Abstract
The objective of this study was to evaluate folic acid and cobalamin production in synbiotic cheese containing Lactobacillus salvarius, Lactococcus lactis, and inulin. Also the physicochemical (pH, fat and protein content), microbiological (viability of Lactobacillus strains), textural, and sensory properties of samples during 28 days of refrigerated storage were investigated. According to the results, the synbiotic cheese samples had lower fat and higher pH and protein content compared to the control cheese (p < 0.01). L. salvarius and L. lactis are able to produce folic acid (B9) and cobalamin (B12) in cheese samples. The viability of L. salivarius reached 10 10 CFU/g (mL) and L. lactis viability reached 10 11 CFU/g (mL) during storage (p < 0.01). The amount of folic acid production in synbiotic cheese was higher than that in control samples (36.6 compared to 21.9 µg/g). Cobalamin concentration in synbiotic cheese was higher than that in control samples (1.33 compared to 0.48 µg/g) (p < 0.05). The results showed better synbiotic cheese texture compared to the control samples (p < 0.01). Regarding the sensory properties, there were no significant differences between the color, salinity, luminosity, and overall acceptance of the samples (p < 0.01), while there was a statistically significant difference between the synbiotic and control cheeses in terms of texture and taste. Synbiotic cheeses had higher and lower scores in terms of texture and taste, respectively. Finally, L. salvarius was able to produce folic acid and cobalamin in synbiotic cheese. Also, the cheeses also retained their desired organoleptic properties by the addition of inulin and L. salvarius strains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Statement of novelty
This can be a novel functional processing method in dairy industries for synbiotic cheese production. Furthermore, the texture and nutritional value of cheese will increase in the presence of inulin (fructooligosaccharide). Inulin as a valuable prebiotic would affect the probiotics viability during storage. Folic acid and cobalamin production in synbiotic cheese will increase and will add to the nutritional value of the dairy products. Furthermore, to the best of our knowledge in the literature, there is no published data on the evaluation of folic acid and cobalamin production in synbiotic cheese containing L. salvarius, L. lactis, and inulin.
2 Introduction
Lactic acid bacteria (LAB) are within the most conventional probiotics, used in food industries, especially dairy industries. Nowadays, LABs have a large market, as they confer the health benefits in humans [1,2,3]. To exert their beneficial effects, probiotics should be alive during food processing as well as passaging through the gastrointestinal tract (GIT) [2, 4]. Also probiotic products should contain at least 106–107 CFU/g(mL) of viable cell at the time of consumption and at least 108 CFU/g in the gut [5]. Dairy products such as yogurt and cheese interest researchers as they are great probiotic carriers [6,7,8]. Cheese can protect probiotics due to its high fat content, pH, and buffering capacity [2]. One of the main health benefits of probiotics is their ability to produce vitamins, especially group B vitamins [9, 10].
There are some scientific reports regarding biosynthesis of vitamins, especially group B vitamins by LAB in food products as well as biosynthesis of vitamins by LAB in dairy products such as yogurt, curd, cultured buttermilk, and cheeses [11,12,13,14,15]. Several probiotics species such as Lactobacillus reuteri, Lactococcus lactis, Lactobacillus salivarius, and Lactobacillus gasseri have been reported as in vitro vitamin producers [11]. Some studies reported the vitamin production by probiotics in food such as production of folic acid in yogurts [16], cobalamin in ricotta cheese [17], folic acid in jeotgal (a traditional Korean fermented seafood) [18], and folate in fermented milks [19]. Thus, appropriate selection and investigation of nutraceutical-producing probiotics are so important to produce fresh foods with a high nutritional value [20, 21].
Simultaneous application of probiotics with prebiotics has sparked the interest of food technologists [3, 22]. Prebiotics such as inulin are non-digestible nutrient components that may lead host health by stimulating the growth or activity of probiotics intestinal flora [23,24,25]. Some investigations have been carried out on the usage of inulin in fermented products like yogurt and cheese, especially white cheese [3].
Up to now, there are no studies on the production of folic acid and cobalamin vitamins by L. salivarius and L. lactis in vitro. In this study, a type of synbiotic cheese containing inulin and L. salivarius has been investigated with the aim of evaluating the contents of vitamins (B9 and 12), probiotics viability, texture parameters, and sensory properties during refrigeration.
