Abstract
The first hypothetical hydrogen (H2) production from biological means was proposed in the early of nineteenth century. However, the biological H2 production technology did not received much attention until the anticipation of H2 production was practically reported through anaerobic digestion of cellulose using microbes present in the ruminant tract in 1930s. Later on, subsequent development on fermentative H2 production has been reported by researchers employing advanced technologies to the fermentative systems. The present review is envisioned to provide a technological devolvement’s towards fermentative H2 production from the late nineteenth to the present twenty-first century. The major technological aspects associated with H2 production through the fermentative process such as genetic engineering, nanomaterial implementations, immobilization techniques, and reactor configuration developments were highlighted in this review.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Indiscriminate use of conventional hydrocarbon fossil fuels and its production not only exhausted the limited reserves but imparted as a causative factor for imbalanced earth’s ecological system [1]. The development of new technologies for sustainable energy production from organic-rich waste appears to be a promising approach in recent decades, which could simultaneously resolve the need for renewable fuels and the burdens of waste management [2, 3]. Waste treatment and simultaneous biofuel (H2, CH4, C2H5OH, etc. ) production have considered a promising approach to mitigate this adverse situation [4]. In this aspect, as an alternative energy carrier, H2 could be the “fuel of the future” as it exhibits higher intrinsic combustion calorific value of 143 MJ.Kg-1 than any other hydrocarbons with environmental credentials [5, 6]. H2 is widely used for the hydrogenation of edible oil and synthesis of ammonia, which has winding its wide range of industrial applications [7, 8]. To address accelerated environmental pollutants, the quest for an advanced and economic way to produce this carbon-free gaseous fuel, various approaches have been applied till yet [5, 9]. The H2 production process is classified into two major categories: chemical-physical and biological [10, 11]. The chemical-physical processes of H2 production are limited due to various substrate characteristics and energy-exhaustive process (as required specific temperature and pressure), while the biological processes overruled these limitations.
Biological means of H2 production is considered a promising approach towards low-cost and environment-friendly fuel production with simultaneous treatment of organic wastes. The application of biological processes for H2 production was initially described in the early nineteenth century. Over a period of time, various technological advancements have been devoted to H2 production using various agro-industrial waste as sustaninable resource. The present article tends to summarize the major technological development in dark fermentative H2 production with diverse applications of genetic engineering to nanotechnological perspective based on available bibliographic literature.
2 Historical background
It was in the early nineteenth century that the first hypothetical production of biological H2 was postulated [12]. However, it was not until the 1930s, when Woodman and his co-researcher has first reported a clear insight into the production of H2 from anaerobic digestion of cellulose in the ruminant tract [13]. Since than, intensive research on H2 production is underway, and several novel approaches have been implemented to surpass drawbacks associated with them. Following sustainable development and minimization of organic waste through fermentative process, H2 is produced as a by-product during the conversion of organic waste into small organic acids with the help of H2-fermenting microbes [14].
The fermentative H2 production from algae in the presence of glucose was reported in the year of 1942 [15]. Later on, it was observed that anaerobic growth of Rhodospirillum rubrum in the absence of light causes metabolism of pyruvate (a metabolite of glucose) into H2 molecules anaerobically [16]. Thereafter, several efforts have been made to enhance the H2 production efficiency using different perspectives of microbiology including co-culture of photosynthetic bacterial species and dark fermentative bacterial species [17]; optimization of physicochemical conditions [18, 19]; application of fermentative immobilized bacterium (Rhodospirillum rubrum) [20]; use of hydrogenases enzymes in H2 metabolism [16, 21]; isolation of efficient H2 producers from various sources [22]; and employing nanotechnological approach [23, 24]. Further developments include study on the involvement of metal ions on H2, CH4, and CO production during batch anaerobic sludge digestion [25]. Moreover, the development of a stable system for the conversion of solar energy into H2 using photosynthetic microorganisms (micro-algae) was an important milestone towards microbe-based H2 production [26]. The isolation of halophilic H2-producing bacterium Haloanaerobium fermentans from pufferfish ovaries and successful application in H2 production from different organic wastes opened a new window of opportunity towards the development of a range of bacteria that have the potential to produce H2 [27, 28]. However, the major interest in H2 production by biological means has been exceptionally grown from early of the twentieth century, both in terms of application of wide range of organic waste and advancement in applied technologies. Table 1 shows some major achievements towards the fermentative H2 production.
The rapid socioeconomic development has enforced all nations to develop an alternative approach for biofuel from sustainable resouscres [45]. The global research on H2 production from sustainable sources increased significantly over the last two decades (Fig. 1). It is worth noting that the number of biological H2-oriented research articles by fermentative means has been published in the year 2000 gradually increased in a significant numbers (including review articles) till 2019. Statistics have shown that China is the one leading contributor in terms of research articles on H2 production followed by the USA and India, (Fig. 2a). As, in the early days of new China, there were limited H2-based industries, while up to the 1990s, it increases about 107.2 times than that of 1949 [46]. Besides, China’s outlook for future H2 has been proposed in a traditional feedstock growth segments and projected 60-million-ton demand by 2050 [47]. A comprehensive review on wide range of organic waste that have been used for treatment with simultaneous production of H2, the industrial waste is accounted for almost 70% (Fig. 2b). It was possibly due to growing concern over industrial effluents which negatively affecting the environmental ecosystems, but at the same, it provides an economic and viable substrate for bioenergy.
This analysis of the historical data and energy technologies proves how fermentative H2 production processes have been developed for the last 4 to 5 decades. The technological development of fermentative process for H2 production has been rapidly evolving since then. These technical advances have been referred by the International Association for Hydrogen Energy [48]. The technological advancement in the fermentative H2 production that has globally received significant consideration is particularly included genetic engineering, nanomaterial applications, immobilization technique, and bioreactor configuration.
This review summarizes the technological developments in fermentative H2 production. So far, the focus has given to the fundamental technological advancement used for improved H2 productivity. These approaches included genetic manipulation followed by nanotechnology. Further, the microbial immobilization technology used for H2 production is being reviewed. Later, the development in reactor configuration towards improved H2 productivity is discussed in this review.
