Abstract
This study employed the Soxhlet extraction method to describe the phyto-oil recovered from Jatropha curcas L., which was planted for the first time in the El Oued region of southeastern Algeria, using normal hexane as the solvent at 60 °C for 8 h. The dry weight-to-weight oil yield was 63.15%. The extracted oil was liquid at room temperature, with a nice sweet aroma and a yellowish-white hue. The physicochemical properties showed that the seed oil has a moisture level of 5.58%, a density of 0.915, a viscosity of 49.85 mm2/s, a peroxide index of 1.1 mEq/kg, an acid value of 2.9%, and an iodine indicator of 96.3 mg/g, a refractive index of 1.458, and a saponification index of 202.87 mg KOH/g. Gas chromatographic analysis showed stearic acid (8.5%), palmitic acid (11.2%), and oleic acid (65.9%). Furthermore, the quality of the extracted oil demonstrated that the seed is an excellent source of oil that might be used in industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The gap between the demand and supply of vegetable oils has widened in the world in general, and among manufacturers in particular, as they mostly rely on common vegetable oils to prepare their various products such as soybean oil, cottonseed oil, palm kernel oil, castor seed oil, peanut oil, and so on [1, 2]. Consequently, the scarcity of vegetable oils and fats for industrial, pharmaceutical, or other purposes has always pursued the resources of these manufacturers. Therefore, research was underway to exploit other wild plants such as Jatropha in Saharan areas. The Jatropha genus has more than 170 species of shrubs and belongs to the Euphorbiaceae family, the subfamily Platilobeae, such as Jatropha platyphylla, Jatropha gossypiifolia, and Jatropha curcas L., the latter producing seeds that are high in oil [3, 4]. Although this species is native to Mexico, it is found in many countries of Asia, Africa, and South America, and has been established to support agriculture and social and economic security in developing regions [5]. Jatropha curcas is a versatile plant with a lot of characteristics and a lot of promise. Its many components have a wide range of uses. Biodiesel, soap, and other products can be made from the oil taken from the seeds [6, 7]. Traditional medicine uses leaves to treat coughing and as an antiseptic [8]. The tree may be utilized as both a firewood source and a protective barrier [9].
Jatropha curcas L. is a drought-resistant plant that thrives in a wide range of environments, including sandy and salt soils, and can tolerate high temperatures and light frosts. It is a fast-growing plant and can produce seeds 2–5 years after planting, resistant to pests and diseases. The annual production of Jatropha ranges from 0.5 to 12 tons [10]. To advance the production of oil used in the biofuel industry, J. curcas L. was cultivated in the El Oued region in southeastern Algeria, which has a dry and a sweltering desert climate in summer and an entirely sandy soil [11].
J. curcas L. kernels make up a large portion of the seeds, accounting for 61.3 3.1% and 40–60% of the oil as a valuable end product. This oil has a higher percentage of unsaturated fatty acids than castor oil, a lower viscosity than castor oil, and lower acidity than soybean oil [12]. Furthermore, the degree of unsaturation is the most important factor that influences the properties of fatty acids and oils. As a result, the average degree of unsaturation is calculated using the iodine value. Under certain conditions, this value is calculated in milligrams as the amount of iodine reacting with double bonds in the sample [13]. The oils are divided into three groups based on their iodine content: non-drying, semi-dried, and dried oils. Non-dried oils have an iodine value of less than 90, while semi-dried oils have of 90 to 130; however, if the iodine value is greater than 130, the oil is dry [14]. The fatty acids palmitic acid (C16: 0), citric acid (C18: 0), oleic acid (C18: 1), and linoleic acid (C18: 2) make up the majority of the Jatropha oil’s fatty acid composition [15]. The chemical makeup of oil varies depending on the climate and location [16].