3 Materials and methods
3.1 Materials and ingredients
Cow milk (3% fat) was prepared from Choopan Dairy industry (Tehran, Iran). Inulin (fructooligosaccharides) was purchased from Sigma-Aldrich (Germany). Calcium chloride was purchased from Merck (Germany). L. salivarius (ATCC 11,741) and L. lactis (ATCC 11,454) as starter cultures were provided by the Iranian Biological and Genetic Resources Center (Tehran, Iran). Enzyme-Max Pills were prepared from Industrial Enzymes (Iran) and cheese vat was prepared by Sabalan Steel (Ardabil, Iran).
3.2 Starter and probiotic microorganisms
L. salivarius and L. lactis probiotics activated cultures were prepared as follows: L. lactis was inoculated in M17 broth with 0.5 g% glucose and incubated at 32 °C for 48–72 h. Then, it was cultured again on M17 agar with 0.5% glucose after incubation for 48–72 h at 32 °C. After that, the bacterial suspensions were prepared according to McFarland standard 10 and added to the milk [26]. Then also L. salivarius was inoculated into MRS broth media, at 37 °C for 72 h, and then cultured on MRS agar and inoculated and incubated at 37 °C for 72 h. For cheese production, 8.5 g/kg of prepared bacterial suspensions was used according to McFarland standard 10 and added to the milk clots after dehydration [26].
3.3 Cheese production
White cheese samples were made from cow milk. The pasteurized milk with 2.4 CC of CaCl2 solution (6.6 M) was inoculated with 10 9 CFU/g of L. lactis after cooling down to 30 °C. After adding 10 enzyme pills at 38 °C for 40 min, the curds were cut. Whey was drained off and cooled; then L. salivarius (10 9 CFU/g) and 2.5 g of inulin were added. Finally, the samples were pressed at room temperature for 16 h. Then, the sample cheeses were packed in containers and stored in a refrigerator for 28 days. Control cheese sample was produced in similar conditions containing L. lactis (10 9 CFU/g).
All the experimental tests are done on control cheese and synbiotic samples in 0, 7, 14, and 28 days of refrigeration in three replicates.
3.3.1 Physicochemical analysis of synbiotic and control cheeses
The pH values of samples were measured using a pH meter (Model 601, Metrohm, Switzerland). Moisture content of the cheese samples was assessed using oven (Sartorius, UK) method and fat content was measured using Gerber-Van and the total protein content using micro-Kjeldahl methods [24].
3.3.2 Viable bacterial counts in cheese
The viability of L. salivarius and L. lactis was monitored in 0, 7, 14, and 28 days of storage at 4 °C. Cheese samples (10 g) were mixed with 90 mL of sterile peptone water; subsequently, a series of dilutions from 10–5 to 10–8 were made in sterile peptone water and plated using surface plate technique. L. salivarius was counted using MRS agar and MRS agar supplemented with 0.002 g% (W/V) of bromophenol blue under anaerobic conditions at 37 °C after 24–72 h. L. lactis was counted using M17 agar supplemented with 0.5 g% (W/V) glucose (GM17) after 24–72 h of aerobic incubation at 32 °C [27].
3.3.3 Analysis of the cheese texture
The texture of cheeses was analyzed using texture profile analysis (Brookfield, USA) at the end of storage. A 0.5-N load cell and a 20-mm diameter probe at a crosshead speed of 5 mm/s were used to carry out a uniaxial compression in two consecutive compressions. The cheese samples were put at room temperature (25 °C) an hour before analysis. The samples were cut into cubic pieces (2.5 cm3) and compressed to 50% of the initial height using two parallel plates. Hardness, adhesiveness, gumminess, cohesiveness, springiness, and chewiness of the samples were assessed in texture analysis [27].
3.3.4 Assessment of vitamins B9 (folic acid) and B12 (cobalamin)
The cobalamin and folic acid in produced cheeses were assessed according to the AOAC method using high-performance liquid chromatography (HPLC) [28]. Folic acid (F7876) and cobalamin (C47869) standards, phosphate buffer, and pancreatin were provided by Sigma-Aldrich (St. Louis, USA). Potassium acetate, acetonitrile, trifluoroacetic acid, ascorbic sodium, potassium cyanide, glacial acetic acid, acetonitrile, and sodium acetate trihydrate were provided by Merck (Germany).
