Abstract
In recent years, humanity has been called upon to redefine how it draws its energy reserves, as well as how to manage and regulate the final disposal of its waste into the environment. Biofuels are considered promising alternatives to fossil fuels. Especially, the second- and third-generation biofuels which are generated from agro-industrial waste can minimize the total disposal of residues, while they contribute to the renewable energy total supply. The current work focuses on the sustainable utilization of potato peel waste (PPW) as a viable raw material in a biorefinery, aiming to produce bioethanol and biogas. Setting dehydration as a necessary pretreatment step, the kinetics and the VFA (volatile fatty acid) production during drying of PPW were also studied. The best results in terms of bioethanol production (71.37 ± 2.47%) were reached when a chemical pretreatment step (NaOH 1% w/v) was included, followed by enzymatic hydrolysis with two different non-commercial enzymes, an amylolytic (NS22109, 50 μL/gstarch) and a cellulolytic (NS22177, 300 μL/gcellulose) enzyme, and alcoholic fermentation with Saccharomyces cerevisiae (2% w/wdry PPW). As far as the integration of the processes is concerned, two different scenarios were investigated. The first included anaerobic digestion as a stand-alone procedure for the utilization of PPW and the second included ethanol production and subsequent valorization of stillage via anaerobic digestion. The latter proved as the best scenario for both the energy production and the solid residue degradation providing 7.15*103 MJ and 390 kg of fertilizer per ton of dry PPW.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
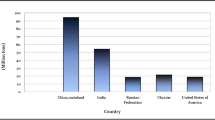
Potato production has a notable annual increase, reaching 388.7 million metric tons of total production at the end of 2021 (Fig. 1), in accordance with the Food and Agriculture Organization of the United Nations (FAO). A considerable fraction, which amounts up to 6%, is being processed by the industry [1]. According to CIP (International Potato Centre), the global potato consumption is shifting from fresh potato to value-added food products. Restaurants and fast-food chains use mainly frozen potatoes with an estimated consumption of around 7 million metric tons per year. Another processed potato product are potato crisps, most commonly called “chips” which is one of the most preferred snacks worldwide.
Potato peeling generates a significant amount of waste (15–40% of the initial raw material depending on the process that is applied, e.g., steam, lye, abrasion peeling [2]). Scraping is mainly used for the production of crisps, while steam peeling is applied in the case of frozen or dehydrated potato products. Furthermore, NaOH has been reported as an alternative to steam peeling [3]. PPW is subject to fast microbial deterioration leading to limited lifespan due to its moisture content. Common utilization pathways of PPW include animal feeding and composting. These methods can be characterized as ineffective in terms of environmental impact as valuable material is lost [4]. The current trend towards the valorization of wastes demands the transformation of industrial and municipal waste into value-added products. PPW is a lignocellulosic substrate that mainly consists of lignin, hemicellulose, cellulose, and starch. Due to its composition, this waste can be considered a potential feedstock for various industrial sectors. Since the early 2000s, research on PPW has dramatically increased. PPW’s valorization possibilities were investigated in various sectors including farming, food industry, medicine, and energy [3]. Valuable compounds contained in PPW such as phenols [5,6,7] and alkaloids [8] have drawn the attention of researchers who are seeking natural resources for the production of chemicals. PPW has a free-radical scavenger ability due to compounds such as caffeic, ferulic, chlorogenic, and p-coumaric acids [7]. Moreover, PPW has been investigated as a food preservative due to the high percentage of phenolic and caffeic acid [9,10,11]. Potato peels have also been examined for their dye absorption properties [12]. Nevertheless, most of these applications are at a low technology readiness level.
Aligned with the sustainable development goals and circular economy, valorization of PPW through the concept of biorefinery has potentially multiple benefits regarding both the minimization of waste and the increase of renewable fuel resources in comparison with fossil fuels. A biorefinery offers multiple possible pathways for liquid and gas biofuel production, in addition to the final solid residue that can be used as a fertilizer, allowing for the oriented utilization of PPW towards the maximum outcome.
To this direction, biofuel production via biochemical processes has gained ground among other alternatives due to the high demand for renewable and sustainable energy resources and is the main target of this paper. Currently, bioethanol is produced worldwide mostly from barley, wheat, maize, and sugarcane. However, these corps are considered food resources, and their use for bioethanol production does not align with the sustainable development goals [13]. On the other hand, industrial waste with high organic load such as PPW can be utilized for bioethanol production being in line with sustainable development without harming the food market and food security. For the degradation of the lignocellulosic structure of PPW into monomers such as glucose, various pretreatment steps have been reported [14,15,16]. Acidic hydrolysis seems to have the best results in terms of saccharification. On the downside, it produces substances that act as inhibitors to the fermentation process [16]. Alkaline pretreatment, on the other hand, has been verified as an effective way to pretreat biomass leading to both high saccharification and ethanol yields [17]. In this study, alkaline pretreatment in series with enzymatic hydrolysis was examined aiming at the highest ethanol yield from PPW.
