polymer generated from petrochemical resources [8, 13], considering that the production cost of PHA is too high. When comparing MMC and pure bacteria to produce PHA, MMC can effectively reduce production costs and tolerate multiple high organic wastewaters as a substrate. Using MMC to make PHA opens up more opportunities [27]. The use of pyruvate to improve the MMC is a novel component of current work. This experiment investigates the production of PHAs adopting SBR with pyruvate to boost MMC to accumulate the PHA with high organic wastewater in SBR and a comparative analysis of the capacity of 3 different sludges to accumulate PHAs.
2 Materials and methods
2.1 Seed sludge collection and preparation
The two kinds of substrates were obtained from the effluent of two acidified fermenters. The first substrate was obtained from the effluent sucrose acidified fermenter, and the second substrate was obtained from molasses waste acidified fermenter both cultivated in Green Products Laboratory, Feng Chia University, Taiwan. Different kinds of seed sludge were collected from the Tainan pig farm, Chiayi pig farm, and Feng Chia University’s wastewater treatment plant. The MMC of the Tainan pig farm was obtained from raw pig manure. The MMC of Chiayi pig farm was obtained from the aerobic tank of the pig farm water treatment system. The MMC of Feng Chia University was the municipal wastewater. This research study includes the comparative ability of its three different MMC to produce PHA which was employed in this research.
The obtained substrates were tested for quality analysis. The pH was measured with a pH meter (HQ2100 Portable Multi-Meter with Gel pH Electrode); COD was calculated using the K2Cr2O7 titration method; TS, VS, and VSS were calculated using methods for chemical analysis of water and wastes [28, 29]; and VFA was calculated using GC-FID (Thermo Scientific GC Focus, USA). Triplicates performed the statistical analysis by the mean, and the standard deviation was calculated.
2.2 Sequencing batch reactor (SBR)
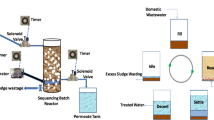
The sequencing batch reactor (SBR) is an activated sludge system for the batch treatment of wastewater [30]. The schematic diagram of SBR is represented in Fig. 1. A sequencing batch reactor operation is flexible and may be adjusted to biomass and changes, typical for municipal and industrial wastewater. The process operation can be optimized and tailored to the current state by adjusting the reactivation rates (duration of phases) and ordering batch phases. A typical cycle of SBR comprises several phases, including filling, reaction, settling, and withdrawal (anaerobic, aerobic, and anoxic). When only the elimination of organic matter and the reduction of nitrogen are considered, three processes have to be performed: removal, nitrification, and denitrification of the chemical oxygen demand (COD) [31, 32].
In this study, the volume of SBR was 250 mL. The working volume was 100 mL, and the temperature was controlled at 30 °C. Fermentation under aerobic conditions was used in this experiment. The tests were terminated when the pH was less than 6 or the PHA concentration had stabilized. Each cycle time was 24 h. After 22.5 h, the reaction was stopped for half an hour to precipitate, and 50 mL sample was extracted in period of 15 min. The supernatant was analyzed including pH, DO, and VFA. A 12.5 mL of bottom solution was taken for another 15 min. The bottom solution analyzed the PHA content and VSS. The fresh substrate was added in the last half an hour. This cycle was continuous for 1 week. The hydraulic retention time (HRT) was 2 days, and the sludge retention time (SRT) was 4 days. As shown in Fig. 1, sequencing batch reactor was utilized for the PHAs production. The reactor was assembled of following parts such as 1, substrate container used for storage of substrates; (2), sequencing batch reactor is the main reactor involved in chemical reactions; 3, the effluent container was engaged in the storage of effluent; 4, constant temperature water bath used for maintaining temperature; 5 and 6, sample storage tank access for sample collection; 7, 8, 9, and 10, pumps were employed in liquid pumping; 11, a gas pump provides air supply; 12, the thermostat was used in controlling temperature; and finally, 13 and 14, sample point was employed in sample collection, as shown in Fig. 1.
