Abstract
The effects of seven mixing ratios (1:0, 3:1, 2:1, 1:1, 1:2, 1:3, and 0:1, swine manure (SM)/rice straw (RS), based on total solid) on anaerobic co-digestion (co-AD) of SM and RS mixtures were investigated in batch tests. The results showed that the highest methane yield (188.79 mL/g VSadded) was obtained at the ratio of 1:1, which was higher than that of the mono-digestion of RS or SM by 178.77% and 18.94%, respectively. For the remained six ratios, the methane yield increased with the increase of SM proportion. The highest organic matter degradation rates were also observed at the ratio of 1:1. The methane content of biogas and the pH, total alkalinity, and total ammonia nitrogen of the digestate all decreased with the increase of RS proportion and decrease of SM proportion. Additionally, the kinetic results of each anaerobic digestion (AD) step suggested that the co-AD of SM and RS at the ratio of 1:1 significantly improved the reaction rates of the four AD steps. Meanwhile, the methane production time was prolonged and the lag time of methanogenesis was shortened at the ratio of 1:1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Anaerobic digestion (AD) is an efficient and low-cost choice for organic waste treatment because it can treat wastes, generate renewable energy (methane and hydrogen), and produce fertilizer [1]. One substrate is used in conventional AD (mono-AD) which has been widely studied, while there are some deficiencies, such as improper carbon/nitrogen (C/N) ratios, low biogas production, and unstable operating performance [2]. Anaerobic co-digestion (co-AD), namely, the simultaneous AD of two or more substrates, can adjust the C/N ratio, enhance biogas yields, dilute potential toxic compounds, and improve stability of the AD process and usually has better performance than mono-AD [3, 4].
Swine manure (SM) is an important organic waste, of which the yield has increased significantly with the rapid development of modern animal husbandry. Approximately 1.7 billion tons of liquid SM are produced globally each year [5]. SM contains high concentrations of chemical oxygen demand (COD), nitrogen, phosphorus, and fecal coliforms, which can pose a profound threat to the environment and human health if left untreated. Traditionally, SM is used as a feedstock in AD globally for energy recovery. However, the mono-AD of SM is not the most effective way to utilize SM because of its low C/N ratio (about 6–8) [6]. In addition, the abundant organic nitrogen in SM poses a potential risk of free ammonia (FA) inhibition of microbial growth in AD [7]. Some researchers have found that the addition of substrates with high carbon content as external carbon sources, such as corn straw [8], maize stalk [9], and wheat straw [10], could receive higher biogas yields.
With the global economic development and population growth, rice straw (RS), as an important agricultural waste, is being generated at an increasingly high rate [11]. More than 0.58 billion tons of RS are available worldwide every year [12], which is difficult to be naturally degraded due to its high contents of cellulose, hemicellulose, and lignin. To alleviate the environmental impacts caused by conventional treatment of RS like burning, AD could be an excellent alternative. However, the high C/N ratio of RS is a non-negligible limitation of AD [13], and co-AD with substrate with a low C/N ratio (such as chicken manure [14], municipal solid waste [15], and food waste [16]) can improve its efficiency.
Since the treatments of SM and RS are very important and their compositions are complementary, co-AD may be an effective method. Although the co-AD of SM or RS has been studied, the properties of SM and other manure are different, and those of RS are also different with other straws. Therefore, the co-AD of SM and RS is different from the co-AD in these previous studies [8,9,10, 14,15,16]. Recently, some studies have emerged with an emphasis on the co-AD of SM and RS and found significant improvements in biogas production [6]. Additionally, the mixing ratio of substrates is also a noteworthy aspect in co-AD [17]. However, few kinetic analyses were performed in previous related studies [18], which focused on biogas production and process stability. By analyzing kinetic results, better understanding of the effects of substrate mixing ratios on methane yields and reaction processes could be achieved. Some kinetic models have been used to predict methane production, such as the modified Gompertz model and the logistic model, in previous researches [19].
However, most studies have focused on the analysis of biogas production [8], and there is little information available concerning the kinetic characterization of reaction processes in the co-AD. AD is a four-step (hydrolysis, acidogenesis, acetogenesis, and methanogenesis) process, and the reaction rates of each step are different [2]. Studying the kinetic characteristics of different steps could enable us to explore the limiting factors in the AD process more deeply. Additionally, carbohydrates, proteins, and lipids, the main components of organic waste, are found in different amounts in SM and RS. Since different compositions of substrates showed diverse degradation kinetics due to the distinct reaction pathways and microbial communities involved [20], studies on the kinetics of different steps at various mixing ratios could provide a deeper understanding about the effects of mixing ratios on the AD process. However, the effects of substrate mixing ratios on the kinetic characteristics of different AD steps are still unclear. Moreover, few studies compare the results of simulations using multiple models.