Another biofuel alternative is biogas that is produced under anaerobic conditions from the bacterial degradation of biomass, and it mainly consists of methane gas (50–75%). Anaerobic digestion is the most common worldwide process used for stillage valorization [18, 19]. In recent years, due to the global need of finding alternative sustainable energy recourses, multiple feedstocks have been proposed and investigated towards their biomethane potential production [20,21,22,23]. In the current study, the anaerobic potential of PPW for both the raw waste and the stillage residue produced after alcoholic fermentation was studied.
This is the first time that PPW serves as a feedstock of a multi-product integrated biorefinery. Two innovative valorization pathways are proposed, optimized, verified, and compared. These include dehydration, alkaline pretreatment, enzymatic hydrolysis, ethanolic fermentation, and anaerobic digestion. In view of the experimental results, a biorefinery conceptual design is also presented.
2 Materials and methods
2.1 Substrate preparation
Potato peel waste (PPW) was supplied by Tsakiris S.A., Coca-Cola 3E, Greece, and delivered to the Unit of Environmental Science and Technology, School of Chemical Engineering, National Technical University of Athens, Greece. The PPW had 77% initial moisture content.
2.2 Dehydration
PPW was milled and dehydrated using a GAIA GC-100 rotary drum dryer. Dehydration took place at 120 °C for 9 h in an effort to minimize microbial development and modifications in the feedstock’s composition. Homogeneous coarse powder PPW was produced after this process. Following the dehydration process, both the dry PPW and the collected moisture condensate were characterized. The present study also investigated the drying kinetics of PPW using the dimensionless moisture ratio Xr which is described by the following equation:
where Xt is the moisture content at time t, X0 is the initial moisture content, and Xe is the equilibrium one. Xr ranges from 1 (for t = 0, X = X0) to 0 (when the equilibrium moisture is reached).
Furthermore, the condensate produced was examined for any existing valuable substances such as volatile fatty acids (VFAs). The dehydrated PPW was then stored at 20 °C prior to further treatment.
2.3 Analytical methods
Hemicellulose, cellulose, acid-insoluble residue, acid-soluble lignin, ash, and moisture were measured following the analytical procedures of NREL laboratory analytical protocols [24]. For the estimation of starch concentration, technique 996.11 [25] was used. In the aqueous phase, total organic carbon (TOC) was also estimated according to standard methods [26], while glucose concentration was calculated following the glucose oxidase–peroxidase method by a commercial kit (Biosis SA). The concentration of volatile fatty acids (VFAs) was determined by using the test kit Spectroquant volatile organic acids (Merck Millipore). Ethanol concentration was measured according to the 2019.11 technique [25].
All characterization analyses were performed three times.
For the investigation of the structure of PPW (raw and pretreated), X-ray diffractometry (Bruker D8-Advance) was applied.
2.4 Bioethanol production
For the bioethanol production treatment train, three successive processes were applied to PPW: chemical pretreatment, enzymatic saccharification, and ethanolic fermentation.
2.4.1 Chemical pretreatment
Ten grams of PPW were pretreated with 1, 2, and 3% w/v dilute NaOH at 50 °C for 6 h in 100 mL final mixture volume (10% w/v) using 250-mL autoclavable bottles. All trials were repeated three times and the mean values are reported.
2.4.2 Enzymatic saccharification
Enzymatic hydrolysis of PPW was carried out in 250-mL autoclavable bottles. After alkaline pretreatment, H2SO4 (78% v/v) solution was used to set the pH near the optimum range (5.0–5.5).
2.5 Starch hydrolysis
After PPW pretreatment, enzymatic hydrolysis of starch was carried out at 65 °C by adding a customized amylolytic formulation, NS22109 (Novozymes, Denmark) for 1 h. The specific activity of NS22109 was 2425 U/mL in accordance with the methodology of Xiao et al. [27]. In the experiments, dosages of 10, 30, and 50 μL/gstarch were used.
2.6 Cellulose hydrolysis
After the hydrolysis of starch, enzymatic hydrolysis of cellulose took place at 50 °C by adding a customized cellulolytic enzyme, NS22177 (Novozymes, Denmark), for 24 h. The total cellulase activity was 227 FPU/mL in accordance with the methodology of IUPAC [28]. In the preliminary experiments, dosages of 100, 200, and 300 μL/gcellulose were adopted. All trials were also repeated three times. The saccharification efficiency was quantified in terms of saccharification yield, SG, as follows (Eq. 2):
where m is the mass of the initial dry substrate (g), cellulose the cellulose content in the initial dry substrate (%), starch the starch content in the initial dry substrate (%),V the volume of the liquid phase (mL), and f = 1.11 (cellulose hydrolysis constant).
2.6.1 Ethanolic fermentation
The fermentation was performed by adding 2% w/w S. cerevisiae at 30 °C for 24 h, aiming to bioconvert the glucose to bioethanol. At the end fermentation, the liquid and solid phases were physicochemically characterized after centrifugation (3600 rpm, 12 min).