3 Strategies for improving fermentative H2 production
3.1 Genetic engineering for enhanced fermentative H2 production
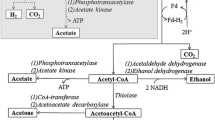
Redirection of the microbial metabolic process by limiting the production of the undesirable microbial product at the genetic level is an emerging approach to improve H2 productivity [49, 50]. The metabolic engineering approach provides enhanced H2 productivity by switching off or by alteration in particular genes that limit the H2 production [51]. Nath and Das (2004) summarized the possible genetic engineering approach to improve H2 production which includes (a) overexpression of H2 evolving hydrogenases, (b) elimination of uptake hydrogenases, and (c) overexpression of cellulases, hemicellulases, and ligninases enzymes that help to maintain substrate availability [52]. Two well-characterized metabolic pathways for H2 production are the formate pathway and nicotinamide adenine dinucleotide (NADH) pathway. Both pathways have been independently investigated by researchers and reported the existence of a linear relationship between the H2 yield with the relative change in NADH pathways [53]. Formate metabolic pathways are catalyzed by pyruvate formate lyase (PFL) and formate hydrogen lyase (FHL) enzyme complexes. The FHL enzyme complex is the core enzyme of formate pathway that further comprises of formate dehydrogenase (FDH) and hydrogenase. Most of the genetic manipulations have been performed on FHL-related genes to regulate the formate pathway and increase the production of H2, as observed in Fig. 3 [54, 55].
H2 production oriented metabolic pathways and genetic enegineering apparoches in E. coli, Adapted from [29]. PEP phosphoenol pyruvate, PFL pyruvate formate lyase, FHL formate hydrogen lyase, LDH lactate dehydrogenase
The successful increased H2 production through in vivo genetic engineered modes using E. coli strains have been investigated by several researchers and comprehensively reviewed by Maeda and his co-authors [51]. Such metabolic modification included the over-expression of particular genes such as cellulases, hemicellulases, and ligninases which increases the complex carbohydrate consuming ability of microbial strains and resulted in increased H2 productivity [56]. Research on targeted regulation of NADH-based metabolic pathways to increase H2 production also has been reported [57]. The reduction of ferredoxin with NADH using reverse electron flow has been anticipated to produce enough reducing power to enhance H2 production by hydrogenases [58]. The major fermentative microorganisms used in the dark fermentation system are E. coli [59], Clostridium sp. [44], Enterobacter sp.[60], and Bacillus sp. [61]. Applications of E. coli and its genetically modified strains were reported for the capability to use maltodextrins as carbon sources plus oversecretion of endogenous alpha amylase [62]. Another attempt of mutant E. coli, HD701, has been reported for unregulated hydrogenase strain that has engineered to metabolize sucrose as feedstocks for H2 production as an alternative to coupling-in and upstream invertase [63]. In a study, mutant E. coli with deleted uptake hydrogenase △hyaAB and △hybABC has reported an increase in H2 yield by 10% over the wild-type strain of BW25113 from glucose. The deletion of lactate dehydrogenase (IdhA) and fumarate reductase (frdBC) increases the H2 yield by 22 and 23%, respectively, in the mixed-acid fermentation pathways [64]. When the Clostridium species were fostered by disabling the uptake of hydrogenases enzyme, it has been reported for more robust H2 production incompared to the wild one [65]. The transcriptomic and proteomic analysis of Clostridium butyricam CWBI1009 was studied by Calussinska et al. where they have provided a bio-molecular overview of the changes that occur during the metabolism shift of H2 production [66]. Metabolically engineered mutant with an inactivated ack gene, which encodes acetate kinase in Clostridium sp. for the inhibition of acetate pathways, was investigated to improve H2 production. Study reported 50% of more H2 by mutant Clostridium sp. than the wild type of strain from glucose [67]. Besides, developing a O2 tolerant H2 producing strain and selectively inactivating the genes to prevent O2 interference with this enzyme’s activity also have been reported for increased H2 production [68, 69]. Thermococcus onnurineus NA1, a genetically modified FrhAGB encoding gene is reported increased H2 produced by increasing its O2 tolerance activity. This strain was able to overcome the inhibitory effects of O2 and demonstrated increased microbial growth and H2 production under oxic conditions [70]. Further, approach to improve H2 production has been reported by altering microbial genes which compete or interferes with the H2 producing metabolic pathways [71]. The genetic manipulation efforts have accelerated the understanding of the H2 research area by providing a deep insight into complex interactions taking place between the various metabolic pathways and hydrogenase enzymes. Evidently, the genetic manipulation of H2-producing microbes seems an effective approach for improved H2 production. It is anticipated that the genetic manipulation will not only help to improve H2 productivity but also it can help to predict a pattern for H2 producers and which will provide new insight on metabolic alteration. In addition, the data mining of microbial genomic and metagenomic sequences could also lead the researchers to revolutionize H2 industries near the future.
3.2 Nanotechnology-based approaches for enhanced fermentative H2 production
The unique physical and chemical properties of NPs are well known for its improved biocatalytic activities in fermentative system [72]. The additive of nano-scaled macro- and micronutrients to the fermentative medium has gained a new direction to heighten H2 productivity by accelerating the microbial bioactivity in different pathways as depicted in Fig. 4 [73]. Hydrogenase and Nitrogenase are considered as key enzymes which are responsible for the microbial H2 production [74] and the presence of metal ions (e.g., Ni, Fe ) at its active get influenced by additive NPs to the culture medium [23, 75].
Over the last few years, several studies have been reported for advanced nanometals and their oxides and investigated its applications for the advancement of fermentative H2 production [5, 76,77,78]. The remarkable assortment of novel structure and exceptional catalytic activity of nanoscale materials has been investigated by several researchers to increase the production of H2 through fermentative process [79]. Among the abundance of nanoscale materials, Ag-oxides [80], Au-oxides [81], CuO2, Fe, Fe2O3, Fe3O4, Ni, NiO, CoO [75], Pd-oxides, SiO2, carbon nanotubes, and TiO2 have been investigated and used as the catalyst for fermentative H2 production. Zhang and Shen have investigated (for the first time as claimed by the authors) the application of gold oxide nanoparticles and concluded that the addition of 5-nm gold nanoparticles resulted in 46% higher H2 productivity from artificial wastewater [82]. The improvement in the yield was explained by the hypothesis that the gold nanoparticles acted as electron-sink due to their higher affinity for electrons, which facilitated the further reduction of protons to molecular H2 in the fermentative medium.
