Abstract
The exploration of domestically available clean and renewable fuel is essential for the attainment of sustainable development goals. The hydrogen (H2)-enriched biogas being renewable and clean can be a highly favorable fuel. In this study, the response surface optimization of dry oxidative reforming using a three-level, three-factor experimental design for hydrogen enrichment of biogas over the nickel-cobalt bimetallic catalyst was investigated. The Box-Behnken design of experimentation was employed to assess the interaction and discrete effect of reforming temperature and ratios of CH4/CO2 and O2/CH4. The effects of CH4/CO2 ratio (1–2) and O2/CH4 ratio (0.3–0.5) on catalytic activity were assessed in the temperature range of 700–800 °C. The conversion of both reactants (CH4 and CO2), yield of products (H2 and CO), and ratio of products (H2/CO ratio) were selected as responses for statistical study. The analysis of variance demonstrated that reforming temperature and O2/CH4 ratio have a statistically considerable impact on the H2 enrichment of biogas by the virtue of higher endothermic nature of the reaction. Experimentally, the maximum H2 enrichment of 44.04% was obtained at 800 °C with 1.5 and 0.5 CH4/CO2 and O2/CH4 ratio, respectively. However, from the statistical model, the optimum H2 enrichment of 36.9% was obtained at 725.68 °C with CH4/CO2 and O2/CH4 ratios of 1.32 and 0.42, respectively. The close agreement between predicted and experimental data shows that the combination of response surface methodology and dry oxidative reforming could be an efficient approach for optimizing the H2 enrichment of biogas and the generation of environment friendly fuel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The non-renewable nature of fossil fuels coupled with harmful gaseous emissions has created adverse impacts on the economies and environment [1]. The locally available sustainable and renewable energy sources could be very helpful in overcoming this issue. Biogas, being a renewable and cleaner source of energy, could be highly beneficial for mitigating the issues related to alternative fuels [2]. It is essentially a blend of methane (CH4) and carbon dioxide (CO2) containing trails of various gases like NH3 (ammonia) and H2S (hydrogen sulfide) and could be produced through a variety of locally available feedstock such as municipal solid waste (MSW), cow dung, and pig manure [3]. Its energy content mainly depends on CH4 content, which makes it an interesting candidate for utilization in internal combustion engines. However, the presence of inert CO2 acts as a coolant in the combustion chamber that reduces the efficiency of the engine [4]. Therefore, hydrogen (H2) as an alternative renewable, sustainable, and cleaner fuel has gained significant attention in recent times. However, the utilization of pure H2 in the internal combustion engines is retarded by the storage issue. In respect of this issue, hydrogen-enriched natural gas and biogas both could be used in the existing technologies [5]. The renewable nature and domestic availability of biogas support its utilization in transportation applications.
Hydrogen-enriched biogas can be efficiently produced through dry reforming reaction as it involves both methane (CH4) and carbon dioxide (CO2) [6,7,8]. However, issues related to economics and catalyst deactivation hinder its industrial applicability. To counter these hurdles, the combination of dry (Eq. 1) and partial oxidative reforming (Eq. 2) (dry oxidative reforming) could be very interesting as it will use the advantages of partial oxidative reforming to overcome the limitations of catalyst deactivation and higher temperature requirements [9,10,11]. Various researchers have shown the impact of oxygen (O2) addition to dry reforming of methane or biogas reforming. The addition of O2 results in the enhanced conversion of CH4 coupled with a higher yield of H2 [12]. Rosha et al. explored the dry oxidative reforming using pure nickel as a catalyst in the temperature range of 800–900 °C. They showed that CH4 conversion and H2 yield increased with increasing temperature and oxygen content; however, CO2 conversion decreased with O2 addition [13]. In their other study, Rosha et al. used Ni nanoparticles for combined reforming of synthetic biogas. It was demonstrated that the addition of oxygen (O2/CH4 = 0.17) at high temperature (≥ 850 °C) resulted in 81% CH4 conversion and 36% H2 selectivity without coke deposition [14]. Moreover, Rosha et al. showed the effects of O2/CH4 ratio on CH4 conversion and H2/CO ratio at a temperature of 650 °C using nickel catalyst supported on the complex of zinc and ceria (CeO2). They obtained 39.3% CH4 conversion and H2/CO ratio of 1.4 at O2/CH4 ratio of 0.57 [11].
