Abstract
In this study, grape pulp (MGP) modified with NaOH and citric acid was used in the production of natural ion exchangers. The effects of parameters such as initial pH, MGP dosage, temperature, initial metal ion concentration, and contact time on the removal of Pb (II) and Cd (II) ions from aqueous solutions using modified materials were investigated by batch experiments. It was found that the experimental kinetic data fit the second-order model, and the activation energy for Pb (II) and Cd (II) adsorption processes were 20.68 and 38.61 kj mol−1, respectively. Although the initial adsorption rate increases with increasing temperature, the adsorption efficiency slightly decreases. It was calculated that the equilibrium data fit the Langmuir isotherm better, and the maximum adsorption capacities for Pb (II) and Cd (II) adsorption processes were approximately 1.496 and 1.022 mmol g−1 at 25 °C, respectively. Thermodynamic analysis has shown that the adsorption processes of Pb (II) and Cd (II) are exothermic (ΔH°Pb = −35.68 kj mol−1, ΔH°Cd = −21.19 kj mol−1) and have a self-developing character.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The fact that the toxic effects of heavy metals are well understood, continues to be a serious pollution factor in wastewater, keeping the environmental interest in these substances alive and efforts to develop different techniques and removal materials from wastewater continue. Extensive research is being carried out on processes using ion exchange, membrane, reverse osmosis, solvent extraction, and electrolytic methods, especially adsorption method, which are investigated as an alternative to the chemical precipitation process widely used in removing heavy metal ions from wastewater streams. Important results of these studies are given in review articles evaluating many publications in this field [1,2,3,4,5]. It has been adopted by many researchers as a general evaluation that the adsorption method to be applied by evaluating the low-cost materials or the modified materials obtained by activating them can be a promising alternative to the chemical precipitation method used in practice [6,7,8,9,10]. As a result of this, the biosorption method, which takes advantage of the adsorption ability of materials obtained from biological materials (of microorganism or agricultural and animal origin), has been a method of much research in this field due to the continuity of the materials and the cheap output materials [11,12,13,14,15]. The walls of biomass cells, which are mainly composed of polysaccharides, proteins and lipids, contain carboxyl, hydroxyl, sulfate, phosphate, and amino acid functional groups, and these groups have the ability to bind metal ions [16]. It has been reported that the galacturonic acid groups contained in pectic substances in polysaccharides are effective in removing cationic contaminants [17,18,19,20], contributing to the ion exchange properties of polysaccharides in removing metal ions [21,22,23]. In general, it has been stated that lignocellulosic substances are interacted with some chemicals and their properties such as water retention and swelling are improved by cross-linking the cellulose fibers in their body, their cation holding/exchange capacity is increased and they can be used effectively in removing cationic contaminants [24, 25]. In one study, it was stated that soybean hulls modified with citric acid could be used as a cation exchange resin, the cost of such a resin was calculated as $1.17 kg−1 and this value was reported to be very low compared to commercial resins of petroleum origin [26].
In some previous studies, it has been revealed that sugar beet pulp can be used to remove some heavy metals and dyestuffs from the water environment by making some changes in its structure. It has been reported that as a result of the modification of sugar beet pulp with sodium hydroxide and citric acid, the cation exchange capacity of the pulp, which is a defect, is partially improved by esterification of the alcoholic hydroxyl groups due to the cellulosic components and attachment of the extra carboxyl groups to the skeleton [27,28,29,30]. This modified material has been investigated in the removal of cations and colored substances from the sugar fabrication intermediate aqueous juice [27, 29], as well as the removal of basic dyes from aqueous media [30]. It was found that lead removal ability of sugar beet pulp modified with citric acid was increased and lead adsorption capacity was 105–120 mg g−1 [27, 29].
In this study, the removal of Pb (II) and Cd (II) ions from aqueous solutions were investigated by using citric acid-modified grape pulp (MGP), which was prepared and determined in our previous studies [27, 29]. With the experiments performed for this purpose, the effects of parameters such as solution pH, MGP dosage, temperature, and initial metal ion concentration on metal ion removal were investigated depending on the contact time. The data obtained were applied to Freundlich’s, Langmuir’s, and Dubinin–Radushkevich’s isotherm equations. Kinetic data were evaluated by pseudo first-order model (Lagergren’s model), second-order model (Ho model) and intraparticle diffusion model. Also, thermodynamic parameters for the adsorption process of Pb (II) and Cd (II) ions by MGP were calculated.