Therefore, the object of this study was to investigate the effects of substrate mixing ratios on the performance and use two models to simulate and compare different kinetic characteristics of four steps in the co-AD of SM and RS.
2 Material and methods
2.1 Swine manure and rice straw
The dry SM, collected from a swine farm in Beijing, China, was homogenized with tap water in a blending machine (QH-400D, QVi, China) and filtered through a sieve with a mesh of 0.85 mm diameter to remove large particles and then stored at 4 °C. The RS, obtained from a rural area of Zhejiang, China, was chopped and ground into small particles less than 0.25 mm in size. The SM and RS were mashed using a blending machine before they were used. The characteristics of the substrates are summarized in Table 1.
2.2 Inoculum
The digestate collected from a mesophilic anaerobic digester in a swine farm in Beijing, China, was used as inoculum. The characteristics of the inoculum are also summarized in Table 1.
2.3 Batch anaerobic digestion
The digesters used in the test were 250 mL serum bottles with a working volume of 180 mL, which could be sealed by a rubber stopper equipped with two openings for sampling biogas and digestate. The slurry of SM and RS were added according to the designed mixing ratios of 1:0, 3:1, 2:1, 1:1, 1:2, 1:3, and 0:1 (SM/RS, based on total solid (TS)). The inoculum to substrate ratio (ISR) was 1:4 (v/v basis) and the characteristics of the mixtures are summarized in Table 2. The inoculum was pre-incubated before inoculation in order to deplete the residual biodegradable organic matter present in it [21]. Prior to operation, the digesters were purged with nitrogen gas for 5 min to ensure anaerobic conditions. Then, the digesters were quickly sealed and agitated on a shaker (HKZ-C, China) at 140–150 rpm and 37 ± 1 °C. After each sampling, nitrogen gas was charged to replenish the reduced volume. The AD was terminated when there was no significant biogas production. All tests were performed in triplicate.
Considering that SM and RS are commonly deficient in trace elements [22,23,24], supplements of essential elements, namely, Fe, Co, and Ni, were added as 10 mg/L of Fe (FeCl2∙4H2O) and 1 mg/L each of Co (CoCl2∙6H2O) and Ni (NiCl2∙6H2O) [25].
2.4 Analytical methods
Tests of each sample were conducted in triplicate, and all results were the average of the replicates.
Biogas and digestate samples were taken twice a week to determine the composition of the biogas and the parameters of the sludge. Biogas production was measured periodically using a liquid displacement device. The methane contents of the biogas were detected using a gas chromatograph (GC) (Agilent 6890N, USA) equipped with a flame ionization detector (FID). The total and soluble indexes of the digestate samples were detected. The samples for the analysis of soluble items were centrifuged at 8000 rpm for 30 min and then passed through 0.45-μm filters before analysis. Carbohydrate and protein contents of sludge samples were measured using the methods of a previous study [26]. The pH was measured using a pH meter (BANTE 900, Shanghai Special Instruments Factory). Another GC (Agilent 6890N, USA), also equipped with a FID, was utilized to detect total volatile fatty acids (TVFA). A portion (1 mL) of the filtrate was collected in a 2-mL GC vial, and 0.5 mL of 0.1 mol/L HCl was added to achieve an acidic pH. TS, volatile solid (VS), COD, total alkalinity (TA), and total ammonia nitrogen (TAN) of sludge samples were determined in accordance with the standard methods [27].
2.5 Calculation
Free ammonia nitrogen (FAN) was calculated according to the Eq. (1) [28].
where FAN is the concentration of FAN (mg/L); TAN is the concentration of TAN (mg/L); pH is the pH value; T is the temperature in Kelvin.
The experimental data of methane yields in the batch experiment were simulated by the modified Gompertz model [29] and the logistic model [30] to obtain the kinetic parameters. The equations of the two models are as follows [8]:
where P(t) is the cumulative methane yield (L/kg VS); P0 is the methane yield potential (L/kg VS); Rmax is the maximum methane yield (L/kg VS/d); λ is the lag phase (d); t is the time (d); e is the exp. (1) = 2.7183; Tef is the effective methane production time (d); and T90 is the technical digestion time (d), which means the time required to achieve 90% of the maximum cumulative methane yield.
