Abstract
The bioprospects of condensed corn distillers solubles (CCDS) from novel selective milling technology was investigated using a modified lab-scale separation. The valued-added products such as mineral fraction (MF), protein fraction (PF), and solvent-extracted fraction (SEF) were obtained. Including characterization of fractions, the enzymatic hydrolysis of PF using trypsin was done to examine the potential of bioactives. The yields of PF, MF, and SEF were 25.43, 8.59, and 65.09%, respectively from CCDS. Glu, Leu, and Pro were the most abundant amino acids (AAs) of PF, thereby expecting greater possibility of bioactives. The significant presence of potassium, phosphorous in MF, and extractable 2,3-butanediol, glycerol in SEF shows promising future applications. Besides, the protein hydrolysate from PF had VFVDHPLFLER, NPESFLSSFSK, ILHTISVPGEFQFFFGPGGR, TGGLGDVLGGLPPAMAANGHR, and FAFSDYPELNLPER as the identified peptides with antioxidant characteristics and inhibition against ACE, DPP-III, and DPP-IV. The bioactivities forecasted by BIOPAP software were verified with larger contents of hydrophobic, aromatic, and other AAs in PF. The porous and amorphous nature of MF and PF as found by microstructural study revealed potentiality of these products for nutritional purposes for feed industries. The prospective of CCDS can be anticipated as per the outcomes and further optimization for value-chain can be established.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
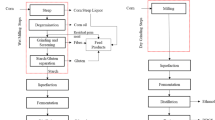
Bioethanol production from corn continues to attract attention across the globe. In 2018, Canada produced 13.90 million tons of corn wherein Ontario contributed to 8.60 million tons [1]. Around corn (~ 33.30%) was utilized for bioethanol and other industrial purposes [1]. With recent advances in selective milling technology (SMT), this more efficient process is being increasingly adopted by leading industries in the sector like IGPC Ethanol Inc., a bioethanol plant in southern Ontario. In SMT, the fiber component is segregated from raw corn before fermentation. Significant variations were observed in co-products from SMT compared to dry/wet milling technologies [2].
Condensed corn distillers solubles (CCDS) from SMT is a co-product acquired by condensing thin stillage (TS) after fermentation and distillation. The CCDS contains proteins, carbohydrates, minerals, glycerol, succinic acid, acetic acid, and lactic acid [3, 4]. Firstly, the dry matter content of CCDS from SMT (~ 30 to 40%) is slightly greater than CCDS of dry-grind process (25.1–28.6%) [5]. Secondly, SMT also increases the ethanol yield (~ 3%) by increasing accessible starch for ethanol conversion (https://icminc.com/icms-selective-milling-technology-new-and-improved/). However, many problems are linked with CCDS despite the processing used in its production that likely restricts its usages. For instance, drying of CCDS is a constraint for market applications [5–7]. The efforts for drying were less efficient due to recalcitrant drying behavior caused by glycerol, oil, and proteins present in the range 5.0–6.5%, 5.0–5.5%, and 4.0–7.0% (wet basis), respectively [5, 8]. Furthermore, thermal (40–50%) and electrical energies (30–40%) for the drying of the mixture comprising CCDS and distillers wet grains were huge [6]. There might be mold growth in undried CCDS during storage [8]. Another concern is higher sulfur in CCDS observed via augmented polioencephalomalacia in cattles (Beef cattle, https://beef-cattle.extension.org/what-are-corn-condensed-distillers-solubles/). The shortcomings of high susceptibility to heat [6] and the altering mixing ratios are also associated with CCDS. The issues with CCDS necessitate further valorization routes of this co-product.
Sedimentation separates a dilute slurry based on the size and densities of particles into solid sediment and a clear fluid product using “g” force. Contrarily, centrifugal sedimentation (CS) uses many folds “g” forces/centrifugal forces which do not change the relative settling velocities of small particles and govern the troubling effects of free convection currents and Brownian motion. Single-step CS was applied for the CCDS from dry-grind processing to generate value-added products [3, 4]. However, the products were largely contaminated with interfering constituents within each other. The influence of centrifugal parameters such as centrifuge force and time on the composition of separated fractions from corn coproducts has been documented [4, 9]. Liu and Barrows [4] found the insignificant effect of centrifuge force on the composition of mineral and glycerol fractions from CCDS. Besides, a centrifuge force of around 3000 g has been stated suitable for producing TS in industries [9]. The mass yield of wet and dry matter did not increase significantly when centrifugal force was raised after 3000 g [9]. Another study has also suggested “3000 g” as an appropriate centrifugal force for creating value-added fractions from CCDS [10]. Moreover, in some cases, insignificant effect of centrifugation time on separation efficiency was observed [11]. The addition of centrifugal stages especially second step has been described very effective in separating fractions [12]. Thus, exploration of similar separation approach for CCDS might provide its new valorization routes. Moreover, the protein in CCDS also comprises a yeast protein from fermentation [13]. The yeast protein further adds value to CCDS protein because dead yeast cells contribute to the enhanced amino acids (AAs) composition in addition to an increment in nutritive value and taste [13].
There has been less previous evidence for value addition to constituents of CCDS. Moreover, few studies have focused on its oil and glycerol [4, 5, 10, 14, 15]. However, CCDS compositional analysis demonstrates valorization prospectives of its proteins, minerals, and chemicals. Consequently, the objectives of the current study were framed as (a) to fractionate CCDS from SMT using modifications in existing separation methods, (b) to investigate the composition of extracted fractions, (c) to analyze the bioactive peptides from PF using enzymatic hydrolysis, (d) to characterize PF and MF using electron microscopy for evaluating their potential as an individual product. The key contribution of this work is the solution it provides for more concentrated fractions of CCDS. The processing steps in SMT are shown in Fig. 1.
Schematic diagram explaining the steps to produce condensed corn distillers solubles from the selective milling technology in corn ethanol industries. SMT - selective milling technology; FST - fiber separation technology; TS - thin stillage; WS - whole stillage; FWS - fiber with syrup; S/L - solid/liquid; PIL/P2L - pass 1 liquid/pass 2 liquid/pass; LQT1, 2 & 3 - liquefaction tanks 1, 2, and 3; DDGS - distillers dried grains with solubles; CCDS: condensed corn distillers solubles; HiPro - high protein; EVAP - evaporation; FERM - fermentation: DIST - distillation
2 Experimental section
2.1 Materials
The CCDS was procured from IGPC Ethanol Inc., Aylmer, Ontario 20 L container and was stored at − 20 °C for further investigation. The moisture content of CCDS sample was computed as 67.00 ± 0.43%, respectively. Ethanol and other chemicals were purchased from Fisher Scientific (Ontario, Canada).