2.3 Fermentation
A sequencing batch reactor which utilized high VFA content effluent from an acidification fermenter was utilized to accumulate PHA. Seed sludge was obtained from the aeration tank of wastewater treatment plants in Central Taiwan mentioned in Section 2.1. Acidification fermentation was under anaerobic fermentation in the bioreactor in Feng Chia University (24.178870011866948, 120.64668745346927). HRT was controlled under 4 days, and the pH was maintained at 5.5. The effluents, after acidification, had a high content of VFA.
The working volume of the sequencing batch reactor was kept at 100 mL, and the temperature was maintained at 30 °C for a time cycle of 24 h. The reaction was held out for 24 h, and it was stopped for half an hour at 22.5 h to precipitate, and a 50 mL sample was extracted every 15 min. The sample supernatant was analyzed for pH, DO, and VFA. After decanting the supernatant, the bottom solution was taken for consecutive 15 min. The sample solution was subjected to PHA content and VSS analysis. Meanwhile, after sample collection, the fresh substrate was added at the end of the reaction, and this reaction cycle was continuously maintained for 1 week. The hydraulic retention time (HRT) was recorded every 2 days, and the sludge retention time (SRT) was measured every 4 days to determine the changes in water quality and PHA content in the biomass [33].
2.4 Addition of pyruvate to enhance PHA synthesis
The addition of exogenous pyruvate may be improved in PHA production. The consecutive experiment was to adopt the best seed from the previous experiment to enhance the production of PHAs. The experiment was enhanced by the addition of external pyruvate (Cheng Yang Instrument Corp. Taiwan). In PHA production experiments, the pyruvate was added to the SBR in the ratio of 0.25 g, 0.5 g, and 1 g in each reactor mixture. All the experiments were carried out in the reactors with a volume of 250 mL. The working volume was maintained as 100 mL, and the temperature was controlled at 30 °C.
2.5 Analytical methods
This study determined dissolved oxygen (DO) with an HQ1130 portable dedicated dissolved oxygen meter with a dissolved oxygen electrode. The pH was determined with a pH meter (HQ2100 Portable Multi-Meter with Gel pH Electrode); total solids (TS) and total volatile solids (VS), total suspended solids (TSS), and volatile suspended solids (VSS) were determined by the protocol based on Walter et al. [34]. Chemical oxygen demand (COD) was quantified using the K2Cr2O7 titration method [35]. In a mixing glass, blend 5 mL of sample and 5 mL of methanol. Take 1.5 mL of the filtered mixture, and VFA was analyzed by GC-FID [36]. The PHA analysis was done by withdrawing biomass from the reactor and being placed on the crucible for drying at 105 ℃ for 24 h. After drying, the solid was subjected to the weight measurement. The sample was dissolved in 5 mL chloroform followed by the addition of 10 mL methanol acid (10% sulfuric acid) and heated at 100 °C for 4 h for esterification. After the process, the reactant was washed with deionized water and then filtered to remove the oil phase. The oil phase was examined using GC-FID. PHB and PHV were used to make the PHA that was generated in this study. To detect 3HB and 3HV, PHA must be dissolved in chloroform and esterified with acidified methanol.
The retention time of 3HB was 9.8067 min on the GC-FID analysis chart, while the retention time of 3HV was 13.733 min, as confirmed by GC-FID standard (Sigma Aldrich, USA). This study confirmed that PHA consisted of PHB and PHV, which GC-FID discovered. The PHA content in the biomass was calculated by Eq. (1):
2.6 Statistical analysis
Statgraphics Centurion 19 was used to conduct the statistical analysis. The experiments in this study were carried out three times. The average standard deviation of three replicates was used to calculate the results. At the 0.05 level, a significant difference was assessed.
3 Results and discussion
3.1 Wastewater analysis
The results obtained from substrate quality analysis for pH, COD, TS, VS, VSS, and VFA are displayed in Table 1. Both substrates had a high COD and VFA, but the effluent from molasses waste acidified fermenter had higher acetic acid and butyric acids such as 4,087 ± 128 and 8,151 ± 152 mg/L, respectively. Effluent from sucrose acidified fermenter has a higher concentration of propionic acid 4,789 ± 36 mg/L. The increasing acetic acid and butyric acid concentration lead to an enhanced level of PHB, while a higher concentration of propionic acid enhance PHV production but inhibit the growth of MMC [36,37,38].