The NPs also behave as an antimicrobial agents as it can easily penetrates the cell membrane and causes cell lysis [83]. Therefore, the immobilization of nanoparticles showed a positive impact on microbial H2 production. A significant increase in H2 yield has been reported by the addition of nanoparticle of Pd, Ag, Cu, and Fe oxides immobilized in a porous matrix of silica [84]. Taherdanak et al. [63] investigated the effect of zero-valent Fe and Ni compared with Fe2+ and Ni2+ nanoparticles (in the range of 0–50 mg/L) on H2 production using glucose as carbon source and heat-shocked anaerobic sludge as inoculum. The results demonstrated a significant increase in H2 yield of 55 and 15%, while the fermentative medium was supplemented with Ni2+ and Fe2+ nanoparticles, respectively [85]. Moreover, the addition of NiO2 and CoO2 nanoparticles to the substrate have reported substantial increase in H2 production by 1.51- and 1.61-fold, respectively [75]. Zho et al. [58] reported a 67.6% increase in the H2 yield using 20 nM Ag-oxide nanoparticles in the medium using glucose as the carbon source and C. butyricum dominated mixed culture as inoculum. Taherdanak et al.[63] described the comparative impact of Fe ions and Fe2+ nanoparticles as supplements (0–50 mg/L) in the fermentative medium containing glucose as substrate and anaerobic sludge as inoculum. A 37% increase in H2 yield was reported with the addition of 52 mg/L of Fe2+ nanoparticles [85]. In addition to these, several nanoparticles of metal ions and oxides have been studied by using different carbon sources and a profound effect on H2 yield enhancement was observes as presented in Table 2.
These nanoparticles mostly increase the H2 production through their substantially effects on the microbial growth, substrate conversion efficiency, and microbial metabolic profile (Fig. 4). It is believed that in the presence of nanoparticles, H2 producer shifts intermediate metabolites towards the higher production of organic acids including acetate and butyrate and reduces the production of alcohol (an inhibitor to H2 production) [52]. However, the uncertainties on optimal concentrations of nanoparticles are still in the quest as the minimal toxicity of nanoparticle on fermentative microbes is of prime requirement. The metalloenzymes need optimal dosages to balance their catalytic activities as well as prevents feedback inhibitions [77]. Further, the identification of novel nanoparticle with significant physicochemical properties from economic sources and their impact on H2 production need to be explored for improved H2 production.
3.3 Immobilization for enhanced fermentative hydrogen production
Immobilization technologies are in existence for many decades and suceesfully applied in various sectors including wastewater treatment, pharmaceuticals, and food industries [92]. The immobilized culture has distinguished property as they cannot move independently in aqueous media which helps to maintain enough biomass concentration in the fermentative medium [11]. The matrices used for microbial immobilization which are inert nature assist in the adsorption of specific nutrients from organic waste during fermentative H2 production [93]. The immobilization can catagorized as entrapment in polymers, confinement in the liquid-liquid emulsion, affinity immobilization, adsorption and covalent coupling [94]. These immobilizations further grouped as “active” (chemical attachment, flocculant agents, and gel encapsulation) and “passive” immobilization (by using microbial natural tendency to attach with the surfaces-natural or synthetic and grow on them) [95]. The schematic representation of the immobilization techniques is illustrated in Fig. 5. Various cell immobilization processes have been adopted to improve H2 productiviton in a continuous system, including biomass immobilization, adsorption to the solid surface, biofilms, granules, and entrapment in polymeric gels. The entrapment of fermentative inoculum within the carrier matrix is a widely used system for providing an adequate anaerobic environment for microbial processes and to improve the H2 productivity [93].
Methods employed for microbial H2 production. a Cell or enzyme immobilization by adsorption/attached to the surface of the matrix. b Immobilization through entrapment/microencapsulation of cell or enzyme in porous matrix. c Covalent binding of cell or enzyme to the nanoparticles. d Covalent cross-linking of cell or enzyme
Recently, the fermentative H2 production using immobilized inoculum have been reported in various studies, as it limits the fermentative medium contamination by unwanted microbes and it also helps to stabilize the inoculum proportions in the medium by preventing cell washout [96, 97]. As the H2 production by using suspended culture is prone to washout during continuous mode, the immobilized culture maintains the culture stability and result improved H2 productivity [98]. Singh et al. have reported the improved H2 production of 380 mLH2/g-COD consumed using Clostridium butyricum LS2 culture immobilized polyethylene glycol in continuous mode at hydraulic retention time (HRT) of 16 h [99]. In another study, threefold increase in H2 production has been reported when the mixed microflora was immobilized in alginate beads supplemented with chitosan and titanium oxides [100]. The increase carbohydrate consumption of 88% with maximum H2 yield of 2.1 mLH2/mL-POME (palm oil mill effluent) has been reported by Ismail et al., when POME wastewater was fermented under a continuous mode for H2 production [101]. Acclimatized sludge immobilized into the composite polymeric matrix (polymethyl methacrylate/collagen/activated carbon) has reporte a significant increase in H2 production from 1.21 mLH2/mL/h (suspended system) to 1.80 mLH2/mL/h (immobilized system) under relatively low organic loading rate (OLR) from synthetic wastewater [102]. Further, the improved H2 production have reported by Zhao et al., when they performed a continuous mode of fermentation using Clostridium sp. T2 immobilized on mycelia pellets. The maximum H2 production rate of 61 mL H2/L/h was reported at HRT of 10 h compared with the suspended one [103]. The number of researchers has been reported the effectiveness of immobilized microbial cells for the enhanced production of H2 as depicted in Table 3.