The ethanolic fermentation efficiency was quantified in terms of ethanol yield, Yeth, as (Eq. 4):
2.6.2 Factorial design
The factorial experimental design was applied in an effort to evaluate the influence of the main controlling parameters on the yields, both saccharification and ethanol (optimization parameters). The chosen parameters were the concentration of NaOH solution and the enzymes’ dosages. The impact of each operational parameter on the optimization parameters along with their statistical significance was assessed by a 23 factorial experiment. Table 1 presents the examined levels of the aforementioned controlling parameters.
The center of the design was repeated 5 times for statistical reasons. Mathematical models were developed upon the experimental results obtained. The Fisher criterion was applied in order to check their adequacy [29, 30].
2.7 Anaerobic digestion
In line with Angelidaki et al.’s work [31], biomethane potential essays (BMPs) were carried out in 250-mL autoclavable bottles in an effort to evaluate the anaerobic digestibility of the untreated PPW and the PPW stillage that had derived under the optimum ethanol yield. The bottles were inoculated with a 50 mL inoculum, 50 mL nutrient medium, and feedstock (1:1–4:1 volatile solids to feedstock ratio). The inoculum was delivered from a full-scale anaerobic plant (Metamorfosis Wastewater Treatment Plant, Attica) treating municipal wastewater (5% TS). Furthermore, one positive sample containing the inoculum, medium, and 0.1 mL acetic acid, and a blank sample containing only the inoculum and the medium were set as control samples. The bottles were agitated at 60 rpm and 36.6 °C.
The biogas production was measured daily following the procedure described by Esposito [32].
In an effort to obtain reproducible results, every BMP test was carried out at 3 different volatile solids ratio loadings with 2 replicates for each loading. Experiments were terminated when no further biogas was measured for three consecutive days. After each BMP test completion, all digestates (including positive and blank) were fully characterized.
3 Results and discussion
3.1 Dehydration of PPW
The dehydration of high moisture biowaste in a treatment train is necessary in order to impede biological degradation and thus protect and reserve its valuable compounds. Hence, when considering a full-scale valorization scheme, such a process should be indispensably included. Nevertheless, dehydration of biowaste is an uncharted territory, and thus, new data on its modelling are essential given the complexity of the various physical processes that take place and because each feedstock has its own unique physical complicated structure. To this end, the main scope in the current study regarding the dehydration stage was the modelling of the process using known mathematical models as described by Gomez de la Cruz et al. [33], as well as the characteristics of the condensate in view of its further valorization. TOC and VFA concentrations of the condensate along with the moisture content ratio (Xr) (Eq. 1) of PPW were the main parameters being examined.
The time evolution of these three parameters was monitored, and the results are presented in Fig. 2.
The maximum VFA and TOC production rate was achieved between 80 and 110 min of dehydration reaching 3.00 and 6.03 mg/kg·min respectively. The total VFA (ΣVFA) and TOC (ΣTOC) production after 540 min of dehydration was observed to be equal to 7.38 and 12.46 g/kg respectively.
In literature, a production rate of 1.56 mg VFA/kg·min was measured in the condensate when restaurant food waste had undergone dehydration under similar conditions (105 °C, 17 h) using a GAIA dryer [34]. Similarly, Lytras et al. [35] reported that 5.1 g VFA was produced per kilogram of dried food waste when a commercial rotary drum dryer (GAIA-300) was used at 94 °C for 9 h verifying the utilization of food waste as co-digestion substrate. Deng et al. [36] examined the formation of VFA such as propionic and acetic acid during the dehydration (air velocity 0.108 m/s, 160 °C) of municipal sewage sludge (MSS) and paper mill sludge (PMS). The production rates of propionic acid reached 36.00 and 54.00 mg/kg·min for MSS and PMS respectively. The acetic acid’s initial production rate was 42.00 and 49.2 mg/kg·min for MSS and PMS respectively with a sharp decrease being observed afterwards. The differences in feedstock composition and the drying temperatures and dryer type could be responsible for the variations that have been observed with VFA production rates reported in this study.
Modelling is critical especially in full-scale plants where significant amounts of feedstock are used leading to elevated energy demands. The most critical parameter that should be described during an air drying process within an integrated biowaste valorization treatment train are the retention time necessary for the efficient dehydration of waste. Thus, a tailored design of biowaste dryers is essential for the viability of such plants. To this end, given that the dehydration process of PPW has not been previously reported in literature, mathematical models for PPW were developed based on the respective of SCG [33]. In Table 2, the mathematical models fitted to the drying curve, the parameters, and the correlation coefficient (R2) for each equation are presented.
The fitted mathematical equation can be, in all cases, described as satisfactory since the correlation coefficients were over 90%. However, the mathematical equations that presented the optimum fit were Page and Modified Page with R2 = 0.99877 for both.