2 Materials and methods
2.1 Modified grape pulp (MGP) used in experiments
NaOH and Citric acid modified grape pulp (MGP) used as adsorbent was prepared and characterized according to the scheme in Fig. 1 in our previous studies [29]. The properties of the adsorbent determined in the related studies [29] are shown in Table 1.
2.2 Preparation of Pb (II) and Cd (II) solutions
By using Pb(CH3COO)2.3H2O (Sigma-Aldrich, %99.99) and CdCl2.XH2O (Sigma-Aldrich, %99.99) salts, 250 mmol L−1 stock Pb (II) and Cd (II) solutions were prepared in distilled water. The working solutions were prepared by taking the appropriate amount from the stock solution and diluting it with distilled water or buffer solution at pH 4.8 (0.07 mol l−1 CH3COONa–0.03 mol L−1 CH3COOH solution) depending on the situation to be used.
2.3 Metal removal experiments
The adsorption experiments performed in the batch system were carried out by adding MGP to the solutions containing 200-mL metal ions in 300-mL flasks and shaking them at 200 rpm for the prescribed time in a shaker (Zcheng 200D) kept at a constant temperature. By using Double Rings-203 filter paper, pH measurements of the solutions separated from the solid by filtration and metal ion analysis after necessary dilution were performed. Studies at different pHs were carried out after adjusting the pH of metal ion solutions at the prescribed concentration to the predicted values with dilute HCl and NaOH solutions. Experiments in pH 4.8 medium were carried out in 0.07 mol L−1 CH3COONa–0.03 mol L−1 CH3COOH solution where this pH was maintained.
The adsorbed metal ion amounts and adsorption efficiency at any time and at equilibrium were calculated using the following equations. In these equations; qt is the amount of metal ions removed (mg g−1) per unit mass of adsorbent at any time t; qe is the amount of metal ions removed per unit mass of adsorbent in its equilibrium (mg g−1); C0 is the initial metal ion concentration (mg L−1); Cs is the final metal ion concentration (mg L−1) at any time t; Ce is the metal ion concentration (mg L−1) at equilibrium; V is the solution volume (L); m indicates the mass of adsorbent used (g) and % Ads., the percentage of metal ions adsorbed by MGP.
2.4 Analytical methods
Solution pH was measured using a Metler Delta 350 pH meter. Lead and cadmium analysis in filtrates Perkin Elmer AAnalyst-400 Atomic Absorption was performed using Spectrophotometer. Calibration was performed with standards prepared at concentrations between 5 and 20 mg L−1 for lead and 0.4 and 2 mg L−1 for cadmium. The concentrations of the samples were determined by comparing them against these calibration systems after the absorbance was determined by making appropriate dilutions when necessary.
3 Results and discussion
As can be seen from the values in Table 1, by modifying the grape pulp, the swelling capacity decreases. Since this feature is related to water absorption in a way, it is in harmony with the decrease in water holding capacity. On the other hand, in the tests of balancing the materials with water, it was determined that the amount of dissolution in water and acid solution decreased in modified products compared to raw grape pulp [24, 26, 27, 29]. This is confirmed in COD (chemical oxygen demand) tests in water balanced with the material. In summary, it can be said that the dissolution of the modified material in water and acid is also reduced and stabilized. With saponification and esterification applied in the processes, the growth of cellulose molecules by cross-linking and the dissolution of small molecule substances in the subsequent washes and the stabilization of the esterified products compared to the raw grape pulp shows that these materials can be used more advantageously in color and ion removal from aqueous media [26, 27, 29].
The most important good property observed in the modified material is the increase in cation exchange capacity. The cation exchange capacity of the ion exchanger obtained especially after the modification increases approximately 3 times the value of the raw grape pulp. The more effective methylene blue binding values obtained with modified materials as a result of the standard methylene blue removal test performed in buffer solution with 500 mg L−1 methylene blue solution are also in agreement with this result [24, 27, 29].