Algeria, like the whole countries of the world, tends to search for the best sources for the production of vegetable oils, used especially in the production of biofuels that are a better and safe alternative for the environment than fossil fuels. The latter tends to run out over time, which is mortgaging the country’s economy, which is almost entirely linked to its hard currency earnings. The end of fossil oil from the reserves in Algeria means that even the national market will not be able to cover it, as well as the international market, whether in terms of energy products and their derivatives or other manufactured products. The negative environmental impact of fossil fuel use and carbon dioxide emissions is one of the most important local motives for searching for green alternatives. The Oued Souf is considered one of the most successful agricultural regions in Algeria, as it is considered the first nationally in the production of many crops of wide consumption such as potatoes, tomatoes, tobacco, olives, and dates. Recently, it has achieved great successes in the production of rapeseed oil destined for the production of table oil [17]. Therefore, it was chosen as the best site to experience the production of Jatropha curcas L. oil in Algeria.
Accordingly, in this work, oil was extracted from Jatropha curcas L. seeds cultivated in the El Oued region (Algeria), and gas chromatography-mass spectroscopy was used to characterize the fatty acid profile. The extraction of oil from this agricultural waste would result in the waste being removed from the environment, resulting in a clean environment. In addition, the oil produced could be used in a variety of industries.
This study aims to (i) experiment the success of the Jatropha curcas L. cultivation in Algeria in terms of the cultivation ease and the crop and oil yield; (ii) study the physicochemical properties of the produced oil in comparison with the oil produced in its original regions; and (iii) the qualitative and quantitative study of its fatty acid components to predict the industrial fields in which it can be used.
2 Materials and methods
2.1 Cultivation of Jatropha curcas L.
The kernels were purchased online (from India). Then, they were presented to the “Algerian Seed Certification and Control Center” to verify their affiliation with Jatropha curcas. In June 2014, these seeds were planted in Akfadou, Debila, near El Oued city, Algeria (33° 29′ 54.2″ N, 6° 56′ 26.1″ E), in polyethylene buckets. Then, they were transferred to the planting site in February 2015. After the placing of organic fertilizers inside a ditch of 50 × 50 × 50 cm, it contains poor marginal land, uncultivated, and characterized by sandy soil. The resulting shrubs were planted in an experimental farm and irrigated throughout the experimental period with 350 to 400 m in depth groundwater, from May 15 to the end of August, twice a day, from the beginning of September to May 15; it was watered from once a day to once a week, as needed. In its third year, for the harvest, the tree’s ripe fruits were collected at the end of July, but it was not all at once. Rather, only the ripe fruits (brown dry) were collected every 3 to 4 days because leaving the fruits ripe for a while affects the acidity of the oil. They were then dried in a well-ventilated place at room temperature, taking care to stir them from time to time for good drying. In the end, the dried seeds were stored in a dry place for laboratory use.
2.2 Obtaining Jatropha curcas oil
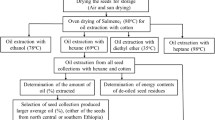
We follow the procedures specified in the following methodology (Fig. 1) to get J. curcas oil: The kernels were peeled to obtain seeds, which were then ground to expose a vast surface to successful extraction. The ground seeds were dried in an oven at a temperature of 60 °C until the weight was stabilized to reduce the moisture content. An amount of 60 g of the product from the previous step was weighed, placed in a thimble, and inserted into a 500-ml reflux flask. An extraction solvent, n-hexane (boiling point 60 °C), was used. The mixture was stirred for 8 h in a Soxhlet extractor which is a hand-installed device. Then, the oil–solvent mixture was separated using rotatory evaporation at 60 °C under reduced pressure. The oil was dried at 60 °C in a drying oven until it reached a constant weight to remove traces of solvent and water. Finally, the pure oil was stored in a refrigerator for later physicochemical analysis [17].
3 J. curcas oil physicochemical analysis
3.1 Oil yield
The yield ratio of fixed oil was calculated using the following equation [18]:
3.2 Measurement of water and volatile content (moisture)
According to NFT 60–201:1984 International Standard [19], the measurement of moisture as a percentage of the mass of seeds is expressed as follows:
\({m}_{1}\) and \({m}_{2}\) are the weight of the sample before and after drying (g), respectively.