2.2 Two-step process of centrifugal sedimentation
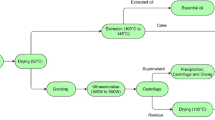
The two-step CS process has been used in our recent work and was modified from previous studies [4, 10, 16]. The reasons for consideration of this modified process were clearly explained [16]. Also, CCDS was de-oiled using physical separation via centrifugation by IGPC Ethanol Inc. Aylmer, although some oil was still observed during preliminary work. Briefly, the lab-scale two-step CS process was created for CCDS with constant centrifugation (3000 g, 30 min) and stirring parameters (30 min, 200 rpm). The centrifugal parameters were selected after carefully studying the previous studies [4, 9, 10] and results of initial experiments as presented in Fig. 4S, Supporting information. Considering our objective of maximum purity, greater centrifugation time (30 min) was considered in our study. The preliminary studies with three-step and four-step CS revealed no significant improvements. In the first step, CCDS weighing 300 g was solubilized in 690 mL water to raise the moisture up to 90% due to greater flow-ability. The mixture was stirred at the conditions with consequent sedimentation (3000 g, 30 min) using a super-centrifuge (Sorvall LYNX 6000, Fiberlite F12-6 × 500L, Max RCF 24,471 × g). Furthermore, the organic fraction (supernatant) was isolated in a container. Second step with precipitates was performed similar to first step. The whole organic fraction was mixed with 95% ethanol in such a manner that ethanol became 60% of the total solution volume. This mixture was stirred (200 rpm, 10 min) and left for 1 h at room temperature to precipitate out. The solution was centrifuged (3500 g, 30 min) to recover suspended particulates and precipitates as a mineral fraction (MF) and a supernatant. The recovered PF and MF were freeze-dried (HRC-7–115, Harvest Right, USA). Lastly, ethanol was evaporated from the supernatant at 80 °C using a rotary evaporator (IKA RV8, HB10, rotovap, USA) until its density approached to 1.3 g.mL−1. This fraction was solvent-extracted fraction (SEF). All the three fractions were kept at − 20 °C for further analysis.
2.3 Compositional analysis
Moisture content was examined using the American Association of Cereal Chemists (AACC) International method 44–01.01 [17]. The protein content (%) was calculated according to the AACC method 46–30.01 [18] using a nitrogen analyzer (LECO FP-528 series, LECO Corporation, St Joseph, MI, USA) calibrated with ethylenediaminetetraacetic acid. The carbohydrates such as cellulose, hemicellulose, and lignin contents were computed using a method previously developed [19]. Ash content in each sample was computed at 575 °C using American society for testing and materials E1755-01 [20]. All the experiments were performed in triplicates. The oil content was determined by Soxhlet apparatus using petroleum ether as a solvent [4]. The glycerol content was determined using a spectrophotometric method using standard curve shown in Fig. 1S (Supporting information) [21].
2.4 Mineral fractions analysis
Elemental analysis for CCDS and MF was carried out using wavelength dispersive X-ray fluorescence (WD-XRF) spectrometer (Panalytical Zetium Model-Zetium 4.0 Kw, the Netherlands) with Rh anode and 4 kV x-ray generator [22], and the average values were presented. Three grams of MF with density and thickness of 1020 kg.m−1 and 3 mm was filled in a primary window with a transparent film of the thickness (0.5 mm) inserted into a secondary window. The actual state parameters were fixed as conductivity (28.57 μS/m), internal flow (4.37 L.min−1), cabinet temperature (35.0 °C), medium pressure (899.61 kPa), and helium environment. The spectrum was examined using the precalibrated SuperQ software envisioned for analysis of 95 elements from hydrogen to americium and each sample was scanned 50 times. The parameters are listed in Table 1S (SI).
2.5 Protein fraction analysis
2.5.1 Amino acid composition
The digestion tubes with PF (0.1 g) were filled with 6 N HCl (5 mL). Then, the tubes were flushed with nitrogen gas for 10 s and sealed. The tubes were kept in heating blocks at 110 °C for 24 h. The tubes were filled with 1 mL of norvaline standard solution (5 mM) followed by cooling after 24 h. Following vortex, the samples were taken out and sedimented by microcentrifuge at 13,000 g for 2 min. The supernatants were mixed with 120 μL of acid and then 100 μL of 6 N NaOH was added, and vortexed. Further 400 μL milliQ water was added and vortexed again. The samples were obtained after microcentrifugaion at 13,000 g for 2 min. The derivatization of samples and AA standards (10 μL) was performed through ACCQTag Ultra derivatization kit (Waters Corporation, Milford, MA, USA) [23]. The UPLC was used to segregate the derivatized AAs at 260 nm (UV detection) using 10 min cycle time for each sample. The 1 μL of derivatized AAs injected in a column (2.1 × 2000 mm, 1.7 μL) held at 55 °C and separated. The peak areas of AAs were matched with their known standards. The data were examined by Waters Empower 2 Software (Waters Corporation, Milford, MA, USA). The oxidized and alkaline hydrolysis of PF were also performed to find contents of Trp, Tyr, Gln, and Asn with similar analysis using UPLC.
2.5.2 Enzymatic hydrolysis
The PF was mixed with 50 mM Tris–HCl (pH 8.0) [solid: liquid ratio = 1:10, protein: liquid = 1:20 (w/v)]. The mixtures were stirred for uniform blending and heated at 90 °C for 30 min in water bath to denature protein and to improve the hydrolysis efficacy [24]. The pH of mixture was adjusted to 8.0 after cooling to room temperature. The concentration of trypsin, i.e., 1:20 (w/w) has been used as an optimal value in previous studies [25, 26]. It is according to the range recommended by Sigma-Aldrich. Preliminary studies also suggested trypsin as more effective, whenever used in 1:20 (w/w). Hence, this was used in the present study for the hydrolysis performed in VWR 1585 shaking incubator (VWR International, Radnor, USA). The reaction was terminated by holding at 95 °C for 15 min to confirm complete inactivation of residual trypsin activity. Subsequently, the hydrolysate was centrifuged at 15,000 × g for 30 min at 4 °C to remove insoluble solids and lipids if present and was lyophilized in a freeze drier and stored at − 20 °C for future analysis.
2.5.3 Protein hydrolysate analysis
Liquid chromatography–mass spectrometry (LC–MS) was used to identify proteins, peptides and to examine molecular weight (Mw) distribution in PF hydrolysate. The distribution of Mw in hydrolysate was examined employing PLRP-S column (1000 Å, 5 μm, Agilent) at Advanced Analysis Centre, University of Guelph using similar conditions below.
The hydrolysate was analyzed for protein and peptide identification by Agilent 1200 HPLC liquid chromatograph interfaced with an Agilent UHD 6530 Q-Tof mass spectrometer equipped with an electrospray ion source. In brief, 30 μL of sample was injected, in triplicate, onto C18 column (Agilent AdvanceBio Peptide Map, 100 mm × 2.1 mm × 2.7 μm) for chromatographic separation at a flow rate of 0.2 mL.min−1. Mobile phases utilized for LC were (A) 0.1% formic acid in water and (B) acetonitrile with 0.1% formic acid. The mobile phase gradient was as follows: initial conditions, 2% B increasing to 45% B in 40 min and then to 55% B in 10 more min followed by column wash at 95% B and 10-min re-equilibration. The first 2 and last 5 min of gradient were sent to waste and not the spectrometer. The mass spectrometer operated in extended dynamic range positive ion auto MS/MS mode, with a drying gas temperature of 350 °C [flow rate = 13 L.min−1] and a capillary voltage of 4.0 kV, in the mass range from m/z 300 to 2000, permitting the successive separation of the three signal intensity ions for collision-induced dissociation at a fragmentation voltage of 150 V. The instrument was externally calibrated with the ESI TuneMix (Agilent). Nebulizer pressure was 40 psi and the fragmentor was set to 150 V. Nitrogen was used as both nebulizing and drying gas, and collision-induced gas.