The Page and Modified Page mathematical models presented similarly high correlation coefficients for spent coffee grounds (R2 = 0.999286) during drying at 100 °C in a convective dryer [33]. Carvalho et al. [37] studied the drying kinetics for potato pulp waste under dissimilar experimental conditions (50–70 °C, 0.06–0.092 m/s air flow). The R2 for the Page model when potato pulp waste was used provided similar results ranging from 0.993550 to 0.998400 for 12 experiments. There has not been any study regarding the drying kinetics of potato peel waste yet but Aghbashlo et al. [38] investigated the drying kinetics of carrot peels at 50–70 °C and 1 m/s air velocity. Once more, the Page and Modified Page models showed a high level of correlation with R2 ranging from 0.9738 reaching up to 0.9998 at 70 °C. Sotiropoulos et al. [39] also studied the kinetics of household food waste drying and concluded that the Midilli model and Page equation presented the optimum fitting results. Additionally, the adoption of a kinetic equation could serve as a valuable tool for the estimation of the drying residence time. For the case of PPW and by applying the Page equation, the drying residence time necessary for PPW to reach 0.2 kg H2O/kg dry matter was estimated to be equal to 478 min (almost 8 h) at 120 °C in a rotary drum dryer. It is presumed that 20% moisture content is the threshold that deactivates/decelerates the biological activity of microorganisms at 25 °C. The Page model and the estimated drying residence time could stand as the basic baseline for the design of PPW industrial dryers.
3.2 Chemical composition
Wet PPW had 77% moisture and the dried substrate had a residual 3.6% moisture content. The compositional analysis on a dry base of the dried PPW is presented in Table 3. Since PPW is an organic agro-industrial substrate, growing and harvesting conditions in addition to different peeling processes may induce variations in its composition. Nevertheless, the characteristics of PPW used in the current paper fall within the literature reported values as indicated in Table 3.
PPW characterization revealed that it mainly contained (in dry base) cellulose (28.5 ± 1.2% w/w) and starch (24.4 ± 3.2% w/w). Considering acid-insoluble residue (AIR) mainly consists of lignin (Raza et al., 2018), the compositions lie in the literature range except for hemicellulose which was measured in higher concentrations than expected.
The high concentration of polysaccharides, especially starch and cellulose, makes PPW an ideal substrate for both bioethanol and biogas production [22, 42].
3.3 Alcoholic fermentation
The results of factorial experiments for both examined optimization parameters, saccharification yield (SG) and ethanol yield (Yeth), are presented in Table 4.
Moderate to high sugar releases were detected after the alkaline pretreatment and enzymatic hydrolysis. The best results for saccharification yield were observed when PPW was pretreated with 3% w/v NaOH for 6 h at 50 °C and hydrolyzed with 10 μLNS22109/gstarch and 100 μLNS22177/gcellulose reaching 82.15%. As far as ethanol production is concerned, the best result reached 71.37% when PPW was pretreated with 1% w/v NaOH for 6 h at 50 °C and hydrolyzed with 50 μLNS22109/gstarch and 300 μLNS22177/gcellulose.
Various ethanol yields and saccharification efficiencies have been reported in the literature [15] and are highly dependent on the procedure applied. Nevertheless, the values that were in this paper are in accordance with the respective literature. More specifically, Ben Taher et al. [14] reported 57.8% as the highest saccharification yield when hydrothermal pretreatment was applied (121 °C for 30 min). In the same paper, when enzymatic hydrolysis was applied, 98% of the reducing sugars was consumed leading to a 0.26 g/gconsumed sugars ethanol production. Ben Atillah et al. [15] achieved 72.38% saccharification yield when commercial enzymes (CellicCTec2-CEL and alpha-amylase from Aspergillus oryzae) were used with 0.46 g/gconsumed sugars ethanol production that corresponds to 96% of the theoretical yield. Khawla et al. [16] managed to reach 70% of theoretical ethanol yield using UEB-S amylase (90U) combined with amyloglucosidase (9U). On the downside of enzymatic hydrolysis, when feasibility is concerned and a chemical pretreatment step is included, parameters such as cost should also be examined. In regard to the microorganism used, Saccharomyces cerevisiae is the leading yeast being used for alcoholic fermentation. Nevertheless, Ben Atillah et al. [15] implemented the fermentation using Wickerhamomyces anomalus achieving the promising results that are mentioned above.
In order to enlighten the degradation mechanism of PPW, Fig. 3 presents the degradation efficiency of the major structural components (TS, starch, cellulose) targeted in the present study after alkaline pretreatment and enzymatic saccharification.
The low degradation of PPW, lower than 30% TS, was measured in the alkaline pretreatment step, mainly caused by the degradation of starch which reached 76.3%, while cellulose degradation was much lower (16%). The cellulolytic enzyme induced a significant cellulose degradation (48.7%) leading to sugar monomers.
Apart from the compositional analysis, a crystallinity characterization using X-ray powder diffraction (XRD) was also performed for the optimum conditions of ethanol yield.