The advantages associated with H2 production using immobilized inoculum systems are well established which include reduced risk of microbial contamination, high cell density maintenance biocatalyst recycling, and increased rate of productivity. However, the reported matrices used for immobilization were synthetic polymers or inorganic materials which possess disposal problem and often toxic to microorganisms. Therefore, cheap, organic, non-toxic, and environmentally friendly matrices should be explored in near future to improve the H2 production. Moreover, the development of genetically engineered tailored for immobilization and implementation of innovative strategies could be the progressive advancements towards the improved H2 productivity.
3.4 Bioreactor configurations and fermentative H2 prodution
Bioreactor configuration affects the microbial homogeneity, hydrodynamic activities, bioprocess activity, substrate accessibility to the microbes, microbial population, mode of operation, etc, [110]. However, every bioreactor exhibits its own benefits and drawbacks. The H2 yield and substrate conversion rate by H2 producers are highly influenced by the reactor type and its operating conditions [111]. Various researches have been investigated for H2 production using the diverse range of bioreactor technologies and concluded that the H2 productivity is not only dependent on bioreactor type but also dependent on the modification tailored for the particular purpose. The reactors tailored for H2 production can be categorized into suspended and immobilized bioreactors. Continuous stirred tank reactor (CSTR), anaerobic membrane bioreactor (AnMBR), and anaerobic sequencing batch reactor (ASBR) are the suspended bioreactors, while upflow anaerobic sludge bioreactor (UASBr), anaerobic fluidized bed reactor (AFBR), and expanded granular sludge bed reactor (EGSBr) are immobilized bioreactors as shown in Fig. 6 [112]. The major advantages and disadvantages of different types of bioreactors for H2 production are listed in Table 4. Generally, the most H2 production experimentation process is accomplished in batch mode bioreactor for lab-scale purposes and continuous type bioreactor for industrial scale [96]. Besides, CSTR has been widely used for a long time fermentative H2 production process both at the lab-scale as well as industrial scale. However, over the time the application of CSTR has declined due to its limitations of biomass washout and short retention time [113].
High sensitivity to the physical conditions (including temperature, pH, HRT) and poor biomass settling are the major constraints of CSTR, which limits it to large-scale production of H2 in continuous mode [114]. Suspended cell bioreactors and CSTR are found to be mostly used bioreactors, while UASBr and AFBR have become popular for their higher H2 production potential [110]. Various reactor designs have been evaluated to examine the continuous H2 production using granular sludge in UASBr and CSTR. The higher production rate of H2 during the continuous process in AFBR, CSTR, and UASBr is mainly correlated with the biomass concentration which influences the reactor performance [115]. CSTR has a relatively short retention period as compared with other reactor types including UASBr because of the better mass transfer performance. However, it requires continuous supervision to prevent cell deposition and its washout at inadequate operating parameters. The washout problem has been troubleshot by performing the fermentative process using membrane bioreactor and by immobilizing the sludge or inoculum in suitable supporting materials (e.g., fixed-bed bioreactors) [96]. The application of UASBr is a promising approach for improved H2 productivity and to treat high-strength organic wastewater. The granulated sludge applied in UASBr can retain maximum inoculum/microorganism, which helps in waste stabilization. In addition, efficient particle separation, high OLR, short HRT, and low set-up space requirement are the features of UASBr which make it an ideal reactor for harnessing H2 by improved productivity. These alternatives demonstrated the process to be more robust and economic with enhanced H2 productivity [111]. The advancement in reactor development would make a worthwhile contribution to overcome the limitations in H2 production and to increase the potential of fermentative H2 production from organic waste. Somehow, the knowledge of adequate configuration is still a prerequisite for optimum process conditions and performance. Recurring this will not only resolve the H2 energy concern but also by economic and environmental means.
4 Conclusive remarks and future prospect
The extensive research on the technological development of fermentative processes in the past three decades has shown the promising improvement in H2 productivity from different types of substrate. A technological breakthrough can be observed with the incorporation of genetic engineering, nanoscale technology, immobilization techniques, and advancement in rector configuration into fermentation technology. To improve the H2, it is important to use highly efficient genetically engineered microorganisms such Clostridium sp. becomes promising trends. Considering the benefits of nanoparticles, various research has been demonstrated for improved H2 productivity under controlled laboratory scale experimentations. Although, the nanoparticles exhibit microbial toxicity the optimum concentrations could drastically influence the H2 productivity. Besides, the use of microbial immobilization for H2 production have evidented beneficial as it increases operational stability, minimizes the contaminations, and extends the fermentation period which subsequently increases the H2 productivity. The H2 production effected by the configurations of bioreactors along with operating conditions. Although various configured reactors are known for H2 production, CSTRs are the widely used bioreactors for H2 production in continuous mode due to its relatively simple, ease of monitoring, and rapid start-up phase. The future research on cost-effective scaling up and broadening H2 production on industrial level needs to be focused on the development of highly active genetically modified H2 producer and new insights on immobilization techniques and matrix. The design and configuration of industrial scale H2 production specialized reactor development is expected to be more effective for H2 production.