This property of lignocellulosic residues is caused by negatively charged active points in the structure. Especially acidic functional groups (such as carboxylic acid and carboxylic acid ester), alcoholic functional groups in free and esterified form, and places such as oxygen bridges formed by carbonyl groups constitute active centers for the retention of cations. In some studies, it has been determined that the cation exchange capacity of cellulosic substances saponified with alkaline solution increases [18, 24, 26, 27, 29]. With the interaction of grape pulp with sodium hydroxide solution, the ester groups in the pectin structure may transform into carboxyl-sodium form by hydrolysis, and possible primary alcohol groups esterified in the cellulose structure may be liberated by hydrolysis (Eq. 3). In the subsequent interaction with the applied citric acid, the primary alcohol groups in the cellulose and citric acid can esterify according to the mechanism represented by the following reaction equation, causing the number of active groups in the molecule to increase (Eq. 4). Other carboxylic acid groups of citric acid can also react with neighboring cellulose molecules and cross-link the fibrils, causing the molecules to grow and thus the material to become more stabilized. The decrease in solubility and decrease in water holding capacity show this [18, 27, 29].
FTIR spectra of grape pulp and the modified product obtained from it are given in Fig. 2. It is obvious that the grape pulp skeleton contains cellulose from the characteristic peak in the fingerprint area of 1000–1200 cm−1. The peak characteristic for free and esterified carboxyl at 1650–1750 cm−1 may also be characteristic for the presence of pectin. The 3200−3600 cm−1 region is determinant for hydroxyl groups in macromolecules (cellulose pectin etc.) or in water. The more pronounced peaks at 1750 cm−1 in the spectra of the MGP sample, which is a product of citric acid esterification, may be the result of the carboxyl groups that have entered the structure extra [7, 25, 28].
The experiments in the first stage were carried out to examine the effect of the change of solution pH using a certain metal ion concentration (5 mM) and a certain adsorbent dosage (10 g 1−1). For lead and cadmium ions in aqueous solutions, as the pH rises, lead and cadmium may precipitate as metal hydroxo complexes after about pH 6.5 and pH 7.5, respectively, as a result of hydrolysis events [28, 30,31,32,33]. It can be stated that it is below. Therefore, experiments investigating the effect of pH were carried out in pH <5 environment. The results of the experiments investigating the effect of pH are given in Table 2. As can be seen from the results, the pH of the solution in contact with the adsorbent slightly decreases compared to the initial value. Looking at the hypothetical nature of the modified material (Eq. 3), it can be stated that the carboxyl groups cause this. According to the results in Table 2, the increase in pH causes the elimination to increase and the removal efficiency reaches higher than 97% around pH 5. According to these results; subsequent experiments were carried out at pH = 4.8 in buffer solution (0.07 mol L−1 CH3COONa–0.03 mol L−1 CH3COOH solution) was carried out. The effect of the MGP dosage on the removal of Pb (II) and Cd (II) from the solution with 5 mM concentration is given in Fig. 3. As can be seen from the figure and as expected, as the MGP dosage is increased, the percentage of Pb (II) and Cd (II) removal from the solution increases, but the amount of adsorbed per unit mass of adsorbent decreases. For example, when using the MGP at a dosage of 0.5 g l−1, after 24 h a removal of about 18% and 30% of the solution is obtained for lead (II) and cadmium (II), respectively, while increasing the dosage to 5 g L−1, the removal is about 83% and 88%, and by increasing the dosage to 10 g L−1, the removal is around 98% and 97%. Considering these results, in this study designed for high metal ion initial concentrations, the MGP dosage was chosen as 10 g l−1, and later experiments were carried out by applying this adsorbent dosage. Experiments were carried out with solutions containing Pb(II) and Cd (II) ions at initial concentrations of 5–25 mmol L−1 at temperatures of 25, 40, and 55 °C and for periods ranging from 5 to 1440 min. The effects of concentration, temperature and time on the removal of Pb(II) and Cd (II) ions from solution were examined (Fig. 4).


If these results are examined in general regardless of kinetic models, it can be said that the percentage of adsorption increases for a certain period of time, and then a practical increase is not observed in the adsorption percentage. It can be stated that when the initial metal ion concentration and temperature are increased, the percentage of adsorption decreases. As it can be understood from the examination of Fig. 4, increasing the temperature shortens the time to equilibrium, but causes a decrease in the adsorption percentage. As in previous studies on this subject, the compatibility of the results obtained in these experiments with some kinetic models frequently applied in adsorption processes was investigated. The three main models among these models are first-order model (Lagergren’s model), second-order model (Ho model) and intraparticle diffusion model (WeberMorris’s model) [34,35,36].