3.3.5 Extraction of folic acid and cobalamin
For folic acid extraction, cheese samples (10 g) were added to phosphate buffer 0.01 M (50 mL) and sodium ascorbate 10% (6 mL) and 4 g pancreatin and homogenized and put in a shaker for 5 min. Then, the mixture was incubated at 37° C for 2 h under continuous agitation. Then they were transferred into boiling water for 20 min under continuous agitation. After that, each sample reached a volume of 100 mL by 0.01 M buffer phosphate. The extractant was then centrifuged at 4000 × g for 10 min and the supernatant was filtered through 0.45-µm PTFE filters and stored at − 20 °C to be measured with HPLC.
For cobalamin extraction, cheese samples (50 g) were added to deionized water (100 mL). After homogenization, the mixtures (30 mL) were added to acetate buffer (50 Mm) containing cyanide potassium 1% (1 mL), followed by 5 min of mixing and then incubated at 100° C for 30 min under continuous agitation. After cooling down to the room temperature, each sample reached a volume of 100 mL by acetate potassium buffer (50 mM). The samples were then centrifuged at 4000 × g for 10 min. The supernatant was filtered through 0.45-µm PTFE filters and stored at − 20 °C to be measured with HPLC.
Vitamin B9 and B12 production was evaluated in control cheese and synbiotic samples in 0, 7, 14, and 28 days of refrigeration in three replicates.
3.3.6 Evaluation of folic acid and cobalamin
Evaluation of folic acid and cobalamin was carried out using HPLC (Hitachi L-6200) (Tokyo, Japan). The conditions for assessing folic acid and cobalamin by HPLC are shown in Table 1. The mobile phase A was trifluoroacetic acid (TFA 0.025% solution in deionized water); mobile phase B was acetonitrile (grade 20%). The extractants of folic acid and cobalamin were filtered through 0.45-µm filters using a C18 Hypersil TM (15 cm × 4.6 mm) (Quest, Runcorn, UK) as the analytical column. The flow rate was measured by an analytical column included 0.25 mL/min with a Fluorescence detector (Hitachi F-1050, Tokyo, Japan) was used. The wavelength set for the measurement of folic acid was 280 and that for cobalamin was 361 nm, respectively.
3.4 Sensory analysis
Sensory evaluation of the cheeses was carried out after the end of storage (28 days) using a 5-point scale (5 = very good; 1 = very bad) by 30 trained panelists for texture, taste, color, and general acceptance properties.
3.5 Statistical analysis
The statistical analysis was carried out using SAS Software version 9.0 (SAS, USA). The analysis of variance (ANOVA) was used to analyze effects of inulin and L. salivarius addition to cheese samples.
4 Results and discussion
4.1 Physicochemical analysis of cheese samples
The chemical composition of control cheese containing L. lactis with no additions of inulin and L. salivarius was as follows: fat 20.3% (W/W), protein 17.7% (W/W), and pH = 5.7 after 28 days of storage. The mean chemical compositions of synbiotic cheeses were as follows: fat 18.7% (W/W), protein 19.9% (W/W), and pH = 6.2 after 28 days of storage. The results were similar to the study by Cardenas et al. [27] as they reported higher pH level and protein content and lower fat content after the addition of L. salivarius CECT5713 during storage at 4 °C.
4.2 Survival of L. salivarius and L. lactis during cheese storage
The viability L. lactis in synbiotic and control samples was increased from 107 to 10 11 CFU/g after 28 days of storage at 4 °C (Fig. 1), the same as previously described by the other researchers Cardenas et al. [27] and Ong et al. [27]. Also, the viability of L. salivarius in synbiotic samples was increased from 107 to 10 9 CFU/g after 28 days of storage at 4 °C. These results are similar to the study by Modzelewska et al. [3] that reported the increase in the viability of Lactobacillus strains in soft cheeses with and without addition of inulin for 45 days.
4.3 Textural analysis of synbiotic and control cheeses
The texture characteristics of cheese samples studied at the end of storage are presented in Table 2. Based on the results, the texture parameters were affected by the addition of inulin and L. salivarius. Cheese sample with L. salivarius and inulin had significantly higher values in all texture parameters, compared to control cheeses at the end of storage (p < 0.05) compared to the control sample. Cardenas et al. [27] reported that the hardness and textural properties of cheeses made with L. salivarius CECT5713 were higher than those of control cheeses.
Texture parameters are expressed as mean ± standard deviation.
Mean values with different letters were significantly different.