3.3 Density (specific weight)
The density was determined according to standard NFT 60–214:2001PR, at a temperature of 30 °C [19] with the following relation:
3.4 Refractive index
Abbe’s refractometer was used to calculate the refractive index. The studies were carried out at 25 °C and the results were presented as a number with four decimal places. The prisms were cleaned and dried with xylene. Adjust the instrument and light to acquire the most distinct reading and calculate the refractive index. Place a few drops of oil on the prisms, shut the prisms, and leave to stand for 1–2 min. The unsaturation and chain length of fatty acids both enhance the oil’s refractive index [20].
3.5 Viscosity
Viscosity was determined using the Brookfield DV1M, rotary-type viscometer from Chemistry Labs, in room temperature; the spindle size S05 was utilized at 100 rpm for 1 min [21].
3.6 Acid value (AV)
The following steps should be followed: 2 g of pure oil was properly weighed into a 250-ml conical flask using the transfer method. A pipette was used to add 20 ml of neutral ethanol, and the flask was heated on a steam bath for 3 min. The flask was then chilled and the contents titrated with 0.1 N alcoholic potassium hydroxide solution using phenolphthalein as an indicator using 0.1 N alcoholic potassium hydroxide solution. In addition, a blank titration was performed side by side. The acid value (\(\mathrm{AV}\)) was determined according to the standard ASTM D 974:2014[22]:
in which \(V\) is the volume of KOH (ml), \(N\) is the normality of KOH, and \(m\) is the mass of the sample (g).
3.7 Saponification index (Si)
The saponification index was calculated according to the standard NBR 14,854–1: 2014. The following is the technique of work in detail: 2 g of oil was properly weighed into a 250-ml round bottom flask using the transfer method. A pipette was used to add a freshly produced 0.5 N alcoholic potassium hydroxide solution (25 ml) to the sample, and the combination was gently refluxed on a water bath using an air condenser for 1 h. The flask was then cooled, the condenser tip cleaned with distilled water, and the contents were titrated using a 0.5 N hydrochloric acid solution and phenolphthalein as an indicator. Simultaneously, a blank titration was performed.
The following equation is used to obtain the Si value [23]:
where \({V}_{0}\) is the volume of hydrochloric acid used for the blank test (ml), \(V\) is the volume of hydrochloric acid used in the test in oil (ml), and \(m\) is the mass of the sample (g).
3.8 Iodine indicator (Ii)
The index of iodine (Ii) was given according to ISO 3961:2018, as follows:
where N is the sodium thiosulfate Na2S2O3 normality (0.1 N), \({V}_{0}\) is the volume of Na2S2O3 solution necessary for titration in blank test (ml), \(V\) is the volume of Na2S2O3 solution necessary for titration of the sample (ml), and \(m\) is the mass of the sample (g).
3.9 Peroxide index (\({\mathrm{P}}_{\mathrm{I}}\))
The peroxide index (PI) was calculated according to the following equation [20]:
where \(N\) is the sodium thiosulfate Na2S2O3 normality (0.002 N), \({V}_{0}\) is the volume of Na2S2O3 solution necessary for titration in blank test (ml), \(V\) is the volume of Na2S2O3 solution necessary for titration of the sample (ml), and \(m\) is the mass of the sample (g).