The reaction rates of the four AD steps, namely, hydrolysis, acidogenesis, acetogenesis, and methanogenesis, were expressed by COD conversion ratios and calculated as follows [31]:
where RHyd., RAcid., RAcet., and RMeth. are cumulative hydrolyzed COD (%), cumulative acidified COD (%), cumulative acetified COD (%), and methane COD (%), respectively; VCH4 is the volume of methane (mL); V is the volume of inoculum (L); CSCOD is the concentration of soluble COD (g/L); CTVFA is the concentration of TVFA (g COD/L); Cacetate is the concentration of acetate acid (g COD/L); CODadded is the COD of substrates added into the bottles (g); and 1 g of COD is equal to 350 mL CH4.
Equations (2) and (3) were also used for reaction rates of the four AD steps; in this case, the parameters were represented as follows: P(t)′ is the conversion ratio of substrates (%), P0′ is the conversion potential (%), and Rmax′ is the maximum conversion rate (%/d).
The present study adopted a nonlinear regression analysis using the CurveExpert program (CurveExpert Professional 2.6.3) and Origin Pro 2016 software for curve fitting and estimation of kinetic parameters of the modified Gompertz model and the logistic model [32]. The experimental data obtained from all reactors were checked for the fitness of the models. The statistical indicators and correlation coefficients (R2) were calculated [33].
3 Results and discussion
3.1 Biogas production
Figure 1a illustrates the daily biogas yields in the AD process. The first biogas yield peak was observed in the first 3 d for all AD tests. The highest peak biogas yield was obtained at the ratio of 1:0 followed by the ratio of 1:1, and the minimum was obtained at the ratio of 0:1. For the ratio of 1:0, the daily biogas yield decreased gradually until the end of the experiment, while that at the ratio of 1:1 fluctuated around 8 L/kg VSadded/d and finally terminated. Meanwhile, those at other ratios were stagnant for about 10 d until another peak was achieved around 20–23 d, which may have been due to the low pH that resulted from accumulation of volatile fatty acids (VFAs) [34]. This result was coincident with previous research [35], which reported that the co-AD of apple waste and SM showed a positive synergetic effect on biogas production with a proper apple waste proportion, but showed an antagonistic effect with excess apple waste.
The methane contents of biogas in AD are summarized in Fig. 1b. For the AD with ratios of 1:0, 3:1, 2:1, and 1:1, the methane contents began to increase significantly after a 2-d lag period and remained stable at 67.41%, 65.00%, 62.50%, and 62.23%, respectively, while the lag period was 10 d, and the ultimate content was 49.08% at a ratio of 0:1. Those at ratios of 1:2 and 1:3 increased in two stages (1–10 d, 11–40 d) and reached stable after 25 d of 57.68% and 52.47%, respectively. The results showed that the maximum methane content increased with the increase in SM proportion, which may have been attributed to the decrease in carbohydrates and increase in proteins in the substrate [36] (Table 2) since the degradation of proteins could contribute more to biogas production than that of carbohydrates [37]. The range of methane content was consistent with the results obtained in a previous study [6], but the differences in methane content between different ratios was larger, which may be due to the higher proportion of protein in the SM used in this study.
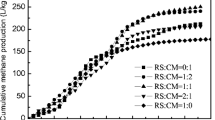
Based on the daily biogas yields and methane contents, the accumulative methane yields of AD could be calculated (Fig. 1c). The cumulative methane yields at the ratios of 1:1 and 1:0 continued to increase until biogas production ceased, while those at other ratios increased in two stages over 20 d, thereby indicating that hydrolysis and acidification were predominant in the early period [38]. The highest accumulative methane yield was obtained at the ratio of 1:1 (188.79 L/kg VSadded), which was 18.49% and 178.78% higher than that of SM mono-AD (159.33 L/kg VSadded) and RS mono-AD (67.72 L/kg VSadded), respectively. The accumulative methane yield obtained by co-digestion at the ratio of 1:1 was roughly the same as the best result of a previous study [39] which studied the effects of particle size reduction of RS on biogas production (0.075 mm, 197 L/kg VSadded). The results indicated that co-digestion could save the cost of pretreatment.