4.4 Sensory evaluation
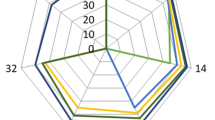
Sensory properties such as odor, texture, flavor, taste, and general acceptance in synbiotic cheeses were evaluated at the end of storage by trained panelists using a 6-point scale (Fig. 2). The analysis showed significant differences between the control and inulin-containing samples with L. salivarius in taste and texture (p < 0.05) and they were acceptable. Synbiotic cheese (compared to control) possesses higher and lower scores in texture and taste, respectively. Furthermore, no significant differences were observed in flavor, odor, and overall acceptance (p > 0.05).
One of the challenges of prebiotics and probiotics in food production is the undesirable changes in sensory properties [29]. Modzelewska et al. [3] reported no significant differences in sensory evaluation between soft fresh cheeses produced by the addition of inulin and L. plantarum, compared to those with no inulin. Similar results were reported by Cardenas et al. [27] in adding L. salivarius CECT5713 and L. salivarius PS2 on sensory qualities of soft cheeses indicating that they had no adverse effects on sensory properties of the products. Ong et al. [30] and Souza and Saad [31] also reported that there were no signficant differences in sensory properties of probiotic cheese by probiotic strains of Lactobacillus acidophilus, paracasei, and casei.
Mendez et al. [32] observed less acidic flavor and more firmness in Arzua-Ulloa cheeses containing L. plantarum and casei compared to the control sample.
4.5 Contents of vitamin B9 (folic acid) and B12 (cobalamin) in cheese
Figures 3 and 4 show the mean vitamin B9 and B12 values produced by L. salivarius and L. lactis in control and synbiotic cheeses, respectively. In Fig. 3, vitamin B9 contents in the cheese samples during the storage are shown. Vitamin B9 values in synbiotic cheese reached from 36.36 to 3.97 µg/g within 28 days of storage (p < 0.05). In control cheese, it reached from 21.97 to 0.77 µg/g at the end of the 7th day and then decreased to 0. Based on the results, vitamin B9 contents in synbiotic cheeses were significantly higher than those in control cheese which means that L. salivarius in the presence of inulin was able to produce vitamin B9 as it was a higher symbiotic sample (36.66 µg/g) than the control one (21.97 µg/g). Also, at the end of storage (28 days), the vitamin B9 amount was 3.97 µg/g but no vitamin B9 was detected in the control sample. The vitamin reduction in cheese during storage was reported in previous studies [18, 19, 33].
Regarding vitamin B12, the results showed significant decreases in contents of cobalamin in all cheeses during the storage (p < 0.05, Fig. 4). The contents of vitamin B12 in synbiotic cheeses were significantly higher than those of control cheese during the storage. In synbiotic samples, vitamin B12 content reached from 1.33 to 0.83 µg/g at the end of day 7 of storage. In control samples, it reached from 0.48 to 0.38 µg/g at the end of day 7 of storage. Moreover, the vitamin B12 content in all cheese samples decreased to 0 after the 7th day of storage. According to the results, vitamin B12 contents in synbiotic cheeses were significantly higher than those in control cheese which means that L. salivarius along with inulin had the ability of vitamin B12 production as it was a higher synbiotic sample (1.33 µg/g) than the control one (0.48 µg/g).
Production of vitamins B9 and B12 and other vitamins depends on various factors such as bacteria strains, and the media conditions such as nutrients and pH [16, 19]. L. lactis and L. salivarius are able to produce vitamins during cheese production [27]. In fact, L. salivarius is one of the bacteria capable of producing folate, B9, and B12 [17, 34]. Similar to L. lactis, this strain produces much more vitamins during cheese production. As shown in Figs. 3 and 4, the contents of vitamin B9 and B12 in synbiotic cheeses (L. lactis with L. salivarius) were higher than those in control cheeses (L. lactis). The ability of vitamin B9 and B12 production by various probiotic species in dairy products such as yogurt has already been reported [33,34,35] and in ricotta cheeses [18]. In previous studies, the measured vitamin amounts in dairy products was in ng/g units [17] whereas based on the results of this study, the initial content of both vitamins are higher (mg/g) during storage in synbiotic cheeses.