3.10 GC–MS analysis
The fatty acid content of the seed oil was analyzed using the TE-CH-208 in-house technique based on AOAC guidelines (2012). Pyrogallic acid (0.2 g) was added to J. podagrica oil to prevent oxidation. Before being injected into the gas chromatography (GC-7890A/MS-5975C model, Agilent Technologies, Santa Clara, CA, USA), 0.1 ml oil was derivatized to fatty acid methyl esters (FAMEs) using 1 ml of 10% (w/w) boron trifluoride-methanol (BF3-methanol). The composition of free fatty acids in the J. equipped with a fused silica capillary DB-5MS column (% phenyl-methylpolysiloxane, 30 0.25 mm, film thickness 0.25 m, Agilent Technologies) was used to analyze curcas oil. The carrier gas was helium at a flow rate of 1 ml/min, and the column was run under the following conditions: The initial temperature was 50 °C, which was held for 1 min; after that, the temperature was increased at a rate of 10 °C per minute up to 250 °C, with a final time of 1 min. After that, the second gradient of 50 °C min−1 to 300 °C was used, followed by a 300 °C hold for 3 min. At 200 °C, 10 l of the sample solution was injected in a 1:10 ratio to collect these samples. The electron multiplier voltage was set to 1400–1500 V and the emission current was set to 10 A in the mass spectrometer. The temperature in the trap was 250 °C, while the temperature in the transfer line was 270 °C. One liter of injection was used. The retention durations of genuine standards evaluated under the identical circumstances were compared to those of FAME peak to identify it [24].
4 Results and discussion
4.1 Jatropha curcas L. cultivation
J. curcas L. plant responded to the irrigation process and gave good production. In this study, a hectare can produce 1.1 tons of oil per hectare (Fig. 2). Compared with India, the motherland and an Asian country with a favorable climate and natural conditions for the growth of this plant, whose production is estimated at 1.5 tons per hectare [21], we can consider the obtained results very encouraging.
4.2 Characterization of Jatropha curcas L. seed
Physical properties of Jatropha curcas L. seed with a dull brownish-black color (without peels) were assessed. Table 1 lists the physical characteristics of seeds.
Chemicals are present in the kernel, and physical characteristics have a direct impact on them. The hardness of seeds is directly related to the average weight and volume of seeds, which affects the analysis process. Physical characteristics of one seed compared to another seed could distinguish product quality. J. curcas seed and its chemical constituents the oil content of the Jatropha seed is 63.15% and the protein content is significant. Protein is said to be responsible for the seed’s toxicity and unpleasant odor.
4.3 Oil physicochemical analysis
Different fats and oils have different properties depending on the degree of hydrogen unsaturation or saturation. As a result, different oils are less or more saturated depending on whether they contain a higher or lower quantity of fatty acid saturation. As a result, researchers need to know how much unsaturation is present in the sample. The various numbers of test parameters like iodine indicator (Ii), saponification value (Si), and acid number (AV) were already applied to our sample.
Table 2 shows the results obtained from the experimental work: oil yield, moisture content, density at 25° C, viscosity at room temperature, saponification index, peroxide index, refractive index (28 °C), iodine indicator, and acid value, compared with Indian and Malaysian J. curcas L. [21, 22].
The Jatropha curcas seed is the plant’s primary organ for storing oil. The oil production of Jatropha curcas seed oil was found to be 63.15% (wt/wt) higher than that of various traditional oil seed crops, including cotton (15.0–24.0%), soybean (17.0–21.0%), safflower (25.0–40.0%), and mustard (24.0–40.0%) [25]. The climatic and geological characteristics of various places may be to blame for such variance in oil content between species and localities [26]. Furthermore, the season in which the seeds were collected had an impact on the oil production of Jatropha curcas L. seed kernels. Seeds collected during the dry season have a greater concentration. Seeds collected in the dry season have a high oil output, making them excellent for biodiesel manufacturing and other industrial purposes.
In the current study, the yield of oil extracted from Jatropha curcas L. seed kernels was higher than that reported for J. curcas seeds that were found to be 27% [27], 32% [28], 32% [29], 34%[30], 43%, 32% [31], and 47% [32] by multiple researches. However, when compared to many potential non-edible oilseed crops for biodiesel generation, such as Raphanus sativus L., the oil output in this research was greater. Sapium sebiferum L., at 26% [33], Sapium sebiferum L., Sapium sebiferum L., sap rubber seed is at 24% [34], Aleurites moluccana is at 20%, Moringa oleifera is at 25%, and Pachira glabra is at 23% [30].