The tolerance values such as 0.05 Da and 15 ppm in case of fragment and parent ions were included in the search measures that permitted one unused oxidation of Met and deamidation of Asn, Gln as variable modifications. The proteins and peptides were scored using Mascot algorithm with the Swiss-Prot database for 95% confidence (p < 0.05). The bioactivities were predicted using online BIOPEP-UWM database (http://www.uwm.edu.pl/biochemia/index.php/pl/biopep/) [27].
2.6 Solvent-extracted fraction analysis
The method adopted in the current study has been taken from earlier work on the identification of volatile compounds by gas chromatography-mass spectroscopy (GC–MS) [28]. SEF weighing 100 mg was mixed with 1000 μL ethanol and 50 mg NaCl. Furthermore, 400 μL of ethyl acetate was added and centrifuged (3000 rpm, 5 min) following 2 min of vortex mixing. The two injections of 1 μL each from the supernatant were taken for GC–MS (Agilent GC 7890, Santa Clara, CA, USA) interfaced with an Agilent 5975C mass selective single quadruple detector and injection run at 70 eV energy. Chromatography was performed using HP-5MS (30 m × 0.25 mm × 0.5 μm) stationary phase column [28]. Helium (grade 5.0) was used as the carrier gas with a constant flow rate of 1 mL/min. The MS was operated in scan mode with a mass range of 50 to 350 amu at 5 scans s − 1. The MS source and quadruple temperatures were 230 °C and 150 °C, correspondingly. The data was analyzed on Agilent MSD ChemStation software (version E02.02.1431). A total of 10 chromatographic runs were executed on SEF sample.
2.7 Scanning electron micrographs
The freeze-dried CCDS, PF, and MF were examined using FEI Quanta FEG 250 scanning electron microscope (SEM) (Thermo Fisher Scientific, Waltham, MA, USA) at 10 kV. All the samples were positioned into an auto sputter coater (Cressington 108) under vacuum and set at a current 25 mA, wherein each sample was coated with a 10-nm fine gold–palladium layer within 90 s − 1 to augment electrical conductivity and to prevent charge build-up before investigation.
2.8 Statistical analysis
The data analysis was executed using R studio software version 3.5.1, 2018. The data were represented as the mean ± standard deviation of triplicate determinations unless otherwise stated. Tukey’s honest significant difference test was performed for statistical significance of pair-wise comparisons of means. A value of p < 0.05 was statistically significant throughout the study.
3 Results and discussion
3.1 Mass balance and chemical composition
The mass balance on CCDS and its extracted fractions is illustrated in Fig. 2. The significant variations were observed in the contents of proteins, carbohydrates, glycerol, and ash among distinct samples, as presented in Table 1. The yield of fractions differed significantly to each other (p < 0.05). The highest yield of SEF (> 65%) agreed with the literature [5]. Besides, glycerol and organic acids in CCDS (db) were also revealed earlier [29]. The yields of MF, PF, and SEF were obtained in order of SEF > PF > MF. The results concurred with earlier studies [5, 8, 29, 30]. The composition of different samples is summarized in Table 1.
The effect of separation can be visualized through the purification of individual components. For instance, the contents of hemicellulose and cellulose in PF were 3.11% and 25.00% greater than dried CCDS. This could be attributed to the water insoluble nature of NDF [31]. Moreover, the content of NDF in PF was 10.11% larger than CCDS. The results were different from literature, wherein carbohydrates in protein extract were 38.29% more than CCDS [10]. This could be attributed to the addition of second step in separation used in our study.
The content of protein accumulated to more than thrice in PF as compared to CCDS in the case of two-step CS. It was significantly distinct compared to doubled protein in PF reported in an earlier study [4]. Although, in our study, protein content in PF extracted via one step CS was 37.94 ± 0.27%. This suggests that addition of a second step in CS significantly concentrates protein in PF (Table 1), which could be verified by observing the composition of other fractions as well (Table 4S, SI). The results concurred with the study by Lin et al. [12], wherein the number of centrifugation steps were altered to analyze purity of extracted products and two-step centrifugations was found to be the most effective in getting the high purity of the products. It could also be supported by Wang et al. [9], in which four-step centrifugal filtration method was developed for separating corn whole stillage into TS and wet grains. They employed multiple washing and redispersion steps to eliminate trapped fine particles from wet grains after first centrifugation. The results showed the efficacy of their developed method by comparing with industrial techniques as well as single step CS. Similar methods have also been used in corn wet milling for protein separation [30]. Additionally, accumulation of protein in PF could be due to the water insoluble nature of CCDS protein, which was identified to be of zein origin [5]. The Mw of zein was in the range of 150–300 kDa and 10–15 kDa contributing to 10–26% and 26–44%, respectively, of total CCDS protein [5] Furthermore, the probability of glycoproteins also exists in PF proteins because CCDS is formed by evaporating TS. This is because sugars and proteins react to create glycoproteins due to the thermal treatment during evaporation. The zein proteins might also get cross-linked in the protein bodies owing to heat treatment [5]. These reasons can also result in concentration of protein and carbohydrates together during two-step CS. This could also be supported by the outcomes of our earlier study in which the glycation was revealed in PF using analytical pyrolysis [16].
The ash analysis is important because ash, due to alkalinity characteristic, was directly related to acid intake during acid hydrolysis for biomass valorization [4]. The ash content in MF was 71.71% greater than CCDS. This infers that MF constitutes more than thrice the amount of ash in comparison with CCDS (Table 1). On the contrary, ash contents in CCDS were 77.87% and 81.45% higher than PF and SEF, respectively. It was inferred that acid hydrolysis of MF would require a higher amount of chemicals than PF, CCDS, and SEF, whenever this route will be followed for value addition. That makes PF and SEF preferable for bioconversion to produce bio-products.
The glycerol content in SEF was 62.86% larger than CCDS. The content of glycerol in CCDS was greater than the previously published results that might be due to the lesser oil in CCDS sample obtained from the industry. Secondly, CCDS was produced through a novel SMT method. On the contrary, PF and MF contained lower contents of glycerol, i.e., 2.39% and 1.78%. Contrary to the previous findings, our study revealed more purified PF and MF fractions in terms of glycerol, whereas SEF was heavily accumulated with glycerol (> 75%) [4, 10]. The results illustrated the improved efficiency of the two-step CS in the purification of individual components.
3.2 Mineral analysis
The qualitative and quantitative analysis of minerals in CCDS and MF were conducted using WD-XRF, and their values in CCDS and MF were calculated, as depicted in Table 2. The mineral content of MF was 61.08% greater than CCDS. The major macro elements such as potassium (K), phosphorus (P), and sulfur (S) with concentrations of 7.00%, 2.59%, and 1.56% were revealed in CCDS, whereas P, K, magnesium (Mg), calcium (Ca), and S with concentrations of 13.95%, 11.00%, 4.41%, 3.81%, and 1.57% were revealed in MF, as stated in Table 2. Besides, major trace elements like iron (Fe) and zinc (Zn) were revealed in CCDS with a concentration of Fe higher than Zn, whereas Zn greater than Fe in MF, as presented in Table 2. The significant differences between CCDS and MF showed that separation had a substantial influence on the elemental composition (p < 0.05). Similar results in corn co-products and mineral fractions were discerned previously [10, 32].