5 Conclusions
In this study, the production of vitamins B9 and B12 by L. salivarius and L. lactis in cheese samples was proved. The viability of the two probiotic strains was increased during 28 days of refrigerated storage at 4 °C. Moreover, the addition of L. salivarius and inulin included no adverse effects on sensory properties of the cheese samples. Inulin was added to the cheese samples for improving the organoleptic properties and the growth of L. salivarius as well as increasing the vitamins (B9 and B12) production in cheese. L. salivarius along with inulin in cheese increased the levels of vitamins B9 and B12. In conclusion, using inulin and L. salivarius can improve levels of vitamins B9 and B12 in synbiotic cheese and can also be applied in other dairy products as novel symbiotic functional foodstuffs.
References
García-Cano I, Rocha-Mendoza D, Ortega-Anaya J, Wang K, Kosmerl E, Jiménez-Flores R (2019) Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl Microb Biotechnol 103:5243–5257
Shah N, Patel A (2014) Recent advances in biosynthesis of vitamin and enzyme from food grade bacteria. Int J Food Ferment Technol 4:79–85
Modzelewska-Kapitula M, Klebukowska L, Kornacki K (2007) Influence of inulin and potentially probiotic Lactobacillus plantarum strain on microbiological quality and sensory properties of soft cheese. Polish J food Nutr Sci 57:143–146
Gu Q, Li P (2016) Biosynthesis of vitamins by probiotic bacteria. In: Probiotics and prebiotics in human nutrition health, Edited by: Venketeshwer Rao In Tech 135–146
Azizkhani M, Parsaeimehr M (2018) Probiotics survival, antioxidant activity and sensory properties of yogurt flavored with herbal essential oils. Int Food Res J 25:921–927
Fenster K, Freeburg B, Hollard C, Wong C, Rønhave Laursen R, Ouwehand AC (2019) The production and delivery of probiotics: a review of a practical approach. Microorganisms 7:75–83
Massoud R, FadaeiNoghani V, KhosraviDarani K (2014) The effect of homogenization pressure and stages on the amounts of lactic and acetic acids of probiotic yoghurt. Appl Food Biotechnol 1(2):25–29
Tharmaraj N, Shah NP (2004) Survival of Lactobacillus acidophilus, Lactobacillus paracasei subsp. paracasei, Lactobacillus rhamnosus, Bifidobacterium animalis and Propionibacterium in cheese-based dips and the suitability of dips as effective carriers of probiotic bacteria. Int Dairy J 14:1055–1066
Fang H, Kang J, Zhang D (2017) Microbial production of vitamin B12: a review and future perspectives. Microb Cell Fact 16:10–15
Patel A, Shah N, Prajapati J (2013) Biosynthesis of vitamins and enzymes in fermented foods by lactic acid bacteria and related genera-a promising approach. Croatian J Food Sci 5:85–91
Capozzi V, Russo P, Dueñas MT, López P, Spano G (2012) Lactic acid bacteria producing B-group vitamins: a great potential for functional cereals products. Appl Microbiol Biotechnol 96:1383–1394
Hamzehlou P, Sepahy AA, Mehrabian S, Hosseini F (2018) Production of vitamins B3, B6 and B9 by Lactobacillus isolated from traditional yogurt samples from 3 cities in Iran, winter 2016. Appl Food Biotechnol 5(2):105–118
Khosravi-Darani K, Zarean S, Ahmadi N, Hadian Z, Mortazavian AM (2019) Fed batch production of a fermented beverage containing vitamin B12. Iran J Chem Chem Engine 38(2):183–192
Massoud R, Khosravi-Darani K, Bagheri SMH, Mortazavian AM, Sohrabvandi S (2019) Vitamin B12: from deficiency to biotechnological solution. Curr Nut Food Sci 15(4):318–326
Massoud R, Khosravi-Darani K, Golshahi M, Sohrabvandi S, Mortazavian AM (2020) Assessment of process variables on vitamin B12 production in fermented dairy product including propionic acid. Curr Nut Food Sci 16(2):155–161
Tamaskoni ZE, Ehsani M, Homayoni RA, Sharifan A, Larijani K (2015) A comparative study concerned with generated folic acid in probiotic and set yogurt produced by milks from cow, sheep and goats. J Food Technol Nut 22:35–46
Repossi A, Zironi E, Gazzotti T, Serraino A, Pagliuca G (2017) Vitamin B12 determination in milk, whey and different by-products of ricotta cheese production by ultra performance liquid chromatography coupled with tandem mass spectrometry. Ital J Food Safe 67:152–155