3.2 Matter degradation
One of the most important parameters to evaluate the performance of AD is the degradation of organic matters. The degradations of some organic matters (VS, TCOD, total carbohydrate (T-carbohydrate), and total protein (T-protein)) in the AD process are summarized in Fig. 2. The co-AD of SM and RS with a ratio of 1:1 obtained the highest degradation of VS and TCOD as 59.38% and 56.17%, respectively, while the mono-AD of RS had the lowest as 51.87% and 42.32%, respectively. Meanwhile, the co-AD at a ratio of 1:1 also had the highest T-carbohydrate and T-protein degradation rates of 59.0% and 49.6%, respectively. The lowest degradation rates of T-carbohydrate (45.3%) and T-protein (40.7%) were obtained at ratios of 0:1 and 1:0, respectively. The degradation rates of T-carbohydrate and T-protein in co-AD were all higher than those in mono-AD. One reason for this phenomenon was that the mixture of SM and RS could adjust the C/N ratio of substrate to the suitable range [40]. Moreover, the synergy of SM and RS produced by diluting the toxicity of inhibitors (like FA) in SM digestion also increased the organic degradation [41]. Additionally, the degradation of organic matter in the AD was consistent with the methane yields (Fig. 1c).
3.3 Characteristics of digestates
Four key parameters for the characteristics of digestates, such as pH, TAN, TA, and TVFA, were analyzed.
The pH changes of digestate in the AD processes are depicted in Fig. 3a. At the beginning, there was a significant decrease in pH below 6.7 in all the AD processes, except for the SM mono-AD. With the progress of AD, the digestate pH of AD processes at ratios of 1:0, 3:1, and 1:1 recovered quickly in the first 5 d due to the degradation of VFAs and production of TAN, while others maintained below 6.5 for 15 d, presumably resulting from the accumulation of VFAs produced by hydrolysis and acidogenesis [42]. The increase in RS proportion decreased the digestate pH, except for the ratio of 1:1, possibly due to the increase in carbohydrate content and decrease in protein content in the substrate (Table 2) because the degradation of carbohydrates lowered the system pH and that of proteins increased it [43]. The lower pH at ratios of 3:1, 2:1, 1:2, 1:3, and 0:1 also accounted for their lower methane yields in the early stage of AD (Fig. 1) [43]. The change trend of pH was a little different with a previous study [44] which found the high RS proportion reactors remained acidic for the rest of the digestion period probably due to high TS contents, while the optimal pH range was reached after 20 d in our test.
As shown in Fig. 3b, the TAN of digestate increased slowly and then remained stable after 13 d. The digestate TAN at different ratios increased from 375.0–825.0 mg/L to 1010.0–2336.8 mg/L in the test, which was within the acceptable range (≤ 3000 mg/L) of anaerobic microorganisms [14]. In addition, the digestate FAN (from 0.77–1.23 mg/L to 5.75–70.09 mg/L) calculated according to Eq. (1) also indicated that there was no inhibition (> 150 mg/L) [45] of methane production. Meanwhile, the higher the proportion of SM in the substrate, the higher the ultimate digestate TAN, which was in accordance with the results of previous research [6]. The TA of the digestate is depicted in Fig. 3c, of which the changes were consistent with the trends in TAN.
VFA is the intermediate product of AD, and its changes are summarized in Fig. 3d. TVFA increased rapidly at the beginning of AD to maximum values due to the hydrolysis and acidogenesis of organic matter and then continuously decreased as a consequence of the consumption of VFAs by methanogenesis [29]. The peak TVFA concentration in the reactors at ratios of 1:0, 3:1, 2:1, 1:1, and 1:2 appeared at 6 d, while that at ratios of 1:3 and 0:1 appeared in two peaks at 6 and 13 d, respectively. The results suggested that methanogenesis in the reactors at ratios of 1:3 and 0:1 was inhibited at first, which probably resulted from the higher C/N ratios [11] (Table 2). Additionally, the high cellulose contents in RS made hydrolysis much slower, thereby resulting in insufficient nutrients for acetogenesis [11]. This TVFA trend led to a similar change in pH, which further affected methanogenesis. The result was consistent with a previous study [46] about SM and RS co-digestion, which also indicated that the growth and metabolism of the methanogens in mono-RS reactor were not good, probably as a consequence of the improper C/N ratio and insufficient micronutrients in RS.
3.4 Kinetic study for methane yield
The kinetic simulation for methane yields of AD was analyzed using the modified Gompertz model and the logistic model [20] (Fig. 4), and the kinetic parameters were calculated (Table 3). The results showed that the kinetic parameters derived from the modified Gompertz model and the logistic model had similar relationships with the different mixing ratios. The highest P0 was obtained at the ratio of 1:1, which suggested that co-AD of SM and RS at proper mixing ratios could improve methane yields. The highest Rmax was found in the mono-AD of SM, because Rmax showed a negative correlation with VS content [47]. Meanwhile, the lowest Rmax was obtained in the mono-AD of RS presumably due to the low pH caused by accumulation of VFAs (Fig. 3d), which could limit the conversion rate of RS.