The variations in minerals play an important role in feed production. Qualitatively, as shown in Fig. 2S (SI), the peaks of K, P, S, Ca, and Cl were visualized in decreasing order. Besides, MF revealed new elements like Si, Cl, and Rb, which could be attributed to SMT involved during corn processing. The results go beyond the previous study that reveals the uniqueness of our results [10]. There could be advantages and disadvantages linked with larger as well as smaller mineral quantities. For instance, the wide range of minerals in corn CDS was investigated with probable negative influence on feed applications [32]. This is because the actual mineral composition could vary as compared to accounted values. In diet preparation, undernourishment can also be avoided with lesser minerals. During this postulation, the overfeeding chances are extremely large that can further cause mineral wastage and nutritional ailments [32].
The P and K were the most important components in MF. The P occurs as phytate in cereals and chelates with proteins and/or other minerals particularly Zn and Ca, affecting nutritional characteristics [33, 34]. The phytate was present nearly 60–82% and 40–50% of the whole P in corn and fermentation mash, correspondingly and thus becomes three times denser in dried distillers grains with solubles (DDGS) [35, 36]. Consequently, P being highest in MF can be a potential nutrient as phytate. Additionally, P usage in chemical and food industries is another interesting application. The lower biological availability (~ 60%) of P in corn DDGS was investigated, which restricts its applications for poultry feed [37]. Besides, the separation of P breaks its linkages with K forming K-rich MF that can be an alternative for animal feeding. The potential of K as fertilizer especially for starch synthesis in corn was shown earlier [34]. We speculate that higher K with or without P could be a viable choice for plant growth. Moreover, MF also purifies PF as verified by its lowest ash content (Table 1).
3.3 Investigation of protein fraction
3.3.1 Amino acid analysis
Total AAs in PF were computed (as is basis) as shown in Fig. 3a. The AA composition is a main nutritive marker of PF that includes AAs from yeast comprising about 40% of CCDS proteins found by multiple linear regression model [38]. The difference in sum of AAs and protein content in PF, i.e., 58.56 ± 1.08 and 57.47 ± 0.79, respectively was insignificant (p > 0.05). This implies the presence of primarily protein nitrogen in CCDS neglecting the other forms of N. The Glu and Leu with contents of 7.56 ± 0.08% and 7.09 ± 0.02% were revealed to be the two most abundant AAs, followed by Pro, Phe, Ala, Asp, Arg, and Ser. Trp was the least content AA in PF. The results were slightly different from Liu [10], wherein prime AAs in PF from CCDS of dry milling process in order of greatest to smallest concentration were Asp > Leu > Glu > Ala > Pro > Val. However, protein content in PF was significantly higher (~ 20%) in our study to that of Liu [10]. Furthermore, Adom et al. [34] investigated the major AAs of CCDS from dry-grind process as Ala ≈ His > Asp > Gly ≈ Thr. Thus, the effects of different processing techniques on AAs could be verified. Figure 3a displays the significant amounts of lipid-soluble AAs in PF, thereby offering a good scope for producing bioactive hydrolysates and peptides. The least quantity of bitterness-causing Trp (0.43 ± 0.03) must result into the desired quality of PF hydrolysates (Fig. 3a). Besides, the essential AAs of PF were similar to AAs pattern in high-quality protein for human application [39]. Hence, PF fulfills all needs excluding Lys. Moreover, Leu and Glu can be recovered from PF considering their huge demands.
a Amino acid composition of protein fraction. Molecular weight (Mw) distribution in trypsin hydrolysate of protein fraction. b deconvoluted spectra from complex, multiply charged protein groups of peaks in m/z raw data of high Mw to obtain actual mass (10.68–11.58 min); c non-deconvoluted spectra from raw data containing low Mw (9.43–10.46 min), d peaks A and B represents the larger and smaller Mw range, respectively
3.3.2 In silico evaluation of bioactivities
Proteins and peptides were identified in the protein hydrolysate prepared using trypsin from PF, as listed in Tables 3 and 4. The peptides were investigated for possible bioactivities using BIOPAP-UWM and PeptideRanker. The peptides with PeptideRanker score > 0.5 were counted as “potentially bioactive” as listed in Table 4. Five of them were found to be bioactive peptides (Table 4). The foremost activities predicted by BIOPEP-UWM were the inhibition of angiotensin-converting enzyme (ACE), dipeptidyl peptidase (DPP)-IV, DPP-III, alpha-glucosidase, renin, and CaMPDE, antiamnestic, antithrombotic, antioxidative, anticancer, hypolipidemic, and stimulating characteristics. The anticipated activities were associated with particular smaller peptides primarily comprised 2–3 AA residues encoded in their sequences. Precisely, LHT, LH, EL, PEL, and YPEL had antioxidant actions. The occurrence of significant quantities of hydrophobic, aromatic, or branched chain AA residues were accountable for the antioxidant behavior, which can easily solubilize the peptides into the lipid bilayer of cell membrane [40]. The ACE inhibition was certainly subsidized by the simultaneous presence of aromatic or hydrophobic AAs at the carbon terminal and Met, Thr, Ser, Glu, and Asp [27]. Explicitly, the smaller sequences DY, GE, GD, TG, SF, VF, and LF were classified under this category. Yet, ACE inhibitor activity was also improved by peptides such as PL, GL, and IL containing hydrophobic and branched chain AAs at nitrogen and carbon terminals, respectively [27]. Besides, ACE inhibition was provided by “GP” sequence owing to its hypothetical strength to trypsin cleavage [41]. The peptides with antioxidant and ACE inhibitory activities have also been studied in the case of corn gluten meal and zein hydrolysates [40, 42].
The predicted activity of DPP-IV inhibition was linked with type 2 diabetes [40]. The di- or tri-peptides containing Pro residue at carbon terminus and hydrophobic AAs in the sequence of length 2–8 AAs exhibited activity of DPP-IV inhibition [27]. The dipeptides containing bulky and aromatic side groups on both AAs were powerful DPP-IV inhibitors [43]. In the present study, FA, FL, and HP fragments were discerned to be responsible for DPP-IV inhibition action, as summarized in Table 4. There were also less common predicted bioactivities of peptides (Table 4). However, antioxidant, ACE inhibition, and DPP-IV inhibition activities were the prime functions retained in the peptides of CCDS even after processing of corn for bioethanol production. A recent study has successfully shown the application of corn DDGS as antioxidant hydrolysates in ground meat, pet food, feed inclosing fish oil, and bulk oils [44]. The proteins in DDGS were degraded to a greater extent as compared to CCDS owing to the severe temperature treatments [45]. The lipid oxidation in pet food comprising 2% alcalase hydrolyzed DDGS was effectively hindered by a decrease of 37.8%. Thus, considering elucidated process-induced alternations in CCDS proteins, we recommend extraction of PF proteins as inappropriate technique; instead, enzymatic hydrolysis can certainly be more effective in this case. In line with the ideas of Hu et al. [44] and previous studies, our future work would focus on evaluation of the major in silico identified bioactivities of protein hydrolysates prepared from PF.