The nickel and cobalt-based catalyst have been extensively utilized in the reforming application due to their low cost and high activity. However, they suffer from the issue related to coke deposition and re-oxidation [15, 16]. The utilization of bimetallic catalysts using nickel and cobalt could be an effective method for overcoming these hurdles through the synergic effects of both the metals [17, 18]. It has been reported that bimetallic nickel-cobalt catalysts provide higher activity and stability when compared to single metal catalyst [19,20,21,22]. The catalytic activity can also be enhanced through optimizing process parameters by using various optimization or design of experiment (DoE) tools. DoE methods are more favored and accurate when compared to the single-factor method due to their higher efficiency and simultaneous study of various factors [23].
Response surface methodology (RSM) is one of these tools that have been reported for optimization in dry reforming applications [24,25,26]. RSM analyzes the interaction effect of various factors on the desired responses and predicts the suitable optimum conditions [27]. The Box-Behnken design requires a lesser number of experimental runs when compared to a normal factorial technique for generating a higher-order response surface [28]. This design method is considered when operating factors are greater than two and the optimum value is expected to lie within the selected range of parameters [29]. The literature review on this aspect indicates that optimization through RSM for H2 enrichment over nickel-cobalt bimetallic catalyst has not been reported. The prospective study is focused on finding the optimized conditions for the dry oxidative reforming of biogas using DoE. The three main reaction-related variables, reforming temperature, carbon dioxide to methane (CH4/CO2) ratio, and oxygen to methane (O2/CH4) ratio, were studied over a previously synthesized nickel-cobalt bimetallic catalyst for optimizing the hydrogen enrichment of biogas.
2 Materials and method
2.1 Materials
The nickel-cobalt bimetallic catalyst was synthesized through the wet-impregnation method. The synthesis procedure and characterization have been presented previously by us [19]. The ultrapure reactant gases methane (CH4; 99.999%), carbon dioxide (CO2; 99.999%), and oxygen (O2; 99.999%) were acquired locally.
2.2 Experimental setup
The dry oxidative reforming of synthetic biogas using bimetallic catalyst was performed using a tube-type fixed-bed down-flow reactor under atmospheric pressure conditions. The details of the reforming reactor and reaction setup have been provided previously by us [19]. The reactions were performed under the temperature range of 700 to 800 °C at 1 bar with varying CH4/CO2 and O2/CH4 ratios. The catalyst (0.15 g) was kept between the two layers of quartz wool, and the required temperature was attained using inert N2 gas with a heating rate of 10 °C/min. The product gases were then taken to the continuous biogas flow analyzer for analyzing the composition of product gases. The analyzer is capable of measuring the various gases (ranges) such as H2 (0–100%), CO (0–100%), CH4 (0–100%), and CO2 (0–50%). The catalytic performance was evaluated by recording the data after 2 h of reaction time at fixed parameters. To ensure the consistency and reproducibility of the data, all the readings were recorded three times and their average values were considered for analysis. The catalytic performance parameters were evaluated using the following equations (Eqs. 3, 4, 5, and 6):
where R represents reactants; Rin, inlet reactant flow rate; Rout, outlet reactant flow rate; CH4 (in), inlet flow rate of methane; H2 (out), outlet flow rate of hydrogen; and CO (out), outlet flow rate of carbon monoxide.
2.3 Design of experiment
The process parameter optimization for hydrogen enrichment of biogas was performed through the Box-Behnken factorial design method of response surface methodology (RSM) using Design-Expert (Version 12.0.12.0) software. A 17-run, three-factors, three-level, randomized design of Box-Behnken (BBD) was employed for interpreting the effects of distinct input variables on output. Three distinct variables, namely, (A) temperature of reforming, (B) the ratio of CH4/CO2, and (C) the ratio of O2/CH4, were investigated at three levels (− 1, 0, and 1) for optimization study. The design matrix has been shown in Table 1. The conversion of both reactants (CH4 and CO2), yield of products (H2 and CO), and ratio of products (H2/CO) were selected as the response for evaluating the catalytic activity in dry oxidative reforming. For the statistical model, the p values less than 0.0500 indicate parameters have a significant impact on the model; values greater than 0.1000 indicate the parameters are not significant.
After the experimentation, a polynomial equation of second order (Eq. 7) was used to evaluate the relationship between independent variables and responses. An analysis of variance (ANOVA) method at 95% confidence level was used to evaluate the data fitting.
where Y represents response predicted; βo represents offset constant; β1, β2, and β3 represent the linear effect; β12, β13, and β23 represent the intersection effects; and β11, β22, and β33 represent squared coefficients.