In an adsorption process that occurs in solution, the necessary boundary conditions for first-order and second-order velocity equations are written and integrated. These equations are written as follows respectively. Here, qe (mmol l−1g−1) indicates the amount of metal ions removed per unit mass of the adsorbent at equilibrium, and qt (mmol l−1g−1) indicates the amount of metal ions removed per unit mass of the adsorbent at time t. k1 (min−1) and k2 (g mmol l−1min−1) are the first- and second-order adsorption rate constants, respectively.
One of the mechanisms that can be valid in adsorption processes is the intraparticle diffusion model (Eq. 6). According to this model,
qt (mmol l−1 g−1) also gives information about the amount of metal ions removed per unit mass of the adsorbent at time t, which is the intraparticle diffusion rate constant (mmol l-1 g-1 min-1/2), and I gives information about the thickness of the layer formed between the adsorbent and the adsorbed substance.
The adsorption data obtained depending on the time at different temperatures were applied to the first and second order and intraparticle diffusion models (Eq. 5, Eq. 6, and Eq. 7). In the adsorption of lead (II) and cadmium (II) ions by MGP, it has been found that both the regression coefficients are closer to 1 and the experimental results are closer to the theoretical results calculated. It can be stated that the test results fit the second-order model better in the applied conditions (Fig. 5) (Table 3).
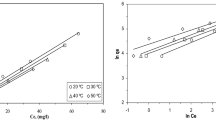
Activation energies were calculated for the initial concentrations of 5–25 mM lead (II) and cadmium (II), as the data conformed to the second-order model. The activation energies were found from the slope of the linear graphs obtained between the logarithms of the velocity constants found by applying the data obtained from the experiments performed at various temperatures to this model and the temperature (T−1) (Fig. 6). It can be obtained from the graph drawn by using the results found in the experiments with 10 mM lead (II) and cadmium (II) solutions.
The activation energies calculated by applying the data obtained to Arrhenius equation (Eq. 8) were found to be 20.68 and 38.61 kj mol−1 for the lead (II) and cadmium (II) adsorption processes, respectively. These values are in agreement with the values of 24–40 kj mol–1 given in the literature for the mechanisms realized by ion exchange [37].
Data were obtained in experiments at various temperatures for initial concentrations of 5–25 mM lead (II) and cadmium (II), as the data conformed to the second-order model. The activation energies were found from the slope of the linear graphs obtained between T–1 and the logarithms of the velocity constants found by applying to this model (Fig. 6). Data were obtained from experiments with 100 mM lead (II) and cadmium (II) solutions. The activation energies calculated by applying the Arrhenius equation (Eq. 8) from the graph drawn using these data were found as 20.68 and 38.61 kj mol−1 for the lead (II) and cadmium (II) adsorption processes, respectively. These values are in agreement with the values of 24–40 kj mol−1 given in the literature for the mechanisms realized by ion exchange [37].
On the other hand, the equilibrium data generally obtained in an adsorption process are applied to different isotherm equations to obtain useful information for the adsorption process. The number of these equations is quite high. The data obtained from adsorption studies are often evaluated according to Freundlich’s, Langmuir’s, and Dubinin–Radushkevich’s (D–R) adsorption isotherms [38,39,40]. Linearized versions of these equations are given below.
In this equation (Freundlich’s equation) [38], x is the amount of substance adsorbed (mM); m is the amount of adsorbent (g); and Ce indicates the equilibrium concentration (mM) of the adsorbed substance in solution. Kf (mM g−1) is Freundlich's constant, a measure of the adsorption capacity. The other Freundlich’s constant n is dimensionless. Another equation to which adsorption data is applied is the Langmuir isotherm [39].
where Ce is the equilibrium concentration of the adsorbed in solution (mmol 1−l); qe is the adsorption capacity at equilibrium (mg g−1), qmax is the maximum adsorption capacity (mg g−1), b is a constant related to the adsorption energy (l mmol−1). One model in which useful evaluations of the adsorption mechanism are made is the Dubinin-Radushkev (D-R) adsorption isotherm [40].
qm is the maximum amount of substance adsorbed per unit adsorbent (mg g−1), a constant for the adsorption energy (mol2 kJ−2), Ɛ is the Polanyi potential.