3.2 Seed sludge
For this investigation, three distinct seed sludges were chosen from Tainan pig farms, Chiayi pig farms, and Feng Chia University’s wastewater treatment facility. These three sludges were fed into various SBRs to compare and analyze PHA generation. The substrate was composed of sucrose acidified fermenter effluent and molasses waste acidified fermenter effluent in a 1:1 ratio. This ratio was selected because VSS in both effluents slightly varies as presented in Table 1. The reaction was maintained at a controlled temperature of 30 °C. The hydraulic retention time (HRT) was recorded every 2 days, and the sludge retention time (SRT) was measured every 4 days during the 1-week reaction. When the experiment was initiated, the pH of the Tainan pig farm reactor was 8.07 ± 0.12, the Chiayi pig farm reactor was 7.87 ± 0.11, and Feng Chia University’s wastewater treatment system reactor was 6.96 ± 0.08. Consecutively, on the fifth day, the Tainan pig farm reactor’s pH decreased to 6.38 ± 0.11, the Chiayi pig farm reactor was decreased to 6.33 ± 0.06, and the wastewater treatment system of Feng Chia University reactor was near to 6.33 ± 0.08. In the first 5 days, the PHA concentration in three reactors increased linearly. On the sixth and seventh days, the PHA concentration in three reactors improved and remained steady. Because the pH of three SBR on the fifth day was less than 6.5, MMC:PHA accumulation was inhibited. As observed in Fig. 2, each of the three seed sludge of the highest PHA concentration from Tainan pig farm, Chiayi pig farm, and the wastewater treatment system of Feng Chia University was 26.88%, 22.88%, and 21.90%.
Among all three sludges, Tainan pig farm sludge was shown the best accumulation of the PHA. Compared to the Chiayi pig farm SBR and wastewater treatment system of Feng Chia University SBR, Tainan pig farm pH was 8.07 ± 0.12 at the beginning, confirming that Tainan pig farm sludge’s initial pH ranged from 8.0 to 9.0, which helped in PHA production. The pH plays a vital role in the process of PHA production [37,38,39]. These studies pointed out that when the pH is 8.0–9.0, the accumulation of PHAs in the organism can be the highest, and when the pH is 6.0–7.0, the bioaccumulation of PHA activity drops [40, 41].
3.3 Metabolites
As per previous study results, the Tainan pig farm showed the highest PHA production. So, this study was focused on seed sludge from the Tainan pig farm. Sludges from the Tainan pig farm were fed in the SBR, and the substrate was proportioned to 1:1 (effluent of sucrose acidified fermenter:effluent from molasses waste acidified fermenter) at the 30 °C. The experiment set up in the seed sludge, as shown in Fig. 2, was stopped for the Chiayi pig farm and Feng Chia University sludge after 7 days. The Tainan pig farm SBR continued for the next consecutive day.
On the eighth day, an experiment was analyzed for VFA metabolism. Butyric acid was metabolized at the fourth hour of the reaction, as shown in Fig. 3. Propionic acid and acetic acid were metabolized by the eighth hour. According to Wang et al. [42], acetic acid and butyric acid mainly produce PHB, while propionic acid and valeric acid can produce PHV. However, propionic acid reduces the efficiency of PHA synthesis. In this experiment, a high concentration of acetic acid and butyric acid was utilized as the substrate. As a result, PHA can be rapidly boosted. When MMC accumulates PHA, butyric acid has been used first, followed by propionic acid. While butyric acid’s metabolic route requires less ATP than propionic acid’s, acetic acid was used after propionic acid consumption [27]. This investigation confirmed the same tendency, and MMC may manufacture PHA in 8 h using VFA efficiency. To make the MMC a viable process for full-scale implementation, various optimal operational strategies must be combined to maximize each step’s performance [43].
3.4 Pyruvate effect
In this study, pyruvate metabolism utilized to generate PHA has been observed using the substrate from sucrose acidified fermenter effluent. PHA production was enhanced by introducing exogenous pyruvate which was introduced to the process. After adding substrate, pyruvate was added in different concentrations such as 0.25 g, 0.5 g, and 1 g in different SBR, and the control was maintained without the addition of pyruvate. The pyruvate effect was monitored and displayed in Fig. 4.