Park SY, Do JR, Kim YJ, Kim KS, Lim SD (2014) Physiological characteristics and production of folic acid of Lactobacillus plantarum JA71 isolated from jeotgal, a traditional korean fermented seafood. Korean J Food Sci Animal Res 34:100–106
Lin M, Young C (2000) Folate levels in cultures of lactic acid bacteria. Int Dairy J 10:409–413
De Melo Pereira GV, De Oliveira CB, Júnio AIM, Thomaz-Soccol V, Soccol CR (2018) How to select a probiotic? A review and update of methods and criteria. Biotechnol Advanc 36:2060–2076
Leblanc JG, Laino JE, Del Valle MJ, Vannini VS, DV, Taranto, MP, Font de Valdez G, Savoy de Giori G, Sesma F, (2011) B-group vitamin production by lactic acid bacteria–current knowledge and potential applications. J Applied microbiol 111:1297–1309
Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Masoumi SJ, Berenjian A, Ghasemi Y (2019) Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods 8:88–92
Massoud R, Khosravi-Darani K (2018) Production of synbiotic corn extract: application against diarrhea causing microorganisms. Biointer Res Appl Chem 8:3351–3355
González Ariceaga CC, Afzal MI, Umer M, Abbas S, Ahmad H, Sajjad M, Parvaiz F, Imdad K, Imran M, Maan AA, Iqbal Khan MK, Hernández-Montes A, Aguirre-Mandujano A, Villegas de Gante A, Jacquot M, Cailliez-Grimal C (2019) Physicochemical, sensorial and microbiological characterization of porocheese, an artisanal mexican cheese made from raw milk. J Foods 8:509
Losada M, Olleros T (2002) Towards a healthier diet for the colon: the influence of fructooligosaccharides and lactobacilli on intestinal health. J Nut Res 22:71–84
McFarland standard -For in vitro use only- Catalogue No. TM50-TM60 (2014) Estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. http://www.dalynn.com/dyn/ck_assets/files/tech/TM53.pdf
Cardenas N, Calzada J, Peiroten A, Jiménez E, Escudero R, Rodríguez JM, Medina M, Fernández L (2014) Development of a potential probiotic fresh cheese using two Lactobacillus salivarius strains isolated from human milk. BioMed Res Int. https://doi.org/10.1155/2014/801918
Martin E, Giménez EC, Konings E (2016) New methods for the analysis of water-soluble vitamins. J AOAC Int 99:10–18. https://doi.org/10.5740/jaoacint.15-0245
Mcsweeney PL (2014) Biochemistry of cheese ripening. Int J Dairy technol 57:127–144
Ong L, Henriksson A, Shah NP (2007) Proteolytic pattern and organic acid profiles of probiotic cheddar cheese as influenced by probiotic strains of Lactobacillus acidophilus, Lb. paracasei, Lb. casei or Bifidobacterium sp. Int Dairy J 17:67–78
Souza CH, Saad SM (2009) Viability of Lactobacillus acidophilus La-5 added solely or in co-culture with a yoghurt starter culture and implications on physico-chemical and related properties of Minas fresh cheese during storage. LWT- Food Sci Technol 42:633–640
Menéndez S, Centeno J, Godınez R, Rodrıguez-Otero J (2000) Effects of Lactobacillus strains on the ripening and organoleptic characteristics of Arzúa-Ulloa cheese. Int J Food Microbiol 59:37–46
Crittenden R, Martinez N, Playne M (2003) Synthesis and utilisation of folate by yoghurt starter cultures and probiotic bacteria. Int J Food Microbiol 80:217–222
Sybesma W, Starrenburg M, Kleerebezem M, Mierau I, De Vos WM, Hugenholtz J (2003) Increased production of folate by metabolic engineering of Lactococcus lactis. J Appl Environmen Microbiol 69:3069–3076
Ong L, Henriksson A, Shah N (2006) Development of probiotic Cheddar cheese containing Lactobacillus acidophilus, Lb. casei, Lb. paracasei and Bifidobacterium spp. and the influence of these bacteria on proteolytic patterns and production of organic acid. Int Dairy J 16:446–456
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arasteh, H.R., Ataee, M. & Sharifan, A. Evaluation of vitamin B9 and B12 production, and physicochemical and organoleptical properties in synbiotic cheese. Biomass Conv. Bioref. 13, 10877–10883 (2023). https://doi.org/10.1007/s13399-021-01876-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01876-1