3.3.3 Molecular weight distribution
The characteristic deconvoluted and non-deconvoluted MS spectra signifying Mw distribution in PF hydrolysate at different retention times are presented in Fig. 3. The various peptide fragments were observed in-between 9.43 and 11.59 min. The Mw was found to be in the range 762.63–86,227.14 Da comprising 53.41% of Mw below 1060.85 Da. The Mw above 1060.85 Da might be attributed to trypsin activity, whose active pocket containing Asp residue cleaves only at C-terminal sideways of Lys and Arg [46]. Although trypsin also leans to cut by Pro [47]. The CCDS had primarily Arg, Ile, Lys, Val, Ala, Asp, Pro, and Glu with radical-scavenging activities and metal-chelating capacities [5]. Moreover, trypsin is an extremely discriminating proteinase that selectivity stimulates the creation of greater as well as lesser Mw peptides. These shorter peptides have better absorption characteristics than longer peptides. Conversely, Wood et al. [5] utilized SDS-PAGE to study Mw distribution in CCDS from dry-grind process. The results displayed three distinct range of Mw, i.e., 10–15 kDa, 50–-75 kDa, and 150–300 kDa contributing a total of 26–44%, 15–20%, and 10–26% of CCDS, respectively. Our outcomes, however, found that trypsin can effectively convert larger Mw polypeptide chains in protein fractions into smaller fragments, among which peptides (< 1.6 kDa) have potential to be applied in feeds and functional foods. Also, Mw range of 6–12 kDa was revealed appropriate for pet food applications [46] and 15–40 kDa were the most common allergens [45, 48]. Since major smaller and the most abundant larger proteins in hydrolysate from PF were lesser than 10 kDa, it might be able to fulfill market demands of pet food industries.
3.4 Chemical analysis of solvent-extracted fraction
GC–MS reveals the chemical footprint of SEF. Excluding glycerol, SEF had 56 volatile constituents, among which 13 the most significant, based on peak purity and concentration (> 1.0%), were selected and represented with molecular structures (Fig. 4A and Table 5). The content was described in terms of area percentage. There were 3 alcohols, 8 acids, 1 furan, and 1 amide, as listed in Table 5. However, glycerol was the most important component of SEF that was excluded from GC–MS analysis because of too much interference with peaks of other chemicals.
3.4.1 Glycerol
Glycerol is highly concentrated in SEF extracted from CCDS due to two-step centrifugation. Glycerol is generally not freely accessible and results from oil portion of corn. Excess glycerol is generated from the sugars fermented using yeast in corn bioethanol industries. By the end of 2021, the production of biodiesel from vegetable oils would raise to 30 × 106 ton. This will cause to the formation of glycerol with an amount nearly 3 × 106 ton [49]. This surplus glycerol has accelerated the research to find its new utilization methods for creating its valorized products. Glycerol was utilized to produce various chemicals such as 1,3-propanediol, acetic acid, and acetone via anaerobic fermentation employing Clostridia [29]. Glycerol was also used to produce succinic acid [29], which could be a potential usage of glycerol consituent in SEF. The obtained crude glycerol of differing purities is refined by methods such as vacuum distillation, nanofiltration, and chemical inclusion to use it at the market level [10]. The deodorizing and bleaching steps are needed to further refine glycerol depending upon its applications, i.e., food or cosmetics or any other [50]. However, no supplementary processing is needed for feed applications. The glycerol from biodiesel production cannot be directly utilized for cosmetics and food applications owing to poor quality; thus, creating the need for an alternative source of glycerol. Since, the content of glycerol (76.34 ± 0.89%) in SEF (Table 1) was significant, it might play an important role in the extraction and usage of glycerol.
3.4.2 Alcohols
The content of alcohols was greater than 1.50%, and the peaks of major alcohols such as 2,3-butanediol (2,3-BDO) (1), benzene ethanol, 4-hydroxy (8), and erythritol (3) perceived with larger intensities, as shown in Fig. 4A. These were detected at retention times of 2.37–2.93, 12.20, and 10.13 min, respectively. Alcohols were products of heterotactic fermentation, which entails the application of fungi and bacteria in addition to yeasts [5].
The 2,3-BDO (34.65%) is a C4 diol potential platform chemical with huge applications in pharmaceutical and cosmetic industries [51], as a bacterial volatile and a constituent of fruit volatiles [52, 53]. The global projected market value of 2,3-BDO is around 43 billion dollars per annum [54]. Previously, 1,3-propanediol was utilized from glycerol through a bioprocessing technique using a bacterial strain isolated from TS [55]. The 2,3-BDO could be used to produce diacetyl and acetoin via dehydrogenation, two value-added food ingredients. Acetoin is utilized in the form of aroma carrier for flavors. Diacetyl is used in butter, cream, and cheese for improving their organoleptic quality. Diacetyl is the foremost identified flavoring constituent containing a pungent buttery aroma. The various sources exploited for 2,3-BDO are corn cob hydrolysate, crude glycerol, starch hydrolysate, and whey permeate. However, CCDS was never testified with the presence of 2,3-BDO; hence, SEF may be an appropriate new feedstock for extraction of this chemical.
The SEF contained a larger quantity of tyrosol (10.42%) in comparison with erythritol (1.52%), as listed in Table 4. Tyrosol was utilized as an antioxidant in defense, an agent to lessen hyperglycemia, and a neuroprotective constituent [56]. Besides, erythritol was examined for characteristics such as zero glycemic index and least calorific value in curing diabetics [57]. Thus, intensifying demands of alcohols need effective extraction procedures from SEF fractions.
3.4.3 Acids
Table 4 illustrates the selected acids of SEF that includes 9,12-octadecadienoic acid (Z, Z)- (C18:0) (15), n-hexadecanoic acid/palmitic acid (C16:0) (14), benzene acetic acid (6), p-coumaric acid, trans (12), 3-hydroxy-4-methoxybenzoic acid (10), and neodecanoic acid (2). The peaks of acids were visualized clearly with their molecular structures (Fig. 4). The 9,12-octadecadienoic acid (Z, Z)- (15), n-hexadecanoic acid (14), and benzene acetic acid (6) with values of peak area% greater than 2.0 instigated to be detected in SEF at a retention time of 15.21 min, 14.52 min, and 11.10 min, respectively. Similarly, p-coumaric acid (12), 3-hydroxy-4-methoxybenzoic acid (10), and neodecanoic acid (2) with peak area% in-between 1.0 and 2.0% were detected at retention times of.acid, and succinic acid were investigated with concentrations of 8.75 g/L, 10.82 g/L, and 5.71 g/L in CCDS from dry-grind milling [5]. In line with this, the acids of oxalic, succinic, lactic, and acetic with quantities 1% (± 0.3%), 1% (± 0.3%), 4% (± 0.1%), and 1% (± 0.04%) weight, respectively, were calculated in defatted CCDS from dry-grind process [29]. The acetate, lactate, propionate, and butyrate were produced from corn fiber during fermentation [58]. From these results, it is clear that absence of such acids in SEF discloses the novelty of SMT, which comprises the elimination of corn fiber before fermentation. The acids were produced by the bacteria and yeast used in fermentation [5]. Besides, the acids accumulated in CCDS may act as a preservative hindering bacterial growth. However, the acids might confine the potential usage of CCDS as an ingredient for feed, which necessitates either their reduction or removal. Moreover, the acids owing to pH variations may cause an inadequate fermentation, resulting in the formation of glycerol from dihydroxyacetone phosphate [5]. This causes the larger quantity of glycerol in CCDS from SMT.
3.4.4 Esters
Table 4 shows the selected esters of SEF, which includes pentanedioic acid, 3-(1,2-diphenylpropylidene)-, monomethyl ester, and linoleic acid ethyl ester. The pentanedioic acid, monomethyl ester (9) initiated to be identified in the fraction at a retention time of 11.92 min. Similarly, 9,12-octadecadienoic acid (Z, Z)- ethyl ester (16) appeared at 15.26 min. The presence of esters in SEF indicated that the fractions extracted with ethanol included longer chain methyl and ethyl esters.