The shortest λ was observed at the ratio of 1:0 presumably because the SM digestate was used as an inoculum, and there were more soluble degradable materials in the substrate (Table 2). Beyond that, the λ at the ratio of 1:1 was much shorter than those at other ratios, which indicated that the ratio of 1:1 could enhance the adaptation ability of microorganisms and shorten the acclimation time. Tef could predict the AD period, the conversion efficiency from organic matter to biogas, and the methane yields. Compared with other ratios, the maximum Tef of 26.92 d and the highest methane yield were obtained at the ratio of 1:1, which implied that the organic-to-methane conversion efficiency was also the highest at this ratio. The longer Tef and shorter λ indicated a higher AD efficiency and less process inhibition in the co-AD of SM and RS at a ratio of 1:1.
In addition, the correlation coefficient R2 values were all above 0.94 for the modified Gompertz model and the logistic model, which indicated that the two models could both be used for the kinetic simulation of co-AD of SM and RS. However, the differences between the measured methane yields and predicated yields of the logistic model were lower than those of the modified Gompertz model, except at the ratio of 1:0 (Table 3). Therefore, the modified Gompertz model was more accurate in terms of fitting and predicting the methane yield at the ratio of 1:0, which was consistent with the findings of Kafle and Kim [35]. In addition, the logistic model was more appropriate at other ratios, which was consistent with a previous study [18].
3.5 Kinetic study for reaction processes
The effects of the substrate mixing ratio on the reaction process of AD in this study were calculated and simulated by the modified Gompertz model and the logistic model.
The calculated and simulated reaction results are shown in Fig. 5, and the conversion potential (P0′) and maximum conversion rate (Rmax′) simulated from the two models are summarized in Table 4. According to the R2 values (Table 4), the logistic model fit better than the modified Gompertz model at ratios of 1:0, 1:1, 1:2, and 1:3, while the fitting results were opposite at ratios of 3:1, 2:1, and 0:1. Combining the best fitting results of the two models, the co-AD of SM and RS could not only accelerate the reaction rates, but also increase the hydrolysis, acidogenesis, acetogenesis, and methanogenesis potential. As shown in Table 4, the P0′ of substrates in the four steps of co-AD were all higher than those of mono-AD, and the highest P0′ was obtained at the ratio of 1:1 which was more than 2 times as high as that of mono-AD. In terms of conversion potentials in different steps, under the ratios of 1:1 and 1:0, the similar P0′ of acidogenesis, acetogenesis, and methanogenesis indicated that VFAs could be mostly converted to acetate by acetogens and then converted to methane by methanogens, thereby leaving few VFAs at the end of AD (Fig. 3d). At the same time, it could be predicted from the P0′ order of methanogenesis, acetogenesis, and acidogenesis that VFAs may accumulate at other ratios as a consequence of inappropriate nutrient ratios for methanogenesis.
The overall reaction rate of AD depends on the slowest reaction, which is called the rate-limiting step, and it was determined by comparing the Rmax′ values. The Rmax′ of the four AD steps under all ratios simulated from the two models was compared, and the order was Rmax′ (Meth.) > Rmax′ (Ace.) > Rmax′ (Acid.) > Rmax′ (Hyd.), which suggested that hydrolysis was the rate-limiting step at all mixing ratios. The lowest Rmax′ was found in the mono-AD of SM because the main component of SM is protein, and proteins generally hydrolyze at a lower rate than carbohydrates under AD [31].
The estimated P0′ (Meth.) and P0′ (Hyd.), and their 95% confidence ellipses with different ratios are shown in Fig. 6. The co-AD achieved higher P0′ (Meth.) and P0′ (Hyd.) values compared with the mono-AD of SM or RS (p < 0.05). The highest P0′ (Meth.) and P0′ (Hyd.) values were obtained at the ratio of 1:1, which was 2.37 and 2.25 times higher than those of the mono-AD of RS, respectively, and 2.12 and 2.12 times higher in comparison with those of the mono-AD of SM, respectively. The higher hydrolysis potential in co-AD may be attributable to the higher degradability of carbohydrates which is the main component of RS and synergetic effects of suitable substrate compositions. The results also showed a positive correlation (R2 = 0.9956) between P0′ (Meth.) and P0′ (Hyd.) for all the ratios [48].
4 Conclusions
The highest biogas yield (188.79 L/kg VSadded) and VS degradation rate (59.38%) were achieved from co-AD of SM and RS at the ratio of 1:1. Although the methane contents decreased with the increase in RS proportion, the highest methane yield was also obtained at the ratio of 1:1. The pH, TA, and TAN of the digestate decreased with the increase in RS ratio in the test. The kinetic results showed that co-AD at the ratio of 1:1 markedly increased the methane yields, shortened the lag time, and extended the effective methane production time. In addition, the mixing of SM with RS, especially at a ratio of 1:1, significantly improved the reaction rates of the four steps of AD.