3 Results and discussion
The impact of input parameters was evaluated for the hydrogen enrichment of biogas through dry oxidative reforming over bimetallic Ni-Co catalyst. The bimetallic catalysts have been reported to possess higher activity and stability when compared to mono-metallic catalyst due to the synergetic effect of constituent metals, higher reducibility, and strong metal-support interaction [19, 30]. The three major input parameters such as inlet temperature, CH4/CO2, and O2/CH4 ratios were selected for the optimization study. The inlet temperature (750–850 °C) was chosen due to the endothermic nature of the reforming reaction; the minimum temperature of 750 °C was selected to counter the side reactions. The CH4/CO2 ratio (1, 1.5, and 2) was adjusted to study the impact of different compositions of the biogas, and O2/CH4 ratio (0.3, 0.4, and 0.5) is essential for analyzing the extent of oxidative reforming and increase in hydrogen enrichment when compared to dry reforming only. A total of 17 experiments were performed for establishing the relation between independent input parameters and output responses. The five responses: CH4 and CO2 conversion, H2 and CO yield, and H2/CO ratio were selected for optimization study. The inlet experimental design and corresponding responses are presented in Table 2.
3.1 Effect of inlet parameters on dry oxidative reforming
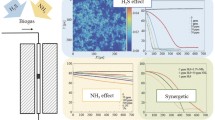
The outcomes of experimentation show that H2 enrichment increase with increasing all the input parameters in the dry oxidative reforming. The three-dimensional (3D) plot depicting the effects of inlet independent parameters (temperature and CH4/CO2 ratio; temperature and O2/CH4 ratio; CH4/CO2 and O2/CH4 ratio) on the yield of hydrogen are presented in Fig. 1. The synergetic interaction among parameters resulted in a significant change in response. Figure 1 a demonstrates the combined impact of temperature and CH4/CO2 ratio on H2 enrichment. The H2 yield was reported to increase with increasing both temperature and reactant (CH4/CO2) ratio and attained a maximum value of 44.04%. The increase in temperature is favorable for enhancing the rate of reforming reaction owning to its high endothermic nature. The maximum H2 yield was obtained at 850 °C with 1.5 and 0.5 CH4/CO2 and O2/CH4 ratio, respectively. The increase in both inlet parameters also results in enhanced conversion of reactant and product yield; however, the temperature has a more pronounced effect when compared to reactant ratio as reforming reactions are more favorable at higher temperature regions. Mageed et al. (2020) reported similar findings that variation of reaction temperature has a statistically significant impact on H2 yield [26]. Nataj et al. (2018) also demonstrated a similar impact of temperature in their study over Ni-Cu bimetallic catalyst and reported that bimetallic catalyst has higher activity and stability when compared to mono-metallic catalyst [31]. Figure 1 b depicts the interaction impact of temperature and O2/CH4 ratio. The H2 yield is significantly dependent on these parameters. The increases in O2/CH4 ratio resulted in increased H2 yield and CH4 conversion due to the synergetic effect of both partial oxidative and dry reforming. Similar trends of increasing CH4 conversion and H2 yield with increasing reaction temperature and O2 proportion were reported by Rosha et al. (2018) [13]. Moreover, a drop in CO2 conversion was reported with an increasing O2/CH4 ratio. The CH4 reacts more favorably with oxygen (O2) when compared to carbon dioxide (CO2), and the presence of two oxidizing agent results in a higher conversion of CH4. Figure 1 c demonstrates the interaction impacts of CH4/CO2 and O2/CH4 ratio. The impacts of O2/CH4 are more detrimental than CH4/CO2 ratio due to the higher reactivity between CH4 and O2. However, the yield of H2 was improved by raising both the parameters. The highest H2 yield of 44.04% was obtained at 850 °C with 1.5 and 0.5 CH4/CO2 and O2/CH4 ratio, respectively. The experimentation demonstrated that for dry oxidative reforming, the effective control of all discrete input parameters (temperature, CH4/CO2, and O2/CH4 ratio) is required for maximum H2 enrichment of biogas. This model provided that optimum conditions for dry oxidative reforming are a temperature of 725.65 °C with 1.32 and 0.42, CH4/CO2, and O2/CH4 ratio, respectively. The optimum H2 enrichment of 36.9% was attained under these reaction conditions.