3.4.5 Quantification of abundant chemicals
The actual quantities of 2,3-BDO and 9,12-octadecadienoic acid (Z, Z)- in SEF were computed using their standard curves (Fig. 3S (SI). The SEF was extracted with ethyl acetate in different concentrations of 0.1 g, 0.5 g, 1.0 g, and 5.0 g of SEF in 2.5 mL of ethyl acetate. The contents of 2,3-BDO and 9,12-octadecadienoic acid (Z, Z)- were found to be 6.05 ± 0.83 mg and 0.75 ± 0.83 mg per gram of SEF. Their contents suggest that these chemicals could be worth to extract from SEF as potential value-added products.
3.5 Micro characteristics affecting feed manufacturing
The characteristics such as microstructure, morphology, and crystallinity of materials play an important role especially during feed manufacturing; thereby, the electron photomicrographs were obtained and analyzed for CCDS, PF, and MF [59]. The dried CCDS exhibited a smooth surface with pores, as shown in Fig. 5a. The broken surface could be owing to corn processing during SMT. The spherical ionic salts interact intensely with glycerol and proteins. Some undamaged cell walls were present because germ and large portions of endosperm were tedious to degrade during bioethanol production [60] (Fig. 5d). Some micro-fractures were perceived, which could be due to the unfolding of proteins. The particles were degraded at the circumference and external surface, indicating the effect of two-step CS using water as a solvent, as presented in Fig. 5b. The particles of PF seemed to have an uneven morphology with a varying particle size dispersal owing to the grinding process. Few tiny empty pockets were noticed within the degenerated particles from where the starch molecules might have left during bioethanol production, as displayed in Fig. 5e [61]. The particles in MF were inconsistent, porous, rough, amorphous, and irregular, as shown in Fig. 5c and f. Some aggregates of mineral particles were noticed in Fig. 5f. The aggregation occurs during precipitation of minerals when aqueous ethanol was added in the organic fraction and forms hydrogen linkages [62]. The variations in particle shape and size can influence the densification efficacy of pellet formation for feed. Besides, the ingredients for animal diets were assessed with particle size as an important parameter [63]. Hence, fine grinding (200–300 μm) is needed to assure uniform feeds. However, average particle size of 600–800 μm is more frequently implemented in actual formulations.
The particles were more amorphous and porous, which suggests lower digestion problems might be linked with PF and MF, as discerned in Fig. 5e and f. The results ties well with previous studies, wherein the issues with digestion and effectiveness of feed enzymes for highly crystalline corn DDGS were described [64]. We speculate that low crystallinity in PF may be from cellulose and/or AAs that creates applications in the development of biodegradable products such as foams, films, and plastics.
4 Conclusion
Condensed corn distillers solubles (CCDS) is a co-product of selective milling technology, an increasingly adopted process by ethanol industries in recent years. There is a lack of detailed investigations on profiles and potentials of CCDS as a sustainable feedstock for value-added products. This study involving the investigation of chemical composition has provided new insights. The components of different fractions from CCDS concentrated to a larger extent by two-step separation especially proteins in protein fraction (PF). The yields of recovered PF, mineral fraction (MF) and SEF were 25.43%, 8.59%, and 65.09%. The PF hydrolysate had peptides VFVDHPLFLER, TGGLGDVLGGLPPAMAANGHR, NPESFLSSFSK, ILHTISVPGEFQFFFGPGGR, and FAFSDYPELNLPER with predicted potential bioactivities such as antioxidant characteristics and inhibition of ACE, DPP-III, and DPP-IV. The MF consisting of potassium and phosphorous can be a significant resource for agricultural and industrial applications. The prime constituents of SEF are glycerol and solvent extractable biochemicals with a significant presence of 2, 3-butanediol, and reveals the scope for further refining. The results are an important finding in the understanding of CCDS. Future research should further develop and confirm these initial findings by creating bio-products from potential fractions of this novel CCDS through separation and purification techniques.
Abbreviations
- AAs:
-
Amino acids
- AACC:
-
American association of cereal chemists
- AA:
-
Amino acid
- ACE:
-
Angiotensin-converting enzyme
- BDO:
-
Butanediol
- CCDS:
-
Condensed corn distillers solubles
- CS:
-
Centrifugal sedimentation
- DDGS:
-
Dried distillers’ grains with solubles
- DPP:
-
Dipeptidyl peptidase
- FST:
-
Fiber separation technology
- GC–MS:
-
Gas chromatography-mass spectroscopy
- GLB1:
-
Globulin 1
- GBSS-I:
-
Granule-bound starch synthase 1
- LC–MS:
-
Liquid chromatography-mass spectrometry
- MF:
-
Mineral fraction
- MS:
-
Mass spectrometry
- Mw :
-
Molecular weight
- NDF:
-
Neutral detergent fiber
- PF:
-
Protein fraction
- SMT:
-
Selective milling technology
- SEF:
-
Solvent-extracted fraction
- SEM:
-
Scanning electron microscopy
- SI:
-
Supporting information
- TS:
-
Thin stillage
- WD-XRF:
-
Wavelength dispersive x-ray fluorescence spectroscopy
References
Network SA-F (2016) Market utilization overview. https://gfo.ca/wp22content/uploads/2018/04/market-utilization-overview-web.pdf (accessed on Jan 21, 2020)
Kim EJ, Parsons CM, Srinivasan R, Singh V (2010) Nutritional composition, nitrogen-corrected true metabolizable energy, and amino acid digestibilities of new corn distillers dried grains with solubles produced by new fractionation processes. Poult Sci 89:44–51. https://doi.org/10.3382/ps.2009-00196
Shurson GC (2017) The role of biofuels coproducts in feeding the world sustainably. Annu Rev Anim Biosci 5:229–256. https://doi.org/10.1146/annurev-animal-022516-022907
Liu K, Barrows FT (2013) Methods to recover value-added coproducts from dry grind processing of grains into fuel ethanol. J Agric Food Chem 61:7325–7332. https://doi.org/10.1021/jf402226r
Wood C, Rosentrater KA, Muthukumarappan K, Gu Z (2013) Quantification of physical and chemical properties, and identification of potentially valuable components from fuel ethanol process streams. Cereal Chem 90:70–79. https://doi.org/10.1094/CCHEM-05-12-0051-R
Kingsly ARP, Ileleji KE, Clementson CL (2010) The effect of process variables during drying on the physical and chemical characteristics of corn dried distillers grains with solubles (DDGS) – Plant scale experiments. Bioresour Technol 101:193–199. https://doi.org/10.1016/j.biortech.2009.07.070