References
Kumar R, Strezov V, Weldekidan H, He J, Singh S, Kan T, Dastjerdi B (2020) Lignocellulose biomass pyrolysis for bio-oil production: a review of biomass pre-treatment methods for production of drop-in fuels. Renew Sust Energ Rev. 123:109763
Grosspietsch D, Saenger M, Girod B (2019) Matching decentralized energy production and local consumption: a review of renewable energy systems with conversion and storage technologies. Wiley Interdisciplinary Reviews: Energy and Environment. 8(4):336
Srivastava RK, Shetti NP, Reddy KR, Aminabhavi TM (2020) Biofuels, biodiesel and biohydrogen production using bioprocesses. A review. Environ Chem Lett 18(4):1049–1072
Qazi A, Hussain F, Rahim NA, Hardaker G, Alghazzawi D, Shaban K, Haruna K (2019) Towards sustainable energy: a systematic review of renewable energy sources, technologies, and public opinions. IEEE Access. 23(7):63837–63851
Mishra P, Krishnan S, Rana S, Singh L, Sakinah M, Ab Wahid Z (2019) Outlook of fermentative hydrogen production techniques: an overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strateg Rev 24:27–37
Singh S, Bahari MB, Abdullah B, Phuong PT, Truong QD, Vo DV, Adesina AA (2018) Bi-reforming of methane on Ni/SBA-15 catalyst for syngas production: Influence of feed composition. Int J Hydrog Energy. 43(36):17230–17243
Ghimire A, Frunzo L, Pirozzi F, Trably E, Escudie R, Lens PN, Esposito G (2015) A review on dark fermentative biohydrogen production from organic biomass: process parameters and use of by-products. Appl Energy 144:73–95
Siang TJ, Singh S, Omoregbe O, Bach LG, Phuc NH, Vo DV (2018) Hydrogen production from CH4 dry reforming over bimetallic Ni–Co/Al2O3 catalyst. J Energy Inst 91(5):683–694
Kumar R, Strezov V, Kan T, Weldekidan H, He J, Jahan S (2019) Investigating the Effect of Mono-and Bimetallic/Zeolite Catalysts on Hydrocarbon Production during Bio-oil Upgrading from Ex Situ Pyrolysis of Biomass. Energ Fuel. 34(1):389–400
Singh S, Kumar R, Setiabudi HD, Nanda S, Vo DV (2018) Advanced synthesis strategies of mesoporous SBA-15 supported catalysts for catalytic reforming applications: A state-of-the-art review. Appl Catal A-Gen. 559:57–74
Mishra P, Ab Wahid Z, Zaid RM, Rana S, Tabassum S, Karim A, Singh L, Islam MA, Jaing X, Sakinah M (2020) Kinetics and statistical optimization study of bio-hydrogen production using the immobilized photo-bacterium. Biomass Convers Bior 10:1–12
Benemann J (1996) Hydrogen biotechnology: progress and prospects. Nat Biotechnol 14(9):1101
Woodman H, Evans R (1938) The mechanism of cellulose digestion in the ruminant organism: IV. Further observations from in vitro studies of the behaviour of rumen bacteria and their bearing on the problem of the nutritive value of cellulose. The J Agricultural Sci. 28(1):43–63
Hallenbeck PC, Ghosh D (2009) Advances in fermentative biohydrogen production: the way forward? Trends Biotechnol. 27(5):287–297
Gaffron H, Rubin J (1942) Fermentative and photochemical production of hydrogen in algae. The Journal of General Physiology 26(2):219–240
Gorrell T, Uffen R (1977) Fermentative metabolism of pyruvate by Rhodospirillum rubrum after anaerobic growth in darkness. J Bacteriol. 131(2):533–543
Miyake J, Mao XY, Kawamura SU (1984) Photoproduction of hydrogen from glucose by a co-culture of a photosynthetic bacterium and Clostridium butyricum. J Ferment Technol. 62(6):531–535
Heyndrickx M, De Vos P, Hibau B, Stevens P, De Ley J (1987) Effect of various external factors on the fermentative production of hydrogen gas from glucose by Clostridium butyricum strains in batch culture. Syst Appl Microbiol. 9:163–168
Stewart CS, MCPHERSON CA, Cansunar E (1987) The effect of lasalocid on glucose uptake, hydrogen production and the solubilization of straw by the anaerobic rumen fungus Neocallimastix frontalis. Lett Appl Microbiol. 5(1):5–7
Ferraiolo G, Del Borghi M, Solisio C, Gardi G (1984). Optimization criteria for the stabilization of sewage sludge and biogas production through anaerobic digestion: an example of an environmental biotechnology application. InHazardous and Industrial Waste Management and Testing: Third Symposium ASTM International.
Mahro B, Küsel AC, Grimme LH (1986) The significance of hydrogenase activity for the energy metabolism of green algae: anaerobiosis favours ATP synthesis in cells of Chlorella with active hydrogenase. Arch Microbiol. 144(1):91–95
Yokoi H, Tokushige T, Hirose J, Hayashi S, Takasaki Y (1998) H2 production from starch by a mixed culture of Clostridium butyricum and Enterobacter aerogenes. Biotechnol Lett. 20(2):143–147
Mishra P, Thakur S, Mahapatra DM, Ab Wahid Z, Liu H, Singh L (2018) Impacts of nano-metal oxides on hydrogen production in anaerobic digestion of palm oil mill effluent–A novel approach. Int J Hydrog Energy 43(5):2666–2676
Chen KF, Li S, Zhang WX (2011) Renewable hydrogen generation by bimetallic zero valent iron nanoparticles. Chem Eng J. 170(2-3):562–567
Hickey RF, Vanderwielen J, Switzenbaum MS (1989) The effect of heavy metals on methane production and hydrogen and carbon monoxide levels during batch anaerobic sludge digestion. Water Res 23(2):207–218
Kumar V, Kothari R, Pathak VV, Tyagi SK (1995) Optimization of substrate concentration for sustainable biohydrogen production and kinetics from sugarcane molasses: Experimental and economical assessment. Waste Biomass Valoriz. 36:903–906
Kobayashi T, Kimura B, Fujii T (2000) Haloanaerobium fermentans sp. nov., a strictly anaerobic, fermentative halophile isolated from fermented puffer fish ovaries. Int J Syst Evol Microbiol. 50(4):1621–1627