3.2 Statistical analysis
Results obtained from experimentation were evaluated through BBD using statistical methods to establish a relationship between independent and dependent variables. The summary statistics of various models checked for data fitting has been shown in Table 3. The suitability of data fitting was determined by using standard deviation (SD), R2, adjusted R2, predicted R2, and PRESS (predicted sum of square).
Table 3 shows that experimental values fit into the quadratic models with value of 0.44, 0.989, 0.976, 0.832, and 21.99 for SD, R2, adjusted R2, predicted R2, and PRESS, respectively. The high value of R2 (0.989) shows the high proximity of input variables and response [26, 32]. The quadratic model infers that 98% fluctuations of responses are around their mean. Furthermore, current model is also satisfactory for forecasting H2 yield as assigned through predicted R2 value [25, 26]. This also implies that experimentally obtained values are in agreement with the predicted values. Additionally, the sequential model of sum of squares suggests that there is two-factor interaction between input variables and quadratic model. The p value of quadratic vs. 2FI is less than 0.0001 which demonstrates that both 2FI and quadratic models are statistically significantly (Table 4).
Table 5 shows the analysis of variance (ANOVA) of discrete as well as linked impacts of independent variable (temperature, and CH4/CO2 and O2/CH4 ratio). Based on ANOVA analysis, the reaction temperature and O2/CH4 ratio have more dominant effect on hydrogen production as depicted by the very low p value (< 0.0001). The reforming reaction and methane (CH4) cracking reactions are responsible for H2 production, and both reactions are favored at higher temperature. It is evident form ANOVA table that reactant ratio (CH4/CO2) is statistically insignificant of H2 production demonstrated by the p value (0.1476) higher than 0.05. The CH4/CO2 ratio has substantial impact on the syngas or product ratio (H2/CO). The interaction among inlet parameters, temperature and CH4/CO2 ratio, temperature and O2/CH4 ratio, and CH4/CO2 ratio and O2/CH4 ratio, is not statistically much significant as their p values are higher than 0.05. The quadratic effects of all distinct variables are statistically critical as their p values are less than 0.05 and these results can be seen in the Fig. 1. The complete analysis of RSM models shows that modes having very low p value (0.0001) have significant impact on the H2 production with good data fitting.
The final expressions of quadratic model concerning coded and actual factors are represented in Eqs. 8 and 9, respectively. The positive value in the equation shows increases with corresponding parameters results in increased H2 yield. It is evident form the equations that increasing both temperature and O2/CH4 ratio has dominant impact on H2 yield as compared to CH4/CO2 ratio.
For further testing the predictability of the model, the results of experimentation were fitted to predict the values of H2 produced. The relationship of actual and predicted values of H2 yield is shown in Fig. 2. The adjusted R2 value is obtained by fitting of data demonstrated the goodness of empirical models. The addition of input parameters that have significant impact on the response results in the increased value of adjusted R2 and vice versa. The adjusted R2 value for H2 yield is 0.976 which indicates that only 2.4% variations of model were not illustrated by input parameters.
4 Conclusion
In the present study, response surface methodology using Box-Behnken DoE was employed for evaluating the domination of input parameters and optimizing the process for H2 enrichment of biogas over nickel-cobalt bimetallic catalyst. The Ni-Co/TiO2 catalyst has been observed to demonstrate suitable catalytic properties for the dry oxidative reforming of synthetic biogas. The following conclusions were drawn from the study:
-
1.
The experimental data obtained from BBD showed that all inlet parameters have a predominant effect on H2 yield under dry oxidative reforming. Moreover, reforming temperature and O2/CH4 ratio have a statistically significant impact on reforming reaction as shown by p value (p < 0.0001).
-
2.
The study manifested that maximum H2 enrichment was obtained at 800 °C along 1.5 and 0.5 CH4/CO2 and O2/CH4 ratio, respectively. The maximum product ratio (H2/CO) was obtained at 800 °C with 1.5 and 0.3 CH4/CO2 ratio and O2/CH4 ratio, respectively.
-
3.
The statistical model predicted that a temperature of 725.68 °C with ratios of 1.32 and 0.42 CH4/CO2 and O2/CH4, respectively, are the optimum conditions for the dry oxidative reforming. The optimum H2 enrichment of 36.9% and product (H2/CO) ratio of 0.93 was obtained under optimum conditions.
-
4.
The experimentation also demonstrated that CH4 conversion and H2 yield escalated with increasing temperature and O2/CH4 ratio due to the endothermic nature of reforming reaction and the presence of two oxidative agents (O2 and CO2). However, the conversion of CO2 was found to decline with escalating O2/CH4 ratio due to higher affinity of CH4 towards O2 when compared to CO2 and manifestation of RWGS (reverse water gas shift reaction).