Evaluations of the nature of adsorption can be made in the calculation using Eq. 12. The E value in this equation is a measure of the energy value required to transfer one mole of an infinitely distant particle in solution to the adsorbent surface. By looking at these values, a judgment can be made about the reaction mechanism. If the detected E value is greater than 16 kj mol−1, the adsorption is more chemical in nature; If it is between 8 and 16 kj mol−1 values, it is stated that the process takes place by ion exchange, if it is less than 8 kj mol−1, the adsorption process is of a physical nature [27, 41,42,43].
First of all, nonlinear isotherm plot was obtained by using the data obtained from the balancing studies for lead (II) and cadmium (II) solutions at different initial metal concentrations and different temperatures (Fig. 7). From the examination of these graphs, it can be stated that both metal ion adsorption systems reach saturation and it can be stated that it is an L-type isotherm in its isotherm form. In this context, the results obtained by applying the experimental data obtained in the range of 5–25 mM metal ion initial concentrations and 720 min equilibration time to the isotherm equations given above are given in Table 4.
The results of the application of equilibrium data to Freundlich’s, Langmuir’s, and D–R’s isotherms show that the Langmuir isotherm is more suitable for both systems of adsorption of lead (II) and cadmium (II) ions by MGP under operating conditions (Fig. 8). The E values found in D–R’s isotherm application and between 10.8 and 15.4 kj mol−1 values (Table 4) show that ion exchange is an effective mechanism in the removal process. The Langmuir constant obtained from the data within the applied operating conditions was calculated by converting it from qmax values to mg g−1 for easy understanding. The adsorption capacities of MGP for lead (II) and cadmium (II) ions were found between 310 and 305 and 115 and 111 mg g−1 for temperatures of 25–55 °C, respectively. These values are compared in Table 5 with the literature values of lead (II) and cadmium (II) removal studies that have been done using various materials. When compared with the results of these studies, which have been carried out under different conditions using different materials, it can be stated that grape pulp (MGP) modified with citric acid is a good adsorbent for lead (II) and cadmium (II) ions.
In adsorption processes that conform to the Langmuir isotherm, the isotherm shape can also be evaluated according to a dimensionless r separation factor [52].
r is a dimensionless separation factor, C0 is the initial concentration of adsorbed (mM), b is the Langmuir constant. The following evaluation is made according to the size of the value obtained when the r factor is calculated. r> 1 adsorption process unfavorable, r = 1 adsorption process linear, 0 <r <1 adsorption process favorable, r = 0 the adsorption process is irreversible.
The r values calculated gradually from the Langmuir constant obtained using the data obtained in this study are shown in Table 6. Between 25 and 55 °C temperatures, r constant values were found to be between 0.72 and 0.98 and 0.53 and 0.92 for lead (II) and cadmium (II), respectively. Accordingly, using MGP, it can be stated that the adsorption processes of lead (II) and cadmium (II) from aqueous solutions are convenient.
In addition, by using the Langmuir constants, the adsorbent dosage required to reach a target solution equilibrium concentration can theoretically be calculated according to the following equation [53].
m/V required adsorbent dosage (g l−1); C0 initial concentration of adsorbed (mM); Ce desired equilibrium to reach adsorbed (mM); and qmax, Langmuir’s constant, maximum adsorption capacity (mg g−1), the Langmuir constant (l mg−1) related to the adsorption energy of b.
The MGP dosage values calculated from Eq. 14 and corresponding to 99% removal are shown in Table 7. Accordingly, for example, 100 mg l−1 concentration of lead (II) and cadmium (II) ions at 25°C while a dosage of approximately 27.6 and 34 g 1−1 MGP was needed to provide 99% removal from the solutions, the dosage that can remove from the solution at the same concentration at 55 °C with the same efficiency was calculated as approximately 86.2 and 64 g 1−1.