The Ii is a unit of measurement for the average amount of unsaturation in fats and oils, and it is expressed in centigrams of iodine absorbed per gram of sample [35]. Because of its high content of unsaturated fatty acids, the oil has a high iodine value (Table 3).
The AV has been discovered to be useful in a variety of chemical and physical characteristics of fats and oils, as well as physiological uses and as a quality assurance procedure for hydrogenation. These applications include use in biodiesel standards and assessing oxidative stability. The iodine value of Jatropha collected from the El Oued region (Algeria) is 96.3% higher than the 105.20% in Nigeria and 135.85% in Malaysia [36].
The moisture content (Table 2) of ground J. curcas L. kernels was low at 5.58%. This can allow the seeds to be stored for a long period without altering their chemical composition and a low potential for microbiological contamination [37]. From the experiment, the density of oil was about 0.915 g/ml, slightly less than that obtained from the Indian crop.
The large molecular mass of vegetable oils, the length of the fatty acid chain, and the degree of unsaturation all impact viscosity [38]. Regarding the viscosity value in this study (49.85 mm2/s), it was in the same range as mentioned by Akbar et al. [35]. The value of the saponification index is 202.87 mg KOH/g, which means that the studied sample contains a high fatty acid molecular weight.
The peroxide index is 1.1 meq/kg, whereas in Akbar et al., it equals 1.63 meq/kg. It is simply an indication that the oil is less prone to lipid degradation due to oxidation in the unsaturated fatty acid double bond that causes necrosis at room temperature [39, 40].
The oil refractive index and iodine indicator were found to be 1.458 and 96.3 g/100 g, respectively, and it is an indication of the high dominance of the long-chain polyunsaturated fatty acids [38]. The acid value was obtained at 2.9 mg KOH/g, which means that oil could be edible, since it is less than the maximum acceptable of 4.0 mg KOH/g.
4.4 GC–MS analysis
Determination of the composition of the oil extracted from the kernel of Jatropha seed via GC–MS was another important feature conducted in this study (Fig. 3).
Though fatty acids, which make up the majority of the total material extracted (85.6%), are analyzed by GC–MS, the most abundant (65.9%) is oleic acid, a monounsaturated acid, followed by palmitic acid (11.2%) and stearic acid (8.5%), and these results are compared to those obtained by Siang et al. [39].
5 Conclusion
Because of the high percentage oil production achieved in this study, Jatropha curcas L. seed is a valuable source of oil. Because of its low peroxide, acid, and free fatty acid values, the oil has an excellent storage quality. Furthermore, the saponification value indicated that the oil may be utilized to make soap in an industrial setting. The presence of phytochemical components in the oil may indicate that it has therapeutic or medicinal potential. For its prospective application, more study on Jatropha curcas seed oil’s toxicity and detoxification process is required. In addition, the government should make an effort to encourage the plant’s production as well as offer good laboratory techniques in order to uncover more of the plant’s potential.