Milczarek RR, Liu K (2015) Drum drying performance of condensed distillers solubles and comparison to that of physically modified condensed distillers solubles. Food Bioprod Process 94:208–217. https://doi.org/10.1016/j.fbp.2014.01.004
Lardy G (2009) Feeding coproducts of the ethanol industry to beef cattle. 1242:1–8. http://library.ndsu.edu/repository/handle/10365/5350%5Cn. https://library.ndsu.edu/repository/bitstream/handle/10365/5350/as1242.pdf?sequence=1
Wang H, Wang T, Pometto AL III, Johnson LA (2009) A laboratory decanting procedure to simulate whole stillage separation in dry-grind corn ethanol process. J Am Oil Chem Soc 86:1241–1250. https://doi.org/10.1007/s11746-009-1451-y
Liu K (2017) Fractionation of condensed distillers solubles and compositional characterization of its co-products. J Am Oil Chem Soc 94:161–168. https://doi.org/10.1007/s11746-016-2923-5
Sinn T, Flegler A, Wolf A, Stübinger T, Witt W, Nirschl H, Gleiß M (2020) Investigation of centrifugal fractionation with time-dependent process parameters as a new approach contributing to the direct recycling of lithium-ion battery components. Metals 10:1617. https://doi.org/10.3390/met10121617
Lin YC, Wang TY, Lan CW, Tai CY (2010) Recovery of silicon powder from kerf loss slurry by centrifugation. Powder Technol 200:216–223. https://doi.org/10.1016/j.powtec.2010.02.028
Pecka-Kielb E, Zachwieja A, Mista D (2017) Use of corn dried distillers grains (DDGS) in feeding of ruminants. https://www.intechopen.com/books/frontiers-in-bioenergy-and-biofuels/use-of-corn-dried-distillers-grains-ddgs-in-feeding-of-ruminants
Majoni S, Wang T (2010) Characterization of Oil Precipitate and Oil Extracted from Condensed Corn Distillers Solubles. J Am Oil Chem Soc 87:205–213. https://doi.org/10.1007/s11746-009-1491-3
Majoni S, Wang T, Johnson LA (2011) Enzyme treatments to enhance oil recovery from condensed corn distillers solubles. J Am Oil Chem Soc 88:523–532. https://doi.org/10.1007/s11746-010-1693-8
Sharma S, Pradhan R, Manickavasagan A, Dutta A (2020) Application of analytical pyrolysis to gain insights into proteins of condensed corn distillers solubles from selective milling technology. Food Bioprod Process 124:354–368. https://doi.org/10.1016/j.fbp.2020.09.011
American Association of Cereal Chemists (AACC) (1999e) Approved methods of analysis (11th Ed.). Method 44–01.01. Calculation of percent moisture. Approved November 3, 1999. St. Paul: AACC International. Available at: https://doi.org/10.1094/AACCIntMethod-44-01.01
American Association of Cereal Chemists (AACC) (2004) Approved methods of analysis (11th Ed.). Method 46–30.01. Calculation of protein. St. Paul: AACC International. Available at: http://methods.aaccnet.org/summaries/46-30-01.aspx
Xiao B, Sun XF, Sun R (2001) Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym Degrad Stab 74:307–319
ASTM E1755–01, Standard test method for ash in biomass, Annual Book of ASTM Standards, Vol. 11.06, ASTM International, West Conshohocken. www.astm.org. https://doi.org/10.1520/E1755-01R15.2
Whyte LK (1946) The spectrophotometric determination of glycerol. Oil Soap 23:323–326. https://doi.org/10.1007/BF02637422
Morgan TJ, George A, Boulamanti AK et al (2015) Quantitative X-ray fluorescence analysis of biomass (switchgrass, corn stover, eucalyptus, beech, and pine wood) with a typical commercial multi-element method on a WD-XRF spectrometer. Energy Fuels 29:1669–1685
Boogers I, Plugge W, Stokkermans YQ, Duchateau ALL (2008) Ultra-performance liquid chromatographic analysis of amino acids in protein hydrolysates using an automated pre-column derivatisation method. J Chromatogr A 1189:406–409. https://doi.org/10.1016/j.chroma.2007.11.052
Jiang Y, Zhang M, Lin S, Cheng S (2018) Contribution of specific amino acid and secondary structure to the antioxidant property of corn gluten proteins. Food Res Int 105:836–844. https://doi.org/10.1016/j.foodres.2017.12.022
Sharma S, Pradhan R, Manickavasagan A, Thimmanagari M, Dutta A (2021) Evaluation of nitrogenous pyrolysates by Py–GC/MS for impacts of different proteolytic enzymes on corn distillers solubles. Food Bioprod Process 127:225–243. https://doi.org/10.1016/j.fbp.2021.03.004
Hao X, Yang W, Zhu Q, Zhang G, Zhang X, Liu L, Li X, Hussain M, Ni C, Jiang X (2021) Proteolysis and ACE-inhibitory peptide profile of cheddar cheese: effect of digestion treatment and different probiotics. LWT 145:111295
Aiello G, Lammi C, Boschin G, Zanoni C, Arnoldi A (2017) Exploration of potentially bioactive peptides generated from the enzymatic hydrolysis of hempseed proteins. J Agric Food Chem 65:10174–10184. https://doi.org/10.1021/acs.jafc.7b03590
Pinu FR, Villas-Boas SG (2017) Rapid quantification of major volatile metabolites in fermented food and beverages using gas chromatography-mass spectrometry. Metabolites 7:1–13. https://doi.org/10.3390/metabo7030037
Adom F, Fan J, Davis J, Dunn P, Shonnard D (2014) Compositional analysis of defatted syrup from a corn ethanol dry-grind process as a feedstock for biobased products. Sustain Chem Eng 2:1139–1146. https://doi.org/10.1021/sc400508p
Jackson DS, Shandera DL (1995) Corn wet milling: separation chemistry and technology. Adv Food Nutr Res 38:271–300. https://doi.org/10.1016/S1043-4526(08)60085-6
Ding J, Yoo CG, Pu Y, Meng X, Bhagia S, Yu C, Ragauskas AJ (2019) Cellulolytic enzyme-aided extraction of hemicellulose from switchgrass and its characteristics. Green Chem 21:3902–3910. https://doi.org/10.1039/c9gc00252a
Belyea RL, Clevenger TE, Singh V, Tumbleson ME, Rausch KD (2006) Element concentrations of dry-grind corn-processing streams. Appl Biochem Biotechnol 134:113–128. https://doi.org/10.1385/ABAB:134:2:113
Reis CER, Rajendran A, Hu B (2017) New technologies in value addition to the thin stillage from corn-to-ethanol process. Rev Environ Sci Biotechnol 16:175–206. https://doi.org/10.1007/s11157-017-9421-6
Bohn L, Meyer AS, Rasmussen SK (2008) Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J Zhejiang Univ Sci B 9:165–191. https://doi.org/10.1631/jzus.B0710640
Liu KS, Han J (2011) Changes in mineral concentrations and phosphorus profile during dry-grind processing of corn into ethanol. Bioresour Technol 102:3110–3118. https://doi.org/10.1016/j.biortech.2010.10.070
Ravindran V, Ravindran G, Sivalogan S (1994) Total and phytate phosphorus contents of various foods and feedstuffs of plant origin. Food Chem 50:133–136. https://doi.org/10.1016/0308-8146(94)90109-0
Kim Y, Mosier NS, Hendrickson R, Ezeji T, Blaschek H, Dien B, Cotta M, Dale B, Ladisch MR (2008) Composition of corn dry-grind ethanol by-products: DDGS, wet cake, and thin stillage. Bioresour Technol 99:5165–5176. https://doi.org/10.1016/j.biortech.2007.09.028
Jianchun H, Keshun L (2010) Changes in composition and amino acid profile during dry grind ethanol processing from corn and estimation of yeast contribution toward DDGS proteins. J Agric Food Chem 58:3430–3437. https://doi.org/10.1021/jf9034833