Kumar AN, Bandarapu AK, Mohan SV (2019) Microbial Electro-hydrolysis of Sewage Sludge for Acidogenic Production of Biohydrogen, Volatile Fatty Acids and Struvite. Chem Eng J 374:1264–1274
Barker HA (1936) On the biochemistry of the methane fermentation. Archiv für Mikrobiologie 7(1-5):404–419
Woodman H (1930) The rgle of cellulose in nutrition. Biol Rev 5(4):273–295
Winter J (1984) Anaerobic waste stabilization. Biotechnol Adv. 2(1):75–99
Von Feiten P, Zürrer H, Bachofen R (1985) Production of molecular hydrogen with immobilized cells of Rhodospirillum rubrum. Appl Microbiol Biot. 23(1):15–20
Tanisho S, Suzuki Y, Wakao N (1987) Fermentative hydrogen evolution by Enterobacter aerogenes strain E. 82005. Int J Hydrog Energy 12(9):623–627
Schropp SJ, Schwarz JR, LaRock PA (1987) Hydrogen production potential of fermentative microorganisms from the Sargasso Sea. Geomicrobiol J. 5(2):149–158
Sparling R, Daniels L (1987) The specificity of growth inhibition of methanogenic bacteria by bromoethanesulfonate. Can J Microbiol 33(12):1132–1136
Yokoi H, Mori S, Hirose J, Hayashi S, Takasaki Y (1988) H2 production from starch by a mixed culture of Clostridium butyricum and Rhodobacter sp. M [h] 19. Biotechnol Lett. 20(9):895–899
Noike T, Mizuno O (2000) Hydrogen fermentation of organic municipal wastes. Water Sci Technol. 42(12):155–162
Fang HH, Liu H, Zhang T (2002) Characterization of a hydrogen-producing granular sludge. Biotechnol Bioeng. 78(1):44–52
Kotay SM, Das D (2007) Microbial hydrogen production with Bacillus coagulans IIT-BT S1 isolated from anaerobic sewage sludge. Bioresour Technol. 98(6):1183–1190
Ren N, Chua H, Chan ST, Sang Y, Wang Y, Sin N (2007) Assessing optimal fermentation type for bio-hydrogen production in continuous-flow acidogenic reactors. Bioresour Technol 98(9):1774–1780
Singh L, Siddiqui MF, Ahmad A, Rahim MH, Sakinah M, Wahid ZA (2013) Biohydrogen production from palm oil mill effluent using immobilized mixed culture. J Ind Eng Chem. 19(2):659–664
Maeda T, Sanchez-Torres V, Wood TK (2007) Enhanced hydrogen production from glucose by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 77(4):879–890
Jung KW, Kim DH, Kim SH, Shin HS (2011) Bioreactor design for continuous dark fermentative hydrogen production. Bioresour Technol 102(18):8612–8620
Mishra P, Thakur S, Singh L, Ab Wahid Z, Sakinah M (2016) Enhanced hydrogen production from palm oil mill effluent using two stage sequential dark and photo fermentation. I Int J Hydrog Energy 41(41):1843–18440
Saratale GD, Saratale RG, Banu JR, Chang JS (2019) Biohydrogen production from renewable biomass resources. In: Pandey A (ed) Biomass, Biofuels and Biochemical: Biohydrogen, Second Edition, Elsevier, pp. 247–277
Mishra P, ab Wahid Z, Singh L, Zaid RM, Tabassum S, Sakinah M, Jiang X (2021) Synergistic effect of ultrasonic and microwave pretreatment on improved biohydrogen generation from palm oil mill effluent. Biomass Convers. Biorefin 12:1–8
Christoffersen G (2019) The rise of China in the global energy governance: an analysis of China's International Energy Policy. China Perspectives 2:15–24
Barbir F (2010) International association for hydrogen energy. In: Tietje C (ed) Handbook of Transnational Economic Governance Regimes, Brill Nijhoff Press, Leiden, Netherlands, pp 915–921
Yi KB, Harrison DP (2005) Low-pressure sorption-enhanced hydrogen production. Ind Eng Chem Res. 44(6):1665–1669
Kumar R, Kumar P (2018) Microbial fuel cells for wastewater treatment, bioremediation, and bioenergy production. In: Chandra P (ed) Advances in Microbial Biotechnology: Current Trends and Future Prospects. Apple Academic Press, Taylor & Francis Group, USA
Maeda T, Sanchez-Torres V, Wood TK (2008) Metabolic engineering to enhance bacterial hydrogen production. Microb Biotechnol 1(1):30–39
Nath K, Das D (2004) Improvement of fermentative hydrogen production: various approaches. Appl Microbiol Biotechnol 65(5):520–529
Müller M, Mentel M, van Hellemond JJ, Henze K, Woehle C, Gould SB, Yu RY, van der Giezen M, Tielens AG, Martin WF (2012) Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol. Mol. Biol. Rev. 76(2):444–495
McDowall JS, Murphy BJ, Haumann M, Palmer T, Armstrong FA, Sargent F (2014) Bacterial formate hydrogenlyase complex. Proc Natl Acad Sci. 111(38):E3948–E3956
Maeda T, Sanchez-Torres V, Wood TK (2012) Hydrogen production by recombinant Escherichia coli strains. Microb Biotechnol. 5(2):214–225
Vardar-Schara G, Maeda T, Wood TK (2008) Metabolically engineered bacteria for producing hydrogen via fermentation. Microb Biotechnol. 1(2):107–125
Zhao H, Lu Y, Wang L, Zhang C, Yang C, Xing X (2015) Disruption of lactate dehydrogenase and alcohol dehydrogenase for increased hydrogen production and its effect on metabolic flux in Enterobacter aerogenes. Bioresour Technol. 194:99–107
Baeyens J, Zhang H, Nie J, Appels L, Dewil R, Ansart R, Deng Y (2020) Reviewing the potential of bio-hydrogen production by fermentation. Renew Sust Energ Rev. 131:110023
Bisaillon A, Turcot J, Hallenbeck PC (2006) The effect of nutrient limitation on hydrogen production by batch cultures of Escherichia coli. Int J Hydrog Energy 31(11):1504–1508
Maru B, López F, Kengen S, Constantí M, Medina F (2016) Dark fermentative hydrogen and ethanol production from biodiesel waste glycerol using a co-culture of Escherichia coli and Enterobacter sp. Fuel 186:375–384