-
5.
The study ascertained that RSM and BBD statistical experimental design give statistically significant results as well as optimum conditions for improved catalytic performance as evident from the close agreement of predicted and experimental data.
References
Verma S, Das LM, Kaushik SC (2017) Effects of varying composition of biogas on performance and emission characteristics of compression ignition engine using exergy analysis. Energy Convers Manag 138:346–359. https://doi.org/10.1016/j.enconman.2017.01.066
Jindal M, Rosha P, Mahla SK, Dhir A (2015) Experimental investigation of performance and emissions characteristics of waste cooking oil biodiesel and n-butanol blends in a compression ignition engine. RSC Adv 5:33863–33868. https://doi.org/10.1039/c4ra14431g
Rosha P, Dhir A, Mohapatra SK (2018) Influence of gaseous fuel induction on the various engine characteristics of a dual fuel compression ignition engine: a review. Renew Sust Energ Rev 82:3333–3349. https://doi.org/10.1016/j.rser.2017.10.055
Das S, Kashyap D, Kalita P, et al (2020) Clean gaseous fuel application in diesel engine: a sustainable option for rural electrification in India. Renew. Sustain. Energy Rev.
Rosha P, Ibrahim H, Nanda AK, et al (2020) Effect of hydrogen-enriched biogas induction on combustion, performance, and emission characteristics of dual-fuel compression ignition engine. Asia-Pacific J Chem Eng 1–10. https://doi.org/10.1002/apj.2435
Singh R, Dhir A, Mohapatra SK, Mahla SK (2019) Dry reforming of methane using various catalysts in the process: review. Biomass Convers. Biorefinery
Usman M, Wan Daud WMA (2016) An investigation on the influence of catalyst composition, calcination and reduction temperatures on Ni/MgO catalyst for dry reforming of methane. RSC Adv 6:91603–91616. https://doi.org/10.1039/c6ra15256b
Usman M, Wan Daud WMA, Abbas HF (2015) Dry reforming of methane: influence of process parameters - a review. Renew Sust Energ Rev 45:710–744
Chen L, Zhu Q, Wu R (2011) Effect of Co-Ni ratio on the activity and stability of Co-Ni bimetallic aerogel catalyst for methane Oxy-CO2 reforming. Int J Hydrog Energy 36:2128–2136. https://doi.org/10.1016/j.ijhydene.2010.11.042
Lau CS, Tsolakis A, Wyszynski ML (2011) Biogas upgrade to syn-gas (H2-CO) via dry and oxidative reforming. Int J Hydrog Energy 36:397–404. https://doi.org/10.1016/j.ijhydene.2010.09.086
Rosha P, Mohapatra SK, Mahla SK, Dhir A (2019) Catalytic reforming of synthetic biogas for hydrogen enrichment over Ni supported on ZnO[sbnd]CeO2 mixed catalyst. Biomass Bioenergy 125:70–78. https://doi.org/10.1016/j.biombioe.2019.04.013
Rosha P, Mohapatra SK, Mahla SK, Dhir A (2018) Biogas reforming for hydrogen enrichment by ceria decorated over nickel catalyst supported on titania and alumina. Int J Hydrog Energy 43:21246–21255. https://doi.org/10.1016/j.ijhydene.2018.09.187
Rosha P, Singh R, Mohapatra SK, Mahla SK, Dhir A (2018) Optimization of hydrogen-enriched biogas by dry oxidative reforming with nickel nanopowder using response surface methodology. Energy and Fuels 32:6995–7001. https://doi.org/10.1021/acs.energyfuels.8b00819
Rosha P, Mohapatra SK, Mahla SK, Dhir A (2019) Hydrogen enrichment of biogas via dry and autothermal-dry reforming with pure nickel (Ni) nanoparticle. Energy 172:733–739. https://doi.org/10.1016/j.energy.2019.02.006
Alves HJ, Bley Junior C, Niklevicz RR, et al (2013) Overview of hydrogen production technologies from biogas and the applications in fuel cells. In: International Journal of Hydrogen Energy. pp. 5215–5225
Abdullah B, Abd Ghani NA, Vo DVN (2017) Recent advances in dry reforming of methane over Ni-based catalysts. J Clean Prod 162:170–185
San-José-Alonso D, Juan-Juan J, Illán-Gómez MJ, Román-Martínez MC (2009) Ni, Co and bimetallic Ni-Co catalysts for the dry reforming of methane. Appl Catal A Gen 371:54–59. https://doi.org/10.1016/j.apcata.2009.09.026