A wide peak around 2θ = 22° was observed for all samples (Fig. 4) indicating the presence of cellulose in crystalline form [45]. A drop at this peak has been noticed at each additional treatment step indicating that cellulose transformed into a more amorphous form or it was broken down into its oligomeric form. Wide peaks after 20° present in the spectrum at the sample after enzymatic hydrolysis possibly correspond to crystalline salts due to the chemical neutralization the sample had undergone.
In view of process optimization and taking into consideration the results of the factorial experiment presented above, the following equations were generated, aiming to identify the optimum operational conditions for maximum saccharification and ethanol yields.
For the saccharification yield, the adequate mathematical models presented below derived:
where X1 and NaOH are the coded and physical values of the concentration of the sodium hydroxide solution (NaOH) (% w/v) respectively, and X3 and NS22177 are the coded and physical values of the dosage of the cellulolytic formulation (NS22177) (μL/g cellulose) respectively.
Likewise, regarding the ethanol yield, the respective adequate mathematical models were developed:
where X2 and NS22109 are the coded and physical values of the loading of the amylolytic formulation (NS22109) (μL/g starch) respectively.
For the sugar release, the statistically important parameters were NaOH concentration and the cellulase loading while for ethanol production, all controlling parameters were statistically important. Nevertheless, not all interactions could be neglected according to statistical analysis in the case of ethanol yield. In accordance with Fisher’s criterion, all mathematical equations were identified as acceptable. An increase of NaOH concentration seems to have a negative effect on ethanol production in contrast to the saccharification yield (Eqs. 6 and 8). This can be attributed to the enzyme’s inactivity when high concentration of salts is present after pH neutralization and to hydrolysis by-products which can act as inhibitors to the yeast. When the enzyme concentration is studied for the saccharification yield, it seems that just the cellulolytic enzyme has a statistically significant effect and it also holds a key role for the ethanol production yield. It should be noted that all results and models generated are only statistically important in the concentration boundaries that were tested.
3.4 Anaerobic digestion
3.4.1 Biogas production
Figure 5 depicts the average biogas production of the untreated PPW and PPW stillage that was produced from the optimum pre-mentioned operational conditions in the fermentation step and whose composition is presented in Table 3. The estimated biogas potentials were 291.6 ± 17.8 mLbiogas/gdry substrate and 119.2 ± 16.5 mLbiogas/gdry substrate for the raw PPW and the stillage PPW respectively.
This reduction corresponds to 60% and is likely caused due to the removal of biodegradable substances such as starch and cellulose. Achinas et al. [23] reported a biogas production potential of 204.3 mLBiogas/gdry PPW for a reactor volume of 0.4 L. Parawira et al. [20] observed a maximum biogas yield of 300 mLBiogas/gdry PPW when the concentration of total solids in the mixture reached 40%. These small deviations could be due to the diverse origins of the raw material, as well as the difference in the peeling method applied.
Another crucial parameter that was also investigated is the rate of biogas production. As shown from Fig. 5, the removal of carbohydrates by enzymatic hydrolysis and fermentation contributes to lower rates of biogas production compared to that of the untreated PPW. Assuming that hydrolysis is the rate-determining stage of the anaerobic digestion, the rate of the biogas production model could be described as follows:
where kh is the 1st-order hydrolysis coefficient, S the substrate, and t the time [31].
For the untreated PPW, the 1st-order hydrolysis coefficient was estimated to be equal to 0.61 ± 0.13 day−1, and for the PPW stillage, it was 0.17 ± 0.02 day−1. A significant reduction of 72% of the hydrolysis coefficient was observed in the case of PPW stillage. This huge decrease in the hydrolysis rate was caused by the low concentration of fast-hydrolyzing substances present in the stillage. Achinas et al. [23] reported an average k value (first-order constant of production rate) for potato peel waste 0.744 day−1 with the highest k value reaching 0.911 day−1.
Mass balances for the main components were employed aiming to assess their biodegradability during anaerobic digestion. All individual experimental trial results along with the performance of the blank samples have been taken into account, with the average results being presented in Table 5.
Similar biodegradability of the individual components was observed for both substrates. Starch appeared to be completely degraded in both cases, while the component with the lowest degradability is insoluble lignin which is the main component of the acid-insoluble residue. Furthermore, regarding the liquid phase, TOC removal was 87.25 ± 3.19% and 86.76 ± 2.84% for the PPW and stillage PPW respectively, indicating that the rate-determining step is hydrolysis.
4 Multi-product biorefinery concept applied on PPW valorization
Waste with a high organic load such as PPW can be efficiently transformed into biofuels, energy, and a plethora of other value-added products. Biorefinery facilities integrate various biomass conversion processes for maximizing the biobased feedstock utilization along with the minimization of the final residue. Biofuels produced from PPW are considered second-generation biofuels, and the potential of using PPW in a biorefinery facility integrates the concepts of the circular economy, pollution prevention, and resource recovery.