References
Pellera F-M, Gidarakos E (2017) Anaerobic digestion of solid agroindustrial waste in semi-continuous mode: evaluation of mono-digestion and co-digestion systems. Waste Manag 68:103–119. https://doi.org/10.1016/j.wasman.2017.06.026
Neshat SA, Mohammadi M, Najafpour GD, Lahijani P (2017) Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew Sust Energ Rev 79:308–322. https://doi.org/10.1016/j.rser.2017.05.137
Patinvoh RJ, Lundin M, Taherzadeh MJ, Sárvári Horváth I (2020) Dry anaerobic co-digestion of Citrus wastes with keratin and Lignocellulosic wastes: batch and continuous processes. Waste Biomass Valorization 11:423–434. https://doi.org/10.1007/s12649-018-0447-y
Pagés-díaz J, Pereda-reyes I, Sanz JL et al (2017) A comparison of process performance during the anaerobic mono- and co-digestion of slaughterhouse waste through different operational modes. J Environ Sci 64:149–156. https://doi.org/10.1016/j.jes.2017.06.004
Makara A, Kowalski Z (2018) Selection of pig manure management strategies: case study of polish farms. J Clean Prod 172:187–195. https://doi.org/10.1016/j.jclepro.2017.10.095
Li D, Liu S, Mi L, Li Z, Yuan Y, Yan Z, Liu X (2015) Effects of feedstock ratio and organic loading rate on the anaerobic mesophilic co-digestion of rice straw and pig manure. Bioresour Technol 187:120–127. https://doi.org/10.1016/j.biortech.2015.03.040
Jurado E, Antonopoulou G, Lyberatos G, Gavala HN, Skiadas IV (2016) Continuous anaerobic digestion of swine manure: ADM1-based modelling and effect of addition of swine manure fibers pretreated with aqueous ammonia soaking. Appl Energy 172:190–198. https://doi.org/10.1016/j.apenergy.2016.03.072
Mao C, Zhang T, Wang X, Feng Y, Ren G, Yang G (2017) Process performance and methane production optimizing of anaerobic co-digestion of swine manure and corn straw. Sci Rep 7:9379. https://doi.org/10.1038/s41598-017-09977-6
Zhang T, Mao C, Zhai N, Wang X, Yang G (2015) Influence of initial pH on thermophilic anaerobic co-digestion of swine manure and maize stalk. Waste Manag 35:119–126. https://doi.org/10.1016/j.wasman.2014.09.004
Wu X, Yao W, Zhu J, Miller C (2010) Biogas and CH4 productivity by co-digesting swine manure with three crop residues as an external carbon source. Bioresour Technol 101:4042–4047. https://doi.org/10.1016/j.biortech.2010.01.052
Gu Y, Chen X, Liu Z, Zhou X, Zhang Y (2014) Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour Technol 158:149–155. https://doi.org/10.1016/j.biortech.2014.02.011
Mancini G, Papirio S, Riccardelli G, Lens PNL, Esposito G (2018) Trace elements dosing and alkaline pretreatment in the anaerobic digestion of rice straw. Bioresour Technol 247:897–903. https://doi.org/10.1016/j.biortech.2017.10.001
Beniche I, El Bari H, Siles JA et al (2020) Methane production by anaerobic co-digestion of mixed agricultural waste: cabbage and cauliflower. Environ Technol 31:1–25. https://doi.org/10.1080/09593330.2020.1770341
Wang X, Lu X, Li F, Yang G (2014) Effects of temperature and carbon-nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, Chicken Manure and Rice Straw: Focusing on Ammonia Inhibition. PLoS One 9:e97265. https://doi.org/10.1371/journal.pone.0097265
Negi S, Dhar H, Hussain A, Kumar S (2018) Biomethanation potential for co-digestion of municipal solid waste and rice straw: a batch study. Bioresour Technol 254:139–144. https://doi.org/10.1016/j.biortech.2018.01.070
Chen X, Yuan H, Zou D, Liu Y, Zhu B, Chufo A, Jaffar M, Li X (2015) Improving biomethane yield by controlling fermentation type of acidogenic phase in two-phase anaerobic co-digestion of food waste and rice straw. Chem Eng J 273:254–260. https://doi.org/10.1016/j.cej.2015.03.067
Chen H, Wu J, Wang H, Zhou Y, Xiao B, Zhou L, Yu G, Yang M, Xiong Y, Wu S (2020) Dark co-fermentation of rice straw and pig manure for biohydrogen production: effects of different inoculum pretreatments and substrate mixing ratio. Environ Technol 0:1–11. https://doi.org/10.1080/09593330.2020.1770340