This supports the conclusion that one of the initial evaluations, temperature has a negative effect on the removal of lead (II) and cadmium (II). Meanwhile, the calculated m/V for 99% elimination can be observed with experiments and a comparison can be made. Some experiments were made for 5–25 mM concentrations at 25 °C by using (dosage, g l−1) values, and the removal efficiencies found in general were determined to be slightly lower than the theoretically found values. For example, the removal efficiency at 25 °C was found to be approximately 98% at the end of the experiment performed with a dosage of 24.2 g 1−1 MGP where a 99 % lead (II) removal was calculated from a 50 mg l−1 solution.
In adsorption studies performed in aqueous solutions, thermodynamic parameters can also be calculated using the Langmuir constant (Fig. 9). Thermodynamic parameters such as free energy (ΔG°), enthalpy (ΔH°) and entropy (ΔS°) change of the adsorption process can be found using the equations given below.
For the adsorption balance b in these equations, the Langmuir constant (l mg−1), b’is a constant, T is the absolute temperature (K), and R is the universal gas constant (8.314 J mol−1K−1). ΔH° can be calculated from the slope of the line in the linear graph to be drawn between lnb and T−1.
The values for the thermodynamic parameters calculated from Eqs. 15, 16, and 17 based on the b values calculated from the Langmuir isotherm are shown in Table 8. According to these, the negative enthalpy change values of the lead (II) and cadmium (II) adsorption processes indicate that the adsorption process of these metal ions from aqueous solutions of the modified grape pulp is exothermic. This confirms preliminary assessments that remediation decreases with temperature. On the other hand, the negative free energy change implies that these adsorption processes will occur spontaneously. This result confirms the earlier finding that the process is feasible by calculating the dimensionless constant r. In addition, it can be stated that the positive entropy change at small values can be caused by the increase of irregularity in the solution adsorbent interface and the changes that may occur on the adsorbent surface during adsorption [54].
4 Conclusion
It has been demonstrated that modified grape pulp (MGP), which has increased cation retention capability by attaching extra carboxyl groups to the structure by interaction with citric acid, can be used effectively in removing metal ions from aqueous solutions containing lead (II) and cadmium (II) in a high concentration range. The kinetic data obtained from the experiments to remove lead (II) and cadmium (II) ions with MGP are based on the second-order kinetic model; equilibrium data fit the Langmuir isotherm model. The thermodynamic and temperature-related findings show that the adsorption processes of both metal ions by MGP are exothermic and are self-running processes. As a result, it can be stated that MGP can effectively remove lead (II) and cadmium (II) ions from aqueous environments and has an adsorption mechanism that has the effect of ion exchange, and these metals can be recovered with such a process. The results obtained may constitute data for the design studies to be done in determining the operating conditions for the applications. In addition, further studies on the recovery of precious metals can be carried out using effective modified adsorbents made from agricultural waste materials of this type.
References
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418 2011
Sheoran AS, Sheoran V (2006) Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Miner Eng 19:105–116 2006
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97:219–243
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33:2469–2479
Renge VC, Khedkar SV, Pande SV (2012) Removal of heavy metals from wastewater using low cost adsorbents: a review. Sci Revs Chem Commun 2:580–584
Arslanoğlu H, Tümen F (2021) Potassium struvite (slow release fertilizer) and activated carbon production: resource recovery from vinasse and grape marc organic waste using thermal processing. Process Saf Environ 147(March):1077–1087