In the current study, PPW was examined as a potential feedstock for a biorefinery with the investigation of two different scenarios (Fig. 6).
To simplify calculations, 1 metric ton of dry PPW was introduced as an influent feedstock for these scenarios. They include pathways for the biofuel production (bioethanol and biogas) as well as digestate as solid fertilizer. All the data that have been considered for the calculations have been extracted from the experiments presented in the current study. The experimental results of chemical composition (Table 3), ethanol production yield (Table 4), biogas production (Fig. 5), and total solids degradation (Fig. 3, Table 5) that were used to set up the biorefinery are well in accordance with literature data.
Scenario 1 suggested anaerobic digestion as a single-step process for utilizing PPW, and biogas and solid digestate are the final products. Scenario 2 includes an alkaline pretreatment step with NaOH 1% w/v accompanied with enzymatic hydrolysis with amylolytic (50 μLNS22109/gstarch) and cellulolytic (300 μLNS22177/gcellulose) enzymes. Afterwards, alcoholic fermentation takes place with the production of bioethanol and stillage, that is further treated with anaerobic digestion. Anaerobic digestion generates the final products consisting of biogas and solid residue.
The total bioenergy production for the valorization of 1 metric ton of dry PPW is 6.71*103 MJ and 7.15*103 MJ for scenarios 1 and 2 respectively, taking into account that the calorific values of biogas and bioethanol are 23 MJ/m3 and 21.1 MJ/L, respectively. The final residue that can be used as fertilizer is 450 kg and 390 kg for scenarios 1 and 2 respectively. The best scenario for both the energy production and the solid residue degradation is the scenario 2 which includes the combination of the procedures. Nevertheless, further technoeconomical assessment needs to take place to upscale the proposed procedures. In this direction, one crucial parameter is the final ethanol concentration of the fermentation broth, which should exceed 4%w/v that is considered the threshold for viable ethanol recovery by distillation. Additionally, parameters such as market demand, product prices, and operational costs including the purchase of significant amounts of enzymes may alter the selection of the final valorization pathway.
5 Conclusions
Conclusively, the high content of carbohydrates included in PPW can be a great platform for a variety of bioprocesses for the production of sustainable energy and biofuels. The global trend for sustainable energy and valuable goods production needs to transform PPW from waste into a valuable raw resource.
Concerning the dehydration process, the Page equation presented the highest correlation results (R2 = 0.99877). Moreover, the total VFA and TOC production during this stage was observed to be 7.38 and 12.46 g/kg respectively that can be further valorized for the production of valuable substances.
The different utilization pathways made evident that PPW could be used as a substrate for producing a variety of biofuels. Regarding the bioethanol pathway, the optimum conditions that were derived from the experimental study were the combination of alkaline pretreatment step (NaOH 1% for 6 h at 50 °C) followed by enzymatic hydrolysis (NS22109 50 μL/g starch for 1 h at 65 °C and NS22177 300 μL/g cellulose for 24 h at 50 °C). Alcoholic fermentation took place for 1 day at 30 °C with the addition of Saccharomyces cerevisiae (2% w/w) with an ethanol yield of 71.37 ± 2.47%. Alcoholic fermentation valorizes just a portion of polysaccharides such as starch and cellulose; thus, the combination with anaerobic digestion is critical.
The combination of both processes (scenario 2) led to final energy production of 7.15*103 MJ and 390 kg digestate when 1 metric ton of waste was utilized.
The alternative scenario (scenario 1) included anaerobic digestion as a single-step process applied to 1 metric ton of PPW and led to the production of 6.80*103 MJ of energy and 450 kg of fertilizer.
Conclusively, PPW can be a promising feedstock in a biorefinery with great abundance across the globe. Hitherto, the valorization of PPW at a full-scale should overcome various obstacles and constraints prior to reaching sustainability.