Valenti F, Zhong Y, Sun M, Porto SMC, Toscano A, Dale BE, Sibilla F, Liao W (2018) Anaerobic co-digestion of multiple agricultural residues to enhance biogas production in southern Italy. Waste Manag 78:151–157. https://doi.org/10.1016/j.wasman.2018.05.037
Mao C, Wang X, Xi J, Feng Y, Ren G (2017) Linkage of kinetic parameters with process parameters and operational conditions during anaerobic digestion. Energy 135:352–360. https://doi.org/10.1016/j.energy.2017.06.050
Nguyen DD, Chang SW, Jeong SY, Jeung J, Kim S, Guo W, Ngo HH (2016) Dry thermophilic semi-continuous anaerobic digestion of food waste: performance evaluation, modified Gompertz model analysis, and energy balance. Energy Convers Manag 128:203–210. https://doi.org/10.1016/j.enconman.2016.09.066
Angelidaki I, Alves M, Bolzonella D et al (2009) Defining the biomethane potential ( BMP ) of solid organic wastes and energy crops : a proposed protocol for batch assays. Water Sci Technol 59:927–934. https://doi.org/10.2166/wst.2009.040
Zhang W, Wu S, Guo J, Zhou J, Dong R (2015) Performance and kinetic evaluation of semi-continuously fed anaerobic digesters treating food waste: role of trace elements. Bioresour Technol 178:297–305. https://doi.org/10.1016/j.biortech.2014.08.046
Bayr S, Pakarinen O, Korppoo A, Liuksia S, Väisänen A, Kaparaju P, Rintala J (2012) Effect of additives on process stability of mesophilic anaerobic monodigestion of pig slaughterhouse waste. Bioresour Technol 120:106–113. https://doi.org/10.1016/j.biortech.2012.06.009
Nordell E, Nilsson B, Nilsson Påledal S, Karisalmi K, Moestedt J (2016) Co-digestion of manure and industrial waste - the effects of trace element addition. Waste Manag 47:21–27. https://doi.org/10.1016/j.wasman.2015.02.032
Xiao B, Zhang W, Yi H, Qin Y, Wu J, Liu J, Li YY (2019) Biogas production by two-stage thermophilic anaerobic co-digestion of food waste and paper waste: effect of paper waste ratio. Renew Energy 132:1301–1309. https://doi.org/10.1016/j.renene.2018.09.030
Xiao B, Qin Y, Zhang W, Wu J, Qiang H, Liu J, Li YY (2018) Temperature-phased anaerobic digestion of food waste: a comparison with single-stage digestions based on performance and energy balance. Bioresour Technol 249:826–834. https://doi.org/10.1016/j.biortech.2017.10.084
APHA (2017) Standard methods for the examination of water and wastewater, 23rd edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington
Niu Q, Qiao W, Qiang H, Hojo T, Li YY (2013) Mesophilic methane fermentation of chicken manure at a wide range of ammonia concentration: stability, inhibition and recovery. Bioresour Technol 137:358–367. https://doi.org/10.1016/J.BIORTECH.2013.03.080
Mancini G, Papirio S, Lens PNL, Esposito G (2018) Increased biogas production from wheat straw by chemical pretreatments. Renew Energy 119:608–614. https://doi.org/10.1016/j.renene.2017.12.045
Prajapati KB, Singh R (2018) Kinetic modelling of methane production during bio-electrolysis from anaerobic co-digestion of sewage sludge and food waste. Bioresour Technol 263:491–498. https://doi.org/10.1016/j.biortech.2018.05.036
Li Q, Qiao W, Wang X, Takayanagi K, Shofie M, Li YY (2015) Kinetic characterization of thermophilic and mesophilic anaerobic digestion for coffee grounds and waste activated sludge. Waste Manag 36:77–85. https://doi.org/10.1016/j.wasman.2014.11.016
Martín MA, Fernández R, Gutiérrez MC, Siles JA (2018) Thermophilic anaerobic digestion of pre-treated orange peel: modelling of methane production. Process Saf Environ Prot 117:245–253. https://doi.org/10.1016/j.psep.2018.05.008
Hagos K, Zong J, Li D, Liu C, Lu X (2017) Anaerobic co-digestion process for biogas production: progress, challenges and perspectives. Renew Sust Energ Rev 76:1485–1496. https://doi.org/10.1016/j.rser.2016.11.184