Arslanoğlu H (2019) Direct and facile synthesis of highly porous low cost carbon from potassium-rich wine stone and their application for high-performance removal. J Hazard Mater 374:238–247
Arslanoğlu H (2021) Production of low-cost asorbent with small particle size from calcium carbonate rich residue carbonatation cake and their high performance phosphate adsorption applications. J Mater Res Technol 11(March-April):428–447
Adelaja OA, Amoo IA, Aderibigbe AD (2011) Biosorption of Lead (II) ions from aqueous solution using Moringa oleifera pods. Arch Appl Sci Res 3:50–60
Abas SNA, Ismail MHS, Kamal ML, Izhar S (2013) Adsorption process of heavy metals by low-cost adsorbent: a review. World Appl Sci J 28:1518–1530
Arslanoglu H (2017) Removal of Cu (II) from aqueous solutions by using marble waste. Pamukkale Univ J Eng Sci 23(7):877–886
Yaraş A, Arslanoğlu H (2019) Utilization of paper mill sludge for removal of cationic textile dyes from aqueous solutions. Sep Sci Technol 54(16):2555–2566
Yaraş A, Arslanoğlu H (2018) Valorization of paper mill sludge as adsorbent in adsorption process of copper (II) ion from synthetic solution: kinetic, isotherm and thermodynamic studies. Arab J Sci Eng 43(5):2393–2402
Farooq U, Kozinski JA, Khan MA, Athar M (2010) Biosorption of heavy metal ions using wheat based biosorbents—a review of the recent literature. Bioresour Technol 101:5043–5053
Febrianto J, Kosasih AN, Sunarso J, Ju YH, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162:616–645
Göksungur Y, Üren S, Güvenç U (2005) Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour Technol 96:103–109
Aksu Z, Isoglu A (2005) Removal of copper (II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochem 40:3031–3044
Dronnet VM, Renard CMGC, Axelos MAV, Thibault J-F (1997) Binding of divalent metalcations by sugar-beet pulp. Carbohydr Polym 34:73–82
Gerente C, Couespel du Mesnil P, Andres Y, Thibault J-F, Le Cloirec P (2000) Removal of metal ions from aqueous solution on low cost natural polysaccharides: sorption mechanism approach. React Funct Polym 46:135–144
Kartel MT, Kupchik LA, Veisov BK (1999) Evaluation of pectin binding of heavy metal ions in aqueous solutions. Chemosphere 38:2591–2596
Sag Y, Kutsal T (1996) The selective biosorption of chromium(VI) and copper(II) ions from binary metal mixtures by R. Arrhizus. Process Biochem 31:561–572
Veglio F, Beolchini F, Gasbarro A (1996) Biosorption of toxic metals an equilibrium study using free cells of Arthrobacter sp. Process Biochem 32:99–105
Zouboulis AI, Rousou EG, Matis KA, Hancock IC (1999) Removal of toxic metals from aqueous mixtures. Part 1: biosorption. J Chem Technol Biotechnol 74:429–436
Dronnet VM, Axelos MAV, Renard CMGC, Thibault J-F (1998) Improvoment of the binding capacity of metal cations by sugar-beet pulp. Part 1. Impact of cross-linking treatments on composition, hydration and binding properties. Carbohydr Polym 35:29–37
Eren MŞ, Arslanoğlu H, Çiftçi H (2020) Production of microporous Cu-doped BTC (Cu-BTC) metal-organic framework composite materials, superior adsorbents for the removal of methylene blue (Basic Blue 9). J Environ Chem Eng 8(5):104247
Marshall WE, Chatters AZ, Wartelle LH, McAloon A (2001) Optimization and estimated production cost of a citricacid-modified soybean hull ion exchanger. Ind Crop Prod 14:191–199
Altundogan HS, Arslan NE, Tumen F (2007) Copper removal from aqueous solutions by sugar beet pulp treated by NaOH and citric acid. J Hazard Mater 149:432–439
Arslanoglu H (2019) Adsorption of micronutrient metal ion onto struvite to prepare slow release multielement fertilizer: copper (II) doped-struvite. Chemosphere 217:393–401
Arslanoglu H, Tumen F (2012) A study on cations and color removal from thin sugar juice by modified sugar beet pulp. J Food Sci Technol 49:319–327
Arslanoğlu H, Orhan R, Turan MD (2019) Application of response surface methodology for the optimization of copper removal from aqueous solution by activated carbon prepared using waste polyurethane. Anal Lett 53(9):1343–1365
Sartape AS, Mandhare AM, Salvi PP, Pawar DK, Kolekar SS (2013) Kinetic and equilibrium studies of the adsorption of Cd(II) from aqueoussolutions by wood apple shell activated carbon. Desalin Water Treat 51:4638–4650