Figure 4a shows that when 0.25 g, 0.5 g, and 1 g of pyruvate were added, the PHV concentrations on the eighth day were 0 mg/L, 75 mg/L, 221 mg/L, and 215 mg/L, respectively. In the control sample with no addition of a pyruvate, there was no PHV production. One of the pyruvate metabolic pathways suggests PHA production is directly proportional to the pyruvate addition [44]. The structure of PHV differs from that of PHB; thus, a higher PHV concentration allows PHA to have reduced crystallinity and more flexibility [4]. Additional 0.5 g and 1 g pyruvate could increase PHV synthesis. On the other hand, it almost produced the same amount of PHV. This result demonstrates that 0.5 g pyruvate was the most beneficial amount in a 100 mL working volume. Figure 4b shows that when 0.25 g, 0.5 g, and 1 g of pyruvate were introduced, the PHB concentration on the eighth day was 0 mg/L, 926 mg/L, 1274 mg/L, and 1580 mg/L. The more pyruvate was added, the higher PHB was produced. The pathway that carries out PHB generation appears to be directly proportional to the amount of pyruvate added [44].
The additional amount of pyruvate supplied was 0.25 g, 0.5 g, and 1 g; PHA concentration on the seventh day was 550 mg/L, 1001 mg/L, 1485 mg/L and 1795 mg/L as observed in Fig. 4c. PHB is the most prevalent by-product of PHA [45]. Figure 4d shows that when 0.25 g, 0.5 g, and 1 g of pyruvate were introduced, the PHAs/VSS (%) on the eighth day were 18.33%, 35.63%, 42.42%, and 53.58%, respectively. As additional pyruvate was introduced to the cycle, the percentage of PHAs in the biomass increased considerably. Sucrose acid manufacturing effluent was used as the substrates. The sucrose effluent may act as the supporting substrate to PHA enhancement. At the same time, sludge from the Tainan pig farm’s aeration tank was mixed with an additional 1 g of pyruvate. It can attain the highest level of PHA production.
Most commonly, the MMC process consists of the following stages: (1) the acidogenic fermentation, which converts organic matter into soluble fermentation products (SFPs), which serve as the precursors for PHA biosynthesis; (2) the selection of the best PHA-producing enriched culture; (3) PHA accumulation, in which a previously selected culture is fed the SFP and accumulated PHA to its maximum capacity [43]; (4) and MMC process modified with boosting the culture with pyruvate provides ease of access of nutrients to MMC for enhanced production.
The use and integration of industrial by-products into current industrial processes to use the infrastructures available in PHA manufacturing enables a sustainable and cost-effective production method [46, 47]. This research used MMC in high organic wastewater with pyruvate to produce PHA acceptable for commercial activities.
As reviewed in Table 2, these studies are focused on wastewater utilization for the production of PHA. Among them [39,40,41,42], PHA production volume was 30.8%, 39.8%, 41.5%, and 53.0%. In this study, the highest PHA production was 53.6 PHA/VSS%. Compared with other methods of producing PHA, the amount of PHA in the biomass in this study was the highest. The substrate used to produce PHA needs high content carbon souses [17]. This study uses kinds of wastewater sources in these two sources to produce PHA very off efficiently. Acidification fermentation is a fundamental way to break the significant carbon source to VFA [26, 38].
According to this analysis, this study’s overall yield was higher than that of other pilot-scale MMC processes. The high overall yield was due to several factors; including acidogenic reactor operating conditions allowed us to achieve a fermentation yield and additionally by pyruvate effect. Since the total COD was used to calculate this parameter and this residue contain insoluble COD, it can be assumed that the fermentation yield cannot be improved further. In both selection and accumulation reactors, SFP stream was critical to maximize yields. Uncoupling carbon from SBR feeding ensured that no SFP was used for growth, resulting in a high selection of PHA-accumulating organisms, thus enhancing the storage yield obtained.