References
Ramesh M, Palanikumar K, Reddy KH (2017) Plant fibre based bio-composites: sustainable and renewable green materials. Renew Sustain Energy Rev 79:558–584
Barghouti S, Cromwell E, Pritchard AJ (1993) Agricultural technologies for market-led development opportunities in the 1990s: The World Bank
Adolf W, Opferkuch H, Hecker E (1984) Irritant phorbol derivatives from four Jatropha species. Phytochemistry 23:129–132
Sabandar CW, Ahmat N, Jaafar FM, Sahidin I (2013) Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): a review. Phytochemistry 85:7–29
Cordova-Albores LC, Rios MY, Barrera-Necha LL, Bautista-Baños S (2014) Chemical compounds of a native Jatropha curcas seed oil from Mexico and their antifungal effect on Fusarium oxysporum f. sp. gladioli. Ind Crops Prod 62:166–172
Achten WM, Mathijs E, Verchot L, Singh VP, Aerts R, Muys B (2007) Jatropha biodiesel fueling sustainability? Biofuels, Bioproducts and Biorefining: Innovation for a sustainable economy 1:283–291
Warra A (2012) Cosmetic potentials of physic nut (Jatropha curcas Linn.) seed oil: a review. Am J Sci Ind Res 3:358–366
Makkar HP, Becker K (2009) Jatropha curcas, a promising crop for the generation of biodiesel and value-added coproducts. Eur J Lipid Sci Technol 111:773–787
Ye M, Li C, Francis G, Makkar HP (2009) Current situation and prospects of Jatropha curcas as a multipurpose tree in China. Agrofor Syst 76:487–497
Openshaw K (2000) A review of Jatropha curcas: an oil plant of unfulfilled promise. Biomass Bioenerg 19:1–15
Mya OB, Amar LB, Zarroud B, Hammami H (2017) Deglet nour dates phoenix dactylifera l.: an alternative source to sugar in Algeria. Sugar tech 19:337–340
Belewu M, Sam R (2010) Solid state fermentation of Jatropha curcas kernel cake: proximate composition and antinutritional components. Journal of Yeast and Fungal Research 1:44–46
Lapuerta M, Rodríguez-Fernández J, De Mora EF (2009) Correlation for the estimation of the cetane number of biodiesel fuels and implications on the iodine number. Energy Policy 37:4337–4344
Earle F, McGuire T, Mallan J, Bagby M, Wolff I, Jones Q (1960) Search for new industrial oils. II. Oils with high iodine values. J Am Oil Chem Soc 37:48–50
Tiwari AK, Kumar A, Raheman H (2007) Biodiesel production from jatropha oil (Jatropha curcas) with high free fatty acids: an optimized process. Biomass Bioenerg 31:569–575
Gutiérrez-Tlahque J, Aguirre-Mancilla CL, Raya-Pérez JC, Ramírez-Pimentel JG, Jiménez-Alvarado R, Hernández-Fuentes AD (2018) Effect of climate conditions on total phenolic content and antioxidant activity of Jatropha dioica Cerv. var. dioica. International Journal of Agriculture and Natural Resources 45:70–81
Mekhzoumi L, Harnane N, Gharbi H. Competitiveness and policy analysis of potato production in Oued Souf region: a policy analysis matrix (PAM) approach
Emil A, Yaakob Z, Satheesh Kumar M, Jahim J, Salimon J (2010) Comparative evaluation of physicochemical properties of jatropha seed oil from Malaysia, Indonesia and Thailand. Journal of the American Oil Chemists’ Society, 87, 689–695
Kandpal J, Madan M (1995) Jatropha curcus: a renewable source of energy for meeting future energy needs. Renewable Energy 6:159–160
Singhal SC, Sekiya J (2003) Modern technology in the oils and fats industry. New Delhi: AOSC-OTA13
Ranken M (1988) Food industries manual, published by AVI van Nostrand Reinhold company, New York
Siang CC (2009) Jatropha curcas: development of a new oil crop for biofuel. East Asian Bureau of Economic Research
Abdullah BM, Salimon J (2009) Physicochemical characteristics of Malaysian rubber (Hevea brasiliensis) seed oil. Eur J Sci Res 31(3):437–445
Premjet D, Obeng AK, Yoo HY, Kim SW, Premjet S (2021) Physicochemical characterization of Jatropha podagrica seed oil for potential biodiesel production and other industrial applications in Thailand. Sains Malaysiana 50(1):85–92