Dixit S, Singh DP (2013) Phycoremediation of lead and cadmium by employing Nostoc muscorum as biosorbent and optimization of its biosorption potential. Int J Phytoremediat 15:801–813
Khani MH (2013) Dynamics and thermodynamics studies on the lead and cadmium removal from aqueous solutions by Padina sp. Algae: studies in single and binary metal systems. Sep Sci Technol 48:2688–2699
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. K Sven Vetenskapsakad Handl 24:1–39
McKay G, Ho YS (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div, ASCE 89:31–60
Inglezakis VJ, Zorpas AA (2012) Heat of adsorption, adsorption energy and activation energy in adsorption and ion exchange systems. Desalin Water Treat 39:149–157
Freundlich H (1907) Ueber die Adsorption in Loesungen. Z Phys Chem 57:385–470
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Dubinin MM, Radushkevich LV (1947) Equation of the characteristic curve of activated charcoal. Proc Acad Sci Phys Chem Sect, USSR 55:331–333
Mahramanlioglu M, Kizilcikli I, Bicer IO (2002) Adsorption of fluoride from aqueous solution by acid treated spent bleaching earth. J Fluor Chem 115:41–47
El-Kamash AM, Zaki AA, El Geleel MA (2005) Modeling batch kinetics and thermodynamics of zinc and cadmium ions removal from waste solutions using synthetic zeolite A. J Hazard Mater 127:211–220
Arslanoglu H, Altundogan HS, Tumen F (2009) Heavy metals binding properties of esterified lemon. J Hazard Mater 164:1406–1413
Nagy B, Manzatu C, Aneanu AM, Indolean C, Silaghi-Dumitrescu L, Majdik C (2014) Effect of alkaline and oxidative treatment on sawdust capacity to remove Cd(II) from aqueous solutions: FTIR and AFM study. J Wood Chem Technol 34:301–311
Azouaou N, Sadaoui Z, Djaafri A, Mokaddem H (2010) Adsorption of cadmium from aqueou solutiononto untreated coffee grounds: equilibrium, kinetics and thermodynamics. J Hazard Mater 184:126–134
Liang S, Guo X, Feng N, Tian Q (2009) Adsorption of Cu(II) and Cd(II) fromaqueous solution by mercapto-acetic acid modified orange peel. Colloids Surf A Physicochem Eng Asp B73:10–14
Kelly-Vargas K, Cerro-Lopez M, Reyna-Tellez S, Bandala ER, Sanchez-Salas JL (2012) Biosorption of heavy metals in polluted water, using different waste fruit cortex. Phys Chem Earth 37–39:26–29
Senthil Kumar P, Ramalingam S, Abhinaya RV, Kirupha SD, Murugesan A, Sivanesan S (2012) Adsorption of metal ions onto the chemically modified agricultural waste. Clean-Soil Air Water 40:188–197
Ofomaja AE, Naidoo EB, Modise SJ (2010) Biosorption of Cu(II) and Pb(II) onto potassium hydroxide treated pine cone powder. J Environ Manag 91:1674–1685
Liu C, Ngo HH, Guo WS (2012) Water melon rind: agro-waste or superior biosorbent. Appl Biochem Biotechnol 167:1699–1715
Saka C, Sahin O, Demir H, Kahyaoglu M (2011) Removal of lead from aqueoussolutions using preboiled and formaldehyde treated onion skins as a newadsorbent. Sep Sci Technol 46:507–517
Ho YS, Wang CC (2004) Pseudo-isotherms for the sorption of cadmium ion onto tree fern. Process Biochem 39:761–765
Arslanoğlu H, Kaya S, Tümen F (2019) Cr (VI) adsorption on low-cost activated carbon developed from grape marc-vinasse mixture. Part Sci Technol 38(6):768–781
Feng N, Guo X, Liang S, Zhu Y, Liu J (2011) Biosorption of heavy metals fromaqueous solutions by chemically modified orange peel. J Hazard Mater 185:49–54
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Ion exchange resins were tested under typical and challenging conditions.
• Performance of resins was better than conventional resins.
• It has been ensured that Pb and Cd are effectively removed from waste water.
• pH 4.8 high Pb and Cd adsorption was obtained with the modified ion exchanger.
• Ion exchange resins can be reused to reduce process cost and byproduct production.
Rights and permissions
About this article
Cite this article
Arslanoğlu, E., Eren, M.Ş.A., Arslanoğlu, H. et al. Modification of grape pulp with citric acid for the production of natural ion exchanger resin and removal of Pb (II) and Cd (II) from aqueous solutions: kinetic, thermodynamics, and mechanism. Biomass Conv. Bioref. 13, 2349–2362 (2023). https://doi.org/10.1007/s13399-021-01521-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01521-x