Xu J, Zhou W, Li Z, Wang J, Ma J (2010) Biogas reforming for hydrogen production over a Ni-Co bimetallic catalyst: effect of operating conditions. Int J Hydrog Energy 35:13013–13020. https://doi.org/10.1016/j.ijhydene.2010.04.075
Sharma H, Dhir A (2020) Hydrogen augmentation of biogas through dry reforming over bimetallic nickel-cobalt catalysts supported on titania. Fuel 279:118389. https://doi.org/10.1016/j.fuel.2020.118389
Takanabe K, Nagaoka K, Nariai K, Aika KI (2005) Titania-supported cobalt and nickel bimetallic catalysts for carbon dioxide reforming of methane. J Catal 232:268–275. https://doi.org/10.1016/j.jcat.2005.03.011
Takanabe K, Nagaoka K, Aika KI (2005) Improved resistance against coke deposition of titania supported cobalt and nickel bimetallic catalysts for carbon dioxide reforming of methane. Catal Letters 102:153–157. https://doi.org/10.1007/s10562-005-5848-4
Xu J, Zhou W, Li Z, Wang J, Ma J (2009) Biogas reforming for hydrogen production over nickel and cobalt bimetallic catalysts. Int J Hydrog Energy 34:6646–6654. https://doi.org/10.1016/j.ijhydene.2009.06.038
Ayodele B V., Abdullah S (2018) An overview of response surface methodology approach to optimization of hydrogen and syngas production by catalytic reforming of greenhouse gases (CH4 and CO2). In: Statistical Approaches With Emphasis on Design of Experiments Applied to Chemical Processes
Braga TP, Santos RCR, Sales BMC, da Silva BR, Pinheiro AN, Leite ER, Valentini A (2014) CO2 mitigation by carbon nanotube formation during dry reforming of methane analyzed by factorial design combined with response surface methodology. Cuihua Xuebao/Chinese J Catal 35:514–523. https://doi.org/10.1016/s1872-2067(14)60018-8
Fan MS, Abdullah AZ, Bhatia S (2011) Hydrogen production from carbon dioxide reforming of methane over Ni-Co/MgO-ZrO2 catalyst: process optimization. Int J Hydrog Energy 36:4875–4886. https://doi.org/10.1016/j.ijhydene.2011.01.064
Mageed AK, Ayodele BV, Mustapa SI (2020) Response surface optimization of hydrogen-rich syngas production by greenhouse gases reforming. Chem Eng Technol 43:742–751. https://doi.org/10.1002/ceat.201900475
Ayodele BV, Khan MR, Nooruddin SS, Cheng CK (2017) Modelling and optimization of syngas production by methane dry reforming over samarium oxide supported cobalt catalyst: response surface methodology and artificial neural networks approach. Clean Techn Environ Policy 19:1181–1193. https://doi.org/10.1007/s10098-016-1318-5
Rao JS, Kumar B (2012) 3D blade root shape optimization. In: institution of mechanical engineers - 10th international conference on vibrations in rotating machinery
Das AK, Dewanjee S (2018) Optimization of extraction using mathematical models and computation. In: Computational Phytochemistry
Turap Y, Wang I, Fu T, Wu Y, Wang Y, Wang W (2020) Co–Ni alloy supported on CeO2 as a bimetallic catalyst for dry reforming of methane. Int J Hydrog Energy 45:6538–6548. https://doi.org/10.1016/j.ijhydene.2019.12.223
Nataj SMM, Alavi SM, Mazloom G (2018) Modeling and optimization of methane dry reforming over Ni–Cu/Al2O3 catalyst using Box–Behnken design. J Energy Chem 27:1475–1488. https://doi.org/10.1016/j.jechem.2017.10.002
Roshan N, Ghader S, Rahimpour MR (2018) Application of the response surface methodology for modeling demulsification of crude oil emulsion using a demulsifier. J Dispers Sci Technol 39:700–710. https://doi.org/10.1080/01932691.2017.1385480
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, H., Dhir, A. Response surface methodology for optimization of dry oxidative reforming for hydrogen enrichment of biogas. Biomass Conv. Bioref. 13, 2875–2883 (2023). https://doi.org/10.1007/s13399-020-01244-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01244-5