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
References
Pandey SK, Marwaha RS, Dinesh K, Singh SV (2009) Indian potato processing story: industrial limitations, challenges ahead and vision for the future. Potato J 36:1–13. http://www.indianjournals.com/ijor.aspx?target=ijor:pj&type=home. Accessed 12/4/2021
Schieber A, Stintzing FC, Carle R (2001) By-products of plant food processing as a source of functional compounds - recent developments. Trends Food Sci Technol 12:401–413. https://doi.org/10.1016/S0924-2244(02)00012-2
Dos Santos RG, Ventura P, Bordado JC, Mateus MM (2016) Valorizing potato peel waste: an overview of the latest publications. Rev Environ Sci Biotechnol 15:585–592. https://doi.org/10.1007/s11157-016-9409-7
Wu D (2016) Recycle technology for potato peel waste processing: a review. Procedia Environ Sci 31:103–107. https://doi.org/10.1016/j.proenv.2016.02.014
Singh PP, Saldaña MDA (2011) Subcritical water extraction of phenolic compounds from potato peel. Food Res Int 44:2452–2458. https://doi.org/10.1016/j.foodres.2011.02.006
Wu ZG, Xu HY, Ma Q et al (2012) Isolation, identification and quantification of unsaturated fatty acids, amides, phenolic compounds and glycoalkaloids from potato peel. Food Chem 135:2425–2429. https://doi.org/10.1016/j.foodchem.2012.07.019
Albishi T, John JA, Al-Khalifa AS, Shahidi F (2013) Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J Funct Foods 5:590–600. https://doi.org/10.1016/j.jff.2012.11.019
Hossain MB, Tiwari BK, Gangopadhyay N et al (2014) Ultrasonic extraction of steroidal alkaloids from potato peel waste. Ultrason Sonochem 21:1470–1476. https://doi.org/10.1016/j.ultsonch.2014.01.023
Sabeena Farvin KH, Grejsen HD, Jacobsen C (2012) Potato peel extract as a natural antioxidant in chilled storage of minced horse mackerel (Trachurus trachurus): Effect on lipid and protein oxidation. Food Chem 131:843–851. https://doi.org/10.1016/j.foodchem.2011.09.056
Sánchez Maldonado AF, Mudge E, Gänzle MG, Schieber A (2014) Extraction and fractionation of phenolic acids and glycoalkaloids from potato peels using acidified water/ethanol-based solvents. Food Res Int 65:27–34. https://doi.org/10.1016/j.foodres.2014.06.018
Amado IR, Franco D, Sánchez M et al (2014) Optimisation of antioxidant extraction from Solanum tuberosum potato peel waste by surface response methodology. Food Chem 165:290–299. https://doi.org/10.1016/j.foodchem.2014.05.103
Anastopoulos I, Kyzas GZ (2014) Agricultural peels for dye adsorption: a review of recent literature. J Mol Liq 200:381–389. https://doi.org/10.1016/j.molliq.2014.11.006
Searchinger T, Heimlich R, Houghton RA et al (2008) Use of U. S. croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 319(5867):1238–1240. https://doi.org/10.1126/science.1151861
Ben Taher I, Fickers P, Chniti S, Hassouna M (2017) Optimization of enzymatic hydrolysis and fermentation conditions for improved bioethanol production from potato peel residues. Biotechnol Prog 33:397–406. https://doi.org/10.1002/btpr.2427
Ben Atitallah I, Antonopoulou G, Ntaikou I et al (2019) On the evaluation of different saccharification schemes for enhanced bioethanol production from potato peels waste via a newly isolated yeast strain of Wickerhamomyces anomalus. Bioresour Technol 289:121614. https://doi.org/10.1016/j.biortech.2019.121614
Khawla BJ, Sameh M, Imen G et al (2014) Potato peel as feedstock for bioethanol production: a comparison of acidic and enzymatic hydrolysis. Ind Crops Prod 52:144–149. https://doi.org/10.1016/j.indcrop.2013.10.025
Stoumpou V, Novakovic J, Kontogianni N et al (2020) Assessing straw digestate as feedstock for bioethanol production. Renew Energy 153:261–269. https://doi.org/10.1016/j.renene.2020.02.021
Drosg B, Fuchs W, Meixner K et al (2013) Anaerobic digestion of stillage fractions — estimation of the potential for energy recovery in bioethanol plants. Water Sci Technol 67:494–505. https://doi.org/10.2166/wst.2012.574
Drosg B, Wirthensohn T, Konrad G et al (2008) Comparing centralised and decentralised anaerobic digestion of stillage from a large-scale bioethanol plant to animal feed production. Water Sci Technol 58:1483–1489. https://doi.org/10.2166/wst.2008.515
Parawira W, Murto M, Zvauya R, Mattiasson B (2004) Anaerobic batch digestion of solid potato waste alone and in combination with sugar beet leaves. Renew Energy 29:1811–1823. https://doi.org/10.1016/j.renene.2004.02.005
Liang S, McDonald AG (2015) Anaerobic digestion of pre-fermented potato peel wastes for methane production. Waste Manag 46:197–200. https://doi.org/10.1016/j.wasman.2015.09.029
Jacob S, Banerjee R (2016) Modeling and optimization of anaerobic codigestion of potato waste and aquatic weed by response surface methodology and artificial neural network coupled genetic algorithm. Bioresour Technol 214:386–395. https://doi.org/10.1016/j.biortech.2016.04.068