4 Conclusions
A sequencing batch reactor SBR was utilized to research aerobic fermentation to produce PHA effectively in this work. Comparing three different seed sludges with high organic wastewater to generate PHA compares the extra pyruvate to enhance PHA production capacity. Pyruvate showed the tendency in the enhancement of PHA production also by producing PHV and PHB.
-
1.
Tainan pig farm has the highest PHA production capacity when compared to other sludge.
-
2.
This investigation confirmed that MMC might manufacture PHA in 8 h using VFA efficiency.
-
3.
PHA production was directly proportional to the pyruvate addition. Also, pyruvate addition enhances the PHV concentration in PHA.
The utilization of high organic wastewater with MMC is the future trend, and the addition of pyruvate can boost PHA output production and change the ratio of PHV in PHA.
According to the best of our knowledge, the global PHA productivity and overall yield achieved are the highest values reported for MMC with wastewaters. These parameters directly influence investment and operational costs. As a result, it can be expected that the conditions applied will contribute to a reduction in the final PHA production costs, demonstrating the potential for full-scale implementation of PHA production from organic wastewaters. In the future, researchers may focus on ways to commercialize this system.
References
Wang C, Zhao L, Lim MK, Chen WQ, Sutherland JW (2020) Structure of the global plastic waste trade network and the impact of China’s import Ban. Resour Conserv Recycl 153:104591
Bhuyar P, Muniyasamy S, Govindan N, (2018) Green revolution to protect environment–an identification of potential micro algae for the biodegradation of plastic waste in Malaysia. Expert Opin Environ Biol 7
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL (2015) Plastic waste inputs from land into the ocean. Science 347:768–771
Dauvergne P (2018) Why is the global governance of plastic failing the oceans? Glob Environ Chang 51:22–31
Siracusa V & Blanco I, (2020) Bio-polyethylene (bio-PE), bio-polypropylene (bio-PP) and bio-poly(ethylene terephthalate) (bio-PET): recent developments in bio-based polymers analogous to petroleum-derived ones for packaging and engineering applications. Polymers (Basel) 12
Saratale RG, Cho SK, Saratale GD, Kadam AA, Ghodake GS, Kumar M, Bharagava RN, Kumar G, Kim DS, Mulla SI, Shin HS (2021) A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour Technol 325:124685
Boonmee C, Kositanont C, Leejarkpai T (2016) Degradation of poly (lactic acid) under simulated landfill conditions. Environ Nat Resour J 14:1–9
Mannina G, Presti D, Montiel-Jarillo G, Suárez-Ojeda ME (2019) Bioplastic recovery from wastewater: a new protocol for polyhydroxyalkanoates (PHA) extraction from mixed microbial cultures. Bioresour Technol 282:361–369
Bugnicourt E, Cinelli P, Lazzeri A, Alvarez VA, (2014) Polyhydroxyalkanoate (PHA): review of synthesis, characteristics, processing and potential applications in packaging
Gahlawat G, Soni SK (2017) Valorization of waste glycerol for the production of poly (3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer by Cupriavidus necator and extraction in a sustainable manner. Bioresour Technol 243:492–501
Sharma V, Sehgal R, Gupta R (2021) Polyhydroxyalkanoate (PHA): properties and modifications. Polymer 212:123–161
Serafim LS, Lemos PC, Albuquerque MG, Reis MA (2008) Strategies for PHA production by mixed cultures and renewable waste materials. Appl Microbiol Biotechnol 81:615–628
Gholami A, Mohkam M, Rasoul-Amini S, Ghasemi Y (2016) Industrial production of polyhydroxyalkanoates by bacteria: opportunities and challenges. Minerva Biotechnol 28:59–74
Davidson PM, Branden AL (1981) Antimicrobial activity of non-halogenated phenolic compounds. J Food Prot 44:623–632
Coats ER, Loge FJ, Wolcott MP, Englund K, McDonald AG (2007) Synthesis of polyhydroxyalkanoates in municipal wastewater treatment. Water Environ Res 79:2396–2403
