Abstract
Biohydrogen produced from cellulosic feedstock is a promising candidate for future energy needs as a renewable energy carrier. The thermochemical route and biological processes have great potential for biohydrogen production. In particular, pyrolysis/gasification and dark fermentation are the methods to enhance the biohydrogen production from cellulose. The review compiles the essential information on both processes, including pretreatment of cellulose since it has a complex structure. The operating conditions for both processes, for example, the influence of cellulose pyrolysis/gasification such as temperature, heating rate, and vapor residence time, while for dark fermentation, including the temperature, inoculum source, hydraulic retention time, and pH, are discussed. The bioreactor configurations and economic aspects of both processes are also discussed. The review aims are to present the current state of knowledge about the two processes using cellulose as substrates. Surprisingly, dark fermentation is a promising method for application of cellulose for biohydrogen production since many works were done on dark fermentation compared to pyrolysis/gasification. The future perspectives on enhancing hydrogen production from cellulose have also been discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The rapid depletion and diminishing of fossil fuels’ supply and their adverse impact on the environment is currently a problem for modern society [1]. Therefore, many studies focused on the development of existing and new processes that use lignocellulosic biomass (LB) as feedstock to reduce the current energy dependence on fossil fuels [2]. Several thermochemical or biochemical processes can treat this LB to produce energy, biofuels, and biochemicals [3