Wu YV, Sexson KR, Wall JS, Regional N (1981) Protein-rich residue from corn alcohol distillation: fractionation and characterization. Cereal Chem 58:343–347. https://pubag.nal.usda.gov/catalog/26089
Zhu B, He H, Hou T (2019) A Comprehensive review of corn protein-derived bioactive peptides: production, characterization, bioactivities, and transport pathways. Compr Rev Food Sci Food Saf 18:329–345. https://doi.org/10.1111/1541-4337.12411
Chen L, Ejima A, Gu R, Lu J, Cai M, Sato K (2019) Presence of exopeptidase-resistant and susceptible peptides in a bacterial protease digest of corn gluten. J Agric Food Chem 67:11948–11954. https://doi.org/10.1021/acs.jafc.9b04444
Bin ZT, He TP, Li HB, Tang HW, Xia EQ (2016) The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 21:1–14. https://doi.org/10.3390/molecules21010072
Khaket TP, Redhu D, Dhanda S, Singh J (2015) In silico evaluation of potential DPP-III inhibitor precursors from dietary proteins. Int J Food Prop 18:499–507. https://doi.org/10.1080/10942912.2013.787626
Hu R, Dunmire KM, Truelock CN, Paulk CB, Aldrich G, Li Y (2020) Antioxidant performances of corn gluten meal and DDGS protein hydrolysates in food, pet food, and feed systems. J Agric Food Res 2:100030. https://doi.org/10.1016/j.jafr.2020.100030
Cookman DJ, Glatz CE (2009) Extraction of protein from distiller’s grain. Bioresour Technol 100:2012–2017. https://doi.org/10.1016/j.biortech.2008.09.059
Walsh K, Wilcox P (1970) Serine Proteases. Methods Enzymol 19:31–41
Rodriguez J, Gupta N, Smith RD, Pevzner PA (2008) Does trypsin cut before proline? J Proteome Res 7:300–305. https://doi.org/10.1021/pr0705035
Singha P, Singh SK, Muthukumarappan K (2019) Textural and structural characterization of extrudates from apple pomace, defatted soy flour and corn grits. J Food Process Eng 42:1–11. https://doi.org/10.1111/jfpe.13046
Koutinas AA, Vlysidis A, Pleissner D, Kopsahelis N, Garcia IL, Kookos IK, Papanikolaou S, Kwan TH, Lin CS (2014) Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem Soc Rev 43:2587–2627. https://doi.org/10.1039/c3cs60293a
Pachauri N, He B (2006) Value-added utilization of crude glycerol from biodiesel production: a survey of current research activities written for presentation at the 2006 ASABE Annual International Meeting Sponsored by ASABE Glycerol Utilization for Specific Products. In: In Proceedings of the ASABE Annual International Meeting. pp 1–16
Zhang Y, Liu D, Chen Z (2017) Production of C2–C4 diols from renewable bioresources: new metabolic pathways and metabolic engineering strategies. Biotechnol Biofuels 10:1–20. https://doi.org/10.1186/s13068-017-0992-9
Sheldon RA (2005) Green solvents for sustainable organic synthesis: state of the art. Green Chem 7:267–278. https://doi.org/10.1039/b418069k
Jordon MJ, Margaria CA, Shaw PE, Goodner KL (2003) Volatile components and aroma active compounds in aqueous essence and fresh pink guava fruit puree ( Psidium guajava L.) by GC-MS and multidimensional GC/GC-O. J Agric Food Chem 51:1421–1426. https://doi.org/10.1021/jf020765l
Ko M, Mihalcea C, Liew F, Tizard JH, Ali MS, Conolly JJ, Al-Sinawi B, Simpson SD (2011) 2, 3-Butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl Environ Microbiol 77:5467–5475. https://doi.org/10.1128/AEM.00355-11
Khan NH, Kang TS, Grahame DAS (2013) Isolation and characterization of novel PM1 from bioethanol thin stillage. Appl Microbiol Biotechnol 97:417–428. https://doi.org/10.1007/s00253-012-4386-4
Larriba M, Omar S, Navarro P, García J, Rodríguez F, Gonzalez-Miquel M (2016) Recovery of tyrosol from aqueous streams using hydrophobic ionic liquids: a first step towards developing sustainable processes for olive mill wastewater (OMW) management. RSC Adv 6:18751–18762. https://doi.org/10.1039/c5ra26510j
Liu X, Yu X, Wang Z, Xia J, Yan Y, Hu L, Wang X, Xu J, He A, Zhao P (2019) Enhanced erythritol production by a Snf1-deficient Yarrowia lipolytica strain under nitrogen-enriched fermentation condition. Food Bioprod Process 119:306–316. https://doi.org/10.1016/j.fbp.2019.11.012
Mohtar SS, TengkuMalimBusu TNZ, Md Noor AM, Shaari N, Mat H (2017) An ionic liquid treatment and fractionation of cellulose, hemicellulose and lignin from oil palm empty fruit bunch. Carbohydr Polym 166:291–299. https://doi.org/10.1016/j.carbpol.2017.02.102
Imam SH, Gordon SH (2002) Biodegradation of coproducts from industrially processed corn in a compost environment. J Polym Environ 10:239–248. https://doi.org/10.1023/A:1021144104458
Khanal SK, Montalbo M, Van Leeuwen J, Srinivasan G, Grewell D (2007) Ultrasound enhanced glucose release from corn in ethanol plants. Biotechnol Bioeng 98:978–985. https://doi.org/10.1002/bit
Payling L, Woyengo TA, Nielsen M, Stein HH, Walsh MC, Romero L, Arent S (2019) Microscopy and protein solubilization of digesta from pigs fed wheat, corn, or soybean meal-based diets, with or without protease and a Bacillus spp. direct-fed microbial. Anim Feed Sci Technol 247:183–193. https://doi.org/10.1016/j.anifeedsci.2018.11.013
Bajwa HK, Santosh O, Koul A, Bisht MS, Nirmala C (2019) Quantitative determination of macroelement and microelement content of fresh and processed bamboo shoots by wavelength dispersive X-ray fluorescence spectrometry. X-Ray Spectrom 48:637–643. https://doi.org/10.1002/xrs.3048
Glencross BD, Booth M, Allan GL (2007) A feed is only as good as its ingredients – a review of ingredient evaluation strategies for aquaculture feeds. Aquac Nutr 13:17–34
Stein HH, Shurson GC (2009) Board-invited review: the use and application of distillers dried grains with solubles in swine diets. J Anim Sci 87:1292–1303. https://doi.org/10.2527/jas.2008-1290
Acknowledgements
This work was supported by Biofuel Net Canada; Ontario Ministry of Agriculture, Food and Rural Affairs, Guelph Ontario, IGPC Ethanol Inc., Aylmer, Ontario, Canada; Shrimp Canada, 67 Watson Rd. S (Unit—2), Guelph, Ontario, N1L 1 E3, Canada.
Author information
Authors and Affiliations
Contributions
Sonu Sharma: conceptualization, methodology, investigation, data curation, formal analysis, visualization, writing—original draft. Ranjan Pradhan: conceptualization, methodology, writing—review and editing, supervision. Annamalai Manickavasagan: writing—review & editing, supervision. Mahendra Thimmanagari: writing—review and editing. Animesh Dutta: writing—review & editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, S., Pradhan, R., Manickavasagan, A. et al. Valorization and potential of condensed corn distillers solubles fractions from selective milling technology. Biomass Conv. Bioref. 13, 5885–5901 (2023). https://doi.org/10.1007/s13399-021-01614-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01614-7