Beccari M, Bertin L, Dionisi D, Fava F, Lampis S, Majone M, Valentino F, Vallini G, Villano M (2009) Exploiting olive oil mill effluents as a renewable resource for production of biodegradable polymers through a combined anaerobic-aerobic process. J Chem Technol Biotechnol 84:901–908
Carvalho G, Oehmen A, Albuquerque MG, Reis MA (2014) The relationship between mixed microbial culture composition and PHA production performance from fermented molasses. N Biotechnol 31:257–263
Bengtsson S, Werker A, Christensson M, Welander T (2008) Production of polyhydroxyalkanoates by activated sludge treating a paper mill wastewater. Bioresour Technol 99:509–516
Lorini L, di Re F, Majone M, Valentino F (2020) High rate selection of PHA accumulating mixed cultures in sequencing batch reactors with uncoupled carbon and nitrogen feeding. New Biotechnol 56:140–148
Huang L, Chen Z, Wen Q, Zhao L, Lee DJ, Yang L, Wang Y (2018) Insights into feast-famine polyhydroxyalkanoate (PHA)-producer selection: microbial community succession, relationships with system function and underlying driving forces. Water Res 131:167–176
Third KA, Newland M, Cord-Ruwisch R (2003) The effect of dissolved oxygen on PHB accumulation in activated sludge cultures. Biotechnol Bioeng 82:238–250
Pederson EN, McChalicher CW, Srienc F (2006) Bacterial synthesis of PHA block copolymers. ACS Publ 7:1904–1911
Wang X, Oehmen A, Freitas EB, Carvalho G, Reis MA (2017) The link of feast-phase dissolved oxygen (DO) with substrate competition and microbial selection in PHA production. Water Res 112:269–278
Jadhav, P., Nasrullah, M., Zularisam, A. W., Bhuyar, P., Krishnan, S., & Mishra, P. (2021). Direct interspecies electron transfer performance through nanoparticles (NPs) for biogas production in the anaerobic digestion process. Int J Environ Sci Technol 1–13
Chandrakant, J. P., Muhammad, N., Bhuyar, P., Krishnan, S., Abd Razak, A. S., Zularisam, A. W., & Nasrullah, M. (2021). A review on the impact of conductive nanoparticles (CNPs) in anaerobic digestion: applications and limitations. Environ Technol Innov 101526
Albuquerque MG, Eiroa M, Torres C, Nunes BR, Reis MA (2007) Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J Biotechnol 130:411–421
Lorini L, Martinelli A, Pavan P, Majone M, Valentino F (2021) Downstream processing and characterization of polyhydroxyalkanoates (PHAs) produced by mixed microbial culture (MMC) and organic urban waste as substrate. Biomass Conversion and Biorefinery 11:693–703
Whangchai, K., Souvannasouk, V., Bhuyar, P., Ramaraj, R., & Unpaprom, Y. (2021). Biomass generation and biodiesel production from macroalgae grown in the irrigation canal wastewater. Water Sci Technol
Robert LB, (1974) Methods for chemical analysis of water and wastes. EPA PUB
Hvala N, Zec M, Roš M, Strmč S, (2001). Design of a sequencing batch reactor sequence with an input load partition in a simulation-based experimental environment. Wiley Online Library 73
Bhuyar P, Farez F, Pragas Maniam G, Govindan N (2021) Removal of nitrogen and phosphorus from agro-industrial wastewater by using microalgae collected from coastal region of peninsular Malaysia. Afr J Biol Sci 3(1):58–66
Bhuyar P, Hong DD, Mandia E, Rahim MHA, Maniam GP, Govindan N (2019) Desalination of polymer and chemical industrial wastewater by using green photosynthetic microalgae, Chlorella sp. Maejo Int J Energy Environ Commun 1(3):9–19
Kim S, Eichhorn P, Jensen JN, Weber AS, Aga DS (2005) Removal of antibiotics in wastewater: effect of hydraulic and solid retention times on the fate of tetracycline in the activated sludge process. Environ Sci Technol 39:5816–5823
Walter WG (1961) Standard methods for the examination of water and wastewater (11th ed.). Am J Public Heal 51:940–940
American Society for Testing and Materials. (1995) Standard test methods for chemical oxygen demand (dichromate oxygen demand) of water Am Soc Test Mater