Xie S, Lawlor PG, Frost JP, Hu Z, Zhan X (2011) Effect of pig manure to grass silage ratio on methane production in batch anaerobic co-digestion of concentrated pig manure and grass silage. Bioresour Technol 102:5728–5733. https://doi.org/10.1016/j.biortech.2011.03.009
Kafle GK, Kim SH (2013) Anaerobic treatment of apple waste with swine manure for biogas production: batch and continuous operation. Appl Energy 103:61–72. https://doi.org/10.1016/j.apenergy.2012.10.018
Xiao B, Qin Y, Wu J, Chen H, Yu P, Liu J, Li YY (2018) Comparison of single-stage and two-stage thermophilic anaerobic digestion of food waste: performance, energy balance and reaction process. Energy Convers Manag 156:215–223. https://doi.org/10.1016/j.enconman.2017.10.092
Nielfa A, Cano R, Vinot M, Fernández E, Fdz-Polanco M (2015) Anaerobic digestion modeling of the main components of organic fraction of municipal solid waste. Process Saf Environ Prot 94:180–187. https://doi.org/10.1016/j.psep.2015.02.002
Mao C, Feng Y, Wang X, Ren G (2015) Review on research achievements of biogas from anaerobic digestion. Renew Sust Energ Rev 45:540–555. https://doi.org/10.1016/j.rser.2015.02.032
Dai X, Hua Y, Dai L, Cai C (2019) Particle size reduction of rice straw enhances methane production under anaerobic digestion. Bioresour Technol 293:122043. https://doi.org/10.1016/j.biortech.2019.122043
Sun Y, Wang D, Qiao W, Wang W, Zhu T (2013) Anaerobic co-digestion of municipal biomass wastes and waste activated sludge: dynamic model and material balances. J Environ Sci 25:2112–2122. https://doi.org/10.1016/S1001-0742(12)60236-8
Lin J, Zuo J, Gan L, Li P, Liu F, Wang K, Chen L, Gan H (2011) Effects of mixture ratio on anaerobic co-digestion with fruit and vegetable waste and food waste of China. J Environ Sci 23:1403–1408. https://doi.org/10.1016/S1001-0742(10)60572-4
Shi X, Lin J, Zuo J, Li P, Li X, Guo X (2017) Effects of free ammonia on volatile fatty acid accumulation and process performance in the anaerobic digestion of two typical bio-wastes. J Environ Sci 55:49–57. https://doi.org/10.1016/J.JES.2016.07.006
Massé DI, Droste RL (2000) Comprehensive model of anaerobic digestion of swine manure slurry in a sequencing batch reactor. Water Res 34:3087–3106. https://doi.org/10.1016/S0043-1354(00)00064-6
Arias DE, Veluchamy C, Dunfield KE, Habash MB, Gilroyed BH (2020) Hygienization and microbial metabolic adaptation during anaerobic co- digestion of swine manure and corn Stover. Bioresour Technol 306:123168. https://doi.org/10.1016/j.biortech.2020.123168
Anthonisen AC, Loehr RC, Prakasam TBS, Srinath EG (1976) Inhibition of nitrification by Ammonia and nitrous acid. J (Water Pollut Control Fed) 48:835–852
Ye J, Li D, Sun Y, Wang G, Yuan Z, Zhen F, Wang Y (2013) Improved biogas production from rice straw by co-digestion with kitchen waste and pig manure. Waste Manag 33:2653–2658. https://doi.org/10.1016/j.wasman.2013.05.014
Li Y, Jin Y, Li J, Li H, Yu Z (2016) Effects of pungency degree on mesophilic anaerobic digestion of kitchen waste. Appl Energy 181:171–178. https://doi.org/10.1016/j.apenergy.2016.08.057
Wei W, Zhou X, Wang D, Sun J, Wang Q (2017) Free ammonia pre-treatment of secondary sludge significantly increases anaerobic methane production. Water Res 118:12–19. https://doi.org/10.1016/j.watres.2017.04.015
Funding
This work was supported by the Major Science and Technology Program for Water Pollution Control and Treatment of China (No. 2017ZX07106003), the China Hunan Provincial Science and Technology Department (No. 2017SK2361), and the State Key Joint Laboratory of Environment Simulation and Pollution Control (Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences) (No. 19Z02ESPCR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, W., Xiao, B., Zhang, K. et al. Effects of mixing ratios on anaerobic co-digestion of swine manure and rice straw: methane production and kinetics. Biomass Conv. Bioref. 13, 1553–1563 (2023). https://doi.org/10.1007/s13399-020-01211-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01211-0