Mishra P, Thakur S, Singh L, Krishnan S, Sakinah M, Ab-Wahid Z (2017) Fermentative hydrogen production from indigenous mesophilic strain Bacillus anthracis PUNAJAN 1 newly isolated from palm oil mill effluent. Int J Hydrog Energy 42(25):16054–16063
Rosales-Colunga LM, Martínez-Antonio A (2014) Engineering Escherichia coli K12 MG1655 to use starch. Microb Cell Fact. 13(1):74
Penfold D, Macaskie L (2004) Production of H 2 from sucrose by Escherichia coli strains carrying the pUR400 plasmid, which encodes invertase activity. Biotechnol Lett. 26(24):1879–1883
Mathews J, Li Q, Wang G (2010) Characterization of hydrogen production by engineered Escherichia coli strains using rich defined media. Biotechnol Bioprocess Eng. 15(4):686–695
Show K, Lee D, Tay J, Lin C, Chang JS (2012) Biohydrogen production: current perspectives and the way forward. Int J Hydrog Energy 37(20):5616–15631
Calusinska M, Hamilton C, Monsieurs P, Mathy G, Leys N, Franck F, Joris B, Thonart P, Hiligsmann S, Wilmotte A (2015) Genome-wide transcriptional analysis suggests hydrogenase-and nitrogenase-mediated hydrogen production in Clostridium butyricum CWBI 1009. Biotechnol Biofuels. 8(1):27
Liu X, Zhu Y, Yang ST (2006) Construction and characterization of ack deleted mutant of Clostridium tyrobutyricum for enhanced butyric acid and hydrogen production. Biotechnol Prog. 22(5):1265–1275
Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M (2000) Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green AlgaChlamydomonas reinhardtii. Plant Physiol. 122(1):127–136
Stapleton JA, Swartz JR (2010) Development of an in vitro compartmentalization screen for high-throughput directed evolution of [FeFe] hydrogenases. PLoS one 5(12):15275
Le SH, Kim MS, Kang SG, Lee HS (2019) Biohydrogen production of obligate anaerobic archaeon Thermococcus onnurineus NA1 under oxic conditions via overexpression of frhAGB-encoding hydrogenase genes. Biotechnol Biofuels. 12(1):24
Saady NMC (2013) Homoacetogenesis during hydrogen production by mixed cultures dark fermentation: unresolved challenge. Int J Hydrog Energy 38(30):13172–13191
Patel SK, Lee JK, Kalia VC (2018) Nanoparticles in biological hydrogen production: an overview. Indian J Microbiol. 58(1):8–18
Yang G, Wang J (2018) Various additives for improving dark fermentative hydrogen production: A review. Renew Sust Energ Rev 95:130–146
Srivastava N, Srivastava M, Malhotra BD, Gupta VK, Ramteke P, Silva RN, Shukla P, Dubey KK, Mishra P (2019) Nanoengineered cellulosic biohydrogen production via dark fermentation: A novel approach. Biotechnol Adv. 37(6):107384
Mishra P, Singh L, Islam MA, Nasrullah M, Sakinah AM, Ab-Wahid Z (2019) NiO and CoO nanoparticles mediated biological hydrogen production: Effect of Ni/Co oxide NPs-ratio. Bioresour Technol Rep. 5:364–368
Kumar G, Mathimani T, Rene ER, Pugazhendhi A (2019) Application of nanotechnology in dark fermentation for enhanced biohydrogen production using inorganic nanoparticles. I Int J Hydrog Energy. 44(26):13106–13113
Shanmugam S, Hari A, Pandey A, Mathimani T, Felix L, Pugazhendhi A (2020) Comprehensive review on the application of inorganic and organic nanoparticles for enhancing biohydrogen production. Fuel 270:117453
Zhang Q, Li Y, Jiang H, Liu Z, Jia Q (2020) Enhanced biohydrogen production influenced by magnetic nanoparticles supplementation using enterobacter cloacae. Waste Biomass Valorization 13:1–9
Mudhoo A, Torres-Mayanga PC, Forster-Carneiro T, Sivagurunathan P, Kumar G, Komilis D, Sánchez A (2018) A review of research trends in the enhancement of biomass-to-hydrogen conversion. Waste Manag. 79:580–594
Zhao W, Zhang Y, Du B, Wei D, Wei Q, Zhao Y (2013) Enhancement effect of silver nanoparticles on fermentative biohydrogen production using mixed bacteria. Bioresour Technol 142:240–245
Khan MM, Lee J, Cho MH (2013) Electrochemically active biofilm mediated bio-hydrogen production catalyzed by positively charged gold nanoparticles. Int J Hydrog Energy. 38(13):5243–5250
Zhang Y, Shen J (2007) Enhancement effect of gold nanoparticles on biohydrogen production from artificial wastewater. Int J Hydrog Energy 32(1):17–23
Mittal AK, Kumar S, Banerjee UC (2014) Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J Colloid Interface Sci. 431:194–199
Beckers L, Hiligsmann S, Lambert SD, Heinrichs B, Thonart P (2013) Improving effect of metal and oxide nanoparticles encapsulated in porous silica on fermentative biohydrogen production by Clostridium butyricum. Bioresour Technol. 133:109–117
Taherdanak M, Zilouei H, Karimi K (2015) Investigating the effects of iron and nickel nanoparticles on dark hydrogen fermentation from starch using central composite design. Int J Hydrog Energy. 40(38):12956–12963
Han H, Cui M, Wei L, Yang H, Shen J (2011) Enhancement effect of hematite nanoparticles on fermentative hydrogen production. Bioresour Technol. 102(17):7903–7909
Mohanraj S, Anbalagan K, Kodhaiyolii S, Pugalenthi V (2014) Comparative evaluation of fermentative hydrogen production using Enterobacter cloacae and mixed culture: Effect of Pd (II) ion and phytogenic palladium nanoparticles. J Biotechnol 192:87–95
Mohanraj S, Anbalagan K, Rajaguru P, Pugalenthi V (2016) Effects of phytogenic copper nanoparticles on fermentative hydrogen production by Enterobacter cloacae and Clostridium acetobutylicum. Int J Hydrog Energy 41(25):10639–10645
Mullai P, Yogeswari M, Sridevi K (2013) Optimisation and enhancement of biohydrogen production using nickel nanoparticles–A novel approach. Bioresour Technol. 141:212–219