Knothe G, Steidley KR (2005) Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 84:1059–1065
Highina BK, Bugaje IM, Umar B (2011) Biodiesel production from Jatropha caucus oil in a batch reactor using zinc oxide as catalyst. Journal of Petroleum Technology and Alternative Fuels 2:146–149
Abou-Arab AA, Abu-Salem FM (2010) Nutritional quality of Jatropha curcas seeds and effect of some physical and chemical treatments on their antinutritional factors. African Journal of Food Science 4(3):93–103
de Oliveira JS, Leite PM, de Souza LB, Mello VM, Silva EC, Rubim JC, Meneghetti SMP, Suarez PAZ (2009) Characteristics and composition of Jatropha gossypiifolia and Jatropha curcas L. oils and application for biodiesel production. Biomass and Bioenergy 33(3): 449–453
Kibazohi O, Sangwan RS (2011) Vegetable oil production potential from Jatropha curcas, Croton megalocarpus, Aleurites moluccana, Moringa oleifera, and Pachira glabra: assessment of renewable energy resources for bio-energy production in Africa. Biomass Bioenerg 35(3):1352–1356
Jaliliannosrati H, Amin NAS, Talebian-Kiakalaieh A, Noshadi I (2013) Microwave assisted biodiesel production from Jatropha curcas L. seed by two-step in situ process: optimization using response surface methodology. Bioresource Technology 136(2013):565–573
Akintayo ET (2004) Characteristics and composition of Parkia biglobbossa and Jatropha curcas oils and cakes. Biores Technol 92(3):307–310
Shah SN, Iha OK, Alves FCSC, Sharma BK, Erhan SZ, Suarez PAZ (2013) Potential application of turnip oil (Raphanus sativus L.) for biodiesel production: physical-chemical properties of neat oil, biofuels and their blends with ultra-low sulphur diesel (ULSD). BioEnergy Research 6(2):841–850
Wang R, Hanna MA, Zhou WW, Bhadury PS, Chen Q, Song BA, Yang S (2011) Production and selected fuel properties of biodiesel from promising non-edible oils: Euphorbia lathyris L., Sapium sebiferum L., and Jatropha curcas L, Bioresource Technology 102(2): 1194–1199
Roschat W, Siritanon T, Yoosuk B, Sudyoadsuk T, Promarak V (2017) Rubber seed oil as potential non-edible feedstock for biodiesel production using heterogeneous catalyst in Thailand. Renewable Energy 101(2017):937–944
Akbar E, Yaakob Z, Kamarudin SK, Ismail M, Salimon J (2009) Characteristic and composition of Jatropha curcas oil seed from Malaysia and its potential as biodiesel feedstock. Eur J Sci Res 29:396–403
Knothe G (2002) Structure indices in FA chemistry. How relevant is the iodine value? Journal of the American Oil Chemists’ Society 79:847–854
Salimon J, Abdullah R (2008) Physicochemical properties of Malaysian Jatropha curcas seed oil. Sains Malaysiana 37:379–382
Hou P, Zhang S, Yang L, Xu Y, Tang L, Wang S et al (2006) Callus induction from Jatropha curcas endosperm and elimination of microbial contamination in culture. Chin J App Environ Biol 12:264
Esteban B, Riba J-R, Baquero G, Rius A, Puig R (2012) Temperature dependence of density and viscosity of vegetable oils. Biomass Bioenerg 42:164–171
Siano F, Straccia MC, Paolucci M, Fasulo G, Boscaino F, Volpe MG (2016) Physico-chemical properties and fatty acid composition of pomegranate, cherry and pumpkin seed oils. J Sci Food Agric 96:1730–1735
Acknowledgements
The authors express their appreciation to the Algerian General Directorate of Scientific Research and Technological Development, and to the research project team PRFU ID number: A16N01UN390120180002, for their scientific support. We are also pleased to extend our sincere thanks and appreciation to Dr. Hadia Hemmami for her valuable assistance in completing this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Serouti, A., Korichi, M. & Ben Mya, O. Characterization and fatty acid profile analysis of Jatropha curcas L. oil cultivated in the Algerian desert. Biomass Conv. Bioref. 13, 12205–12212 (2023). https://doi.org/10.1007/s13399-021-02013-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02013-8