Achinas S, Li Y, Achinas V, Euverink GJW (2019) Biogas potential from the anaerobic digestion of potato peels: process performance and kinetics evaluation. Energies 12(12):2311. https://doi.org/10.3390/en12122311
Sluiter A, Hames B, Ruiz R, et al (2012) Determination of structural carbohydrates and lignin in biomass: Laboratory Analytical Procedure (LAP) (NREL/TP-510–42618). Natl Renew Energy Lab 17. NREL/TP-510–42618
Association of Official Analytical Chemists (AOAC) (1995) Official methods of analysis of the Association of Official Analytical Chemists, 15th Edn. AOAC, Arlington
APHA (2005) Standard methods for the examination of water and wastewater. 21st Edition, American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC
Xiao Z, Storms R, Tsang A (2006) A quantitative starch-iodine method for measuring alpha-amylase and glucoamylase activities. Anal Biochem 351:146–148. https://doi.org/10.1016/j.ab.2006.01.036
Ghose TK (1987) Measurement of cellulose activities. Pure Appl Chem 59:257–268. https://doi.org/10.1111/j.1468-2389.1995.tb00038.x
Li SZ, Jain A (2009) Fisher criterion. Encyclopedia of biometrics. Springer, Boston, pp 549–549
Allen TT, Sauter R (2007) Introduction to engineering statistics and six sigma, 3rd Edn. Springer, Colombus
Angelidaki I, Alves M, Bolzonella D et al (2009) Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays. Water Sci Technol 59:927–934. https://doi.org/10.2166/wst.2009.040
Esposito G (2012) Bio-methane potential tests to measure the biogas production from the digestion and co-digestion of complex organic substrates. Open Environ Eng J 5:1–8. https://doi.org/10.2174/1874829501205010001
Gómez-De La Cruz FJ, Cruz-Peragón F, Casanova-Peláez PJ, Palomar-Carnicero JM (2015) A vital stage in the large-scale production of biofuels from spent coffee grounds: the drying kinetics. Fuel Process Technol 130:188–196. https://doi.org/10.1016/j.fuproc.2014.10.012
Salimi E, Taheri ME, Passadis K et al (2020) Valorisation of restaurant food waste under the concept of a biorefinery. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00613-4
Lytras G, Koutroumanou E, Lyberatos G (2020) Anaerobic co-digestion of condensate produced from drying of household food waste and waste activated sludge. J Environ Chem Eng 8(4):103947. https://doi.org/10.1016/j.jece.2020.103947
Deng WY, Yan JH, Li XD et al (2009) Emission characteristics of volatile compounds during sludges drying process. J Hazard Mater 162:186–192. https://doi.org/10.1016/j.jhazmat.2008.05.022
de Carvalho WT, de Oliveira TF, da Silva FA et al (2014) Drying kinetics of potato pulp waste. Food Sci Technol 34:116–122. https://doi.org/10.1590/S0101-20612014000100017
Aghbashlo M, Kianmehr MH, Khani S, Ghasemi M (2009) Mathematical modelling of thin-layer drying of carrot. Int Agrophysics 23:313–317
Sotiropoulos A, Malamis D, Michailidis P et al (2016) Research on the drying kinetics of household food waste for the development and optimization of domestic waste drying technique. Environ Technol (United Kingdom) 37:929–939. https://doi.org/10.1080/21622515.2015.1092588
Chintagunta AD, Jacob S, Banerjee R (2016) Integrated bioethanol and biomanure production from potato waste. Waste Manag 49:320–325. https://doi.org/10.1016/j.wasman.2015.08.010
Puttongsiri T, Choosakul N, Sakulwilaingam D (2012) Moisture content and physical properties of instant mashed potato. Int Conf Nutr Food Sci 39:92–95
Liang S, McDonald AG (2014) Chemical and thermal characterization of potato peel waste and its fermentation residue as potential resources for biofuel and bioproducts production. J Agric Food Chem 62:8421–8429. https://doi.org/10.1021/jf5019406
Hijosa-Valsero M, Paniagua-García AI, Díez-Antolínez R (2018) Industrial potato peel as a feedstock for biobutanol production. N Biotechnol 46:54–60. https://doi.org/10.1016/j.nbt.2018.07.002
Arapoglou D, Varzakas T, Vlyssides A, Israilides C (2010) Ethanol production from potato peel waste (PPW). Waste Manag 30:1898–1902. https://doi.org/10.1016/j.wasman.2010.04.017
Teater C, Yue Z, MacLellan J et al (2011) Assessing solid digestate from anaerobic digestion as feedstock for ethanol production. Bioresour Technol 102:1856–1862. https://doi.org/10.1016/j.biortech.2010.09.099
Funding
This work was supported by an EU LIFE project “CIRCforBIO” (LIFE Ref. No: LIFE18 CCM/GR/001180).
Author information
Authors and Affiliations
Contributions
Study conception and design: Dimitris Malamis, Sofia Mai, and Elli Maria Barampouti; acquisition of data: Andreas Christofi; analysis and interpretation of data: Sofia Mai and Elli Maria Barampouti; drafting of the manuscript: Andreas Christofi; critical revision: Sofia Mai and Elli Maria Barampouti; supervision: Dimitris Malamis and Sofia Mai. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barampouti, E.M., Christofi, A., Malamis, D. et al. A sustainable approach to valorize potato peel waste towards biofuel production. Biomass Conv. Bioref. 13, 8197–8208 (2023). https://doi.org/10.1007/s13399-021-01811-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01811-4