Tu W, Zhang D, Wang H (2019) Polyhydroxyalkanoates (PHA) production from fermented thermal-hydrolyzed sludge by mixed microbial cultures: the link between phosphorus and PHA yields. Waste Manag 96:149–157
Carvalho G, Oehmen A, Albuquerque MG, Reis MA (2014) The relationship between mixed microbial culture composition and PHA production performance from fermented molasses. New Biotechnol 31(4):257–263
Albuquerque MGE, Concas S, Bengtsson S, Reis MAM (2010) Mixed culture polyhydroxyalkanoates production from sugar molasses: the use of a 2-stage CSTR system for culture selection. Biores Technol 101(18):7112–7122
Chua ASM, Takabatake H, Satoh H, Mino T (2003) Production of polyhydroxyalkanoates (PHA) by activated sludge treating municipal wastewater: effect of pH, sludge retention time (SRT), and acetate concentration in influent. Water Res 37:3602–3611
Villano M, Beccari M, Dionisi D, Lampis S, Miccheli A, Vallini G, Majone M (2010) Effect of pH on the production of bacterial polyhydroxyalkanoates by mixed cultures enriched under periodic feeding. Process Biochem 45:714–723
Kourmentza C, Kornaros M (2016) Biotransformation of volatile fatty acids to polyhydroxyalkanoates by employing mixed microbial consortia: the effect of pH and carbon source. Bioresour Technol 222:388–398
Wang X, Carvalho G, Reis MA, Oehmen A (2018) Metabolic modeling of the substrate competition among multiple VFAs for PHA production by mixed microbial cultures. J Biotechnol 280:62–69
Matos, M., Cruz, R. A., Cardoso, P., Silva, F., Freitas, E. B., Carvalho, G., & Reis, M. A. (2021). Combined strategies to boost polyhydroxyalkanoate production from fruit waste in a three-stage pilot plant. ACS Sustainable Chemistry & Engineering.
Guerra-Blanco P, Cortes O, Poznyak T, Chairez I, García-Peña EI (2018) Polyhydroxyalkanoates (PHA) production by photoheterotrophic microbial consortia: effect of culture conditions over microbial population and biopolymer yield and composition. Eur Polym J 98:94–104
Bhuwal AK, Singh G, Aggarwal NK, Goyal V, Yadav A, (2013) Isolation and screening of polyhydroxyalkanoates producing bacteria from pulp, paper, and cardboard industry wastes. Int J Biomater
De Donno ML, Moreno S, Rene ER (2021) Polyhydroxyalkanoate (PHA) production via resource recovery from industrial waste streams: a review of techniques and perspectives. Bioresour Technol 331:124985
Chu CY, Zheng JL, Chen TH, Bhuyar P (2021) High performance of biohydrogen production in packed-filter bioreactor via optimizing packed-filter position. Int J Environ Res Public Health 18(14):7462
Almeida JR, Serrano E, Fernandez M, Fradinho JC, Oehmen A, Reis MAM (2021) Polyhydroxyalkanoates production from fermented domestic wastewater using phototrophic mixed cultures. Water Res 197:117101
Morgan-Sagastume F, Hjort M, Cirne D, Gérardin F, Lacroix S, Gaval G, Werker A (2015) Integrated production of polyhydroxyalkanoates (PHAs) with municipal wastewater and sludge treatment at pilot scale. Biores Technol 181:78–89
Roibás-Rozas A, Del Rio AV, Hospido A, Mosquera-Corral A (2021) Strategies for the valorization of a protein-rich saline waste stream into polyhydroxyalkanoates (PHA). Bioresour Technol 334:124964
Acknowledgements
The authors would like to gratefully acknowledge the Ministry of Science and Technology, Taiwan (grant number: MOST 109-2221-E-035-003). The Institute of Green Products, Feng Chia University, is great acknowledged in assisting the experiment and providing materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, MY., Chu, CY., Sawatdeenarunat, C. et al. Production, downstream processing, and characterization of polyhydroxyalkanoates (PHAs) boosted by pyruvate supplement using mixed microbial culture (MMC) and organic wastewater. Biomass Conv. Bioref. 13, 15861–15869 (2023). https://doi.org/10.1007/s13399-021-02170-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02170-w