Malik SN, Pugalenthi V, Vaidya AN, Ghosh PC, Mudliar SN (2014) Kinetics of nano-catalysed dark fermentative hydrogen production from distillery wastewater. Energy Procedia. 54:417–430
Reddy K, Nasr M, Kumari S, Kumar S, Gupta SK, Enitan AM, Bux F (2017) Biohydrogen production from sugarcane bagasse hydrolysate: effects of pH, S/X, Fe 2+, and magnetite nanoparticles. Environ Sci Pollut Res Int. 24(9):8790–8804
Sekoai PT, Awosusi AA, Yoro KO, Singo M, Oloye O, Ayeni AO, Bodunrin M, Daramola MO (2018) Microbial cell immobilization in biohydrogen production: a short overview. Crit Rev Biotechnol. 38(2):157–171
Singh L, Wahid ZA, Siddiqui MF, Ahmad A, Rahim MHA, Sakinah M (2013) Biohydrogen production from palm oil mill effluent using immobilized Clostridium butyricum EB6 in polyethylene glycol. Process Biochem. 48(2):294–298
Martins SCS, Martins CM, Fiúza LMCG, Santaella ST (2013) Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial wastewater. Afr J Biotechnol 12(28)
Vasilieva S, Lobakova E, Lukyanov A, Solovchenko A (2016) Immobilized microalgae in biotechnology. Moscow Univ Biol Sci Bull. 71(3):170–176
Singh L, Wahid ZA (2015) Methods for enhancing bio-hydrogen production from biological process: a review. J Ind Eng Chem. 21:70–80
Boshagh F, Rostami K, Moazami N (2019) Biohydrogen production by immobilized Enterobacter aerogenes on functionalized multi-walled carbon nanotube. Int J Hydrog Energy 44(28):14395–14405
Kourkoutas Y, Bekatorou A, Banat IM, Marchant R, Koutinas A (2004) Immobilization technologies and support materials suitable in alcohol beverages production: a review. Food Microbiol. 21(4):377–397
Singh L, Siddiqui MF, Ahmad A, Rahim MHA, Sakinah M, Wahid ZA (2013) Application of polyethylene glycol immobilized Clostridium sp. LS2 for continuous hydrogen production from palm oil mill effluent in upflow anaerobic sludge blanket reactor. Biochem Eng J. 70:158–165
Wu KJ, Chang JS, Chang C (2006) Biohydrogen production using suspended and immobilized mixed microflora. J Taiwan Inst Chem Eng. 37(6):545
Ismail I, Hassan MA, Rahman AA, Soon CS (2011) Effect of retention time on biohydrogen production by microbial consortia immobilised in polydimethylsiloxane. Afr J Biotechnol. 10(4):601–609
Wu KJ, Chang JS (2007) Batch and continuous fermentative production of hydrogen with anaerobic sludge entrapped in a composite polymeric matrix. Process Biochem. 42(2):279–284
Zhao L, Gl C, Wang AJ, Guo WQ, Liu BF, Ren H, Ren N, Ma F (2012) Enhanced bio-hydrogen production by immobilized Clostridium sp. T2 on a new biological carrier. Int J Hydrog Energy 37(1):162–166
Rai PK, Singh S, Asthana R (2012) Biohydrogen production from cheese whey wastewater in a two-step anaerobic process. Appl Biochem Biotechnol. 167(6):1540–1549
Seol E, Manimaran A, Jang Y, Kim S, Oh YK, Park S (2011) Sustained hydrogen production from formate using immobilized recombinant Escherichia coli SH5. Int J Hydrog Energy 36(14):8681–8686
Wu SY, Lin CN, Chang JS, Chang JS (2005) Biohydrogen production with anaerobic sludge immobilized by ethylene-vinyl acetate copolymer. Int J Hydrog Energy. 30(13-14):1375–1381
Wu KJ, Lo Y, Chen S, Chang J-S (2007) Fermentative production of biofuels with entrapped anaerobic sludge using sequential HRT shifting operation in continuous cultures. J Taiwan Inst Chem Eng. 38(3-4):205–213
Gokfiliz P, Karapinar I (2017) The effect of support particle type on thermophilic hydrogen production by immobilized batch dark fermentation. Int J Hydrog Energy 42(4):2553–2561
Kirli B, Kapdan IK (2016) Selection of microorganism immobilization particle for dark fermentative biohydrogen production by repeated batch operation. Renew Energy. 87:697–702
Kumar G, Shobana S, Nagarajan D, Lee DJ, Lee KS, Lin CY, Chen CY, Chang JS (2018) Biomass based hydrogen production by dark fermentation—recent trends and opportunities for greener processes. Curr Opin Biotechnol 50:136–145
Pandey A, Srivastava S (2018). Fermentative hydrogen production. Bioenergy and Biofuels.
Spier MR, Vandenberghe L, Medeiros ABP, Soccol CR (2011) Application of different types of bioreactors in bioprocesses. Bioreactors: Design, Properties and Applications; Nova Science Publishers, Hauppauge, pp 53–87
Krishnan S, Din MFM, Taib SM, Ling YE, Puteh H, Mishra P, Nasrullah M, Sakinah M, Wahid ZA, Rana S (2019) Process constraints in sustainable bio-hythane production from wastewater. Bioresour Technol Rep. 5:359–363
Neshat SA, Mohammadi M, Najafpour GD, Lahijani P (2017) Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew Sust Energ Rev 79:308–322
Gunasekaran M, Merrylin J, Usman TM, Kumar G, Kim SH, Banu JR (2019) Biohydrogen production from industrial wastewater. In: Biofuels: Alternative feedstocks and conversion processes for the production of liquid and gaseous biofuels. Academic Press. Elsevier, United States, pp 733–760
Acknowledgment
The author acknowledges the support of the Ministry of Higher Education Malaysia (Ref. code FRGS/1/2018/STG05/UMP/01/1; UMP Ref.: RDU190121). Puranjan Mishra would like to acknowledge the Postdoctoral Research Fellowship awarded by the Research and Innovation Department, Universiti Malaysia Pahang.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mishra, P., Wahid, Z.A., Karim, A. et al. Chronological perspective on fermentative-hydrogen from hypothesis in early nineteenth century to recent developments: a review. Biomass Conv. Bioref. 12, 3711–3723 (2022). https://doi.org/10.1007/s13399-020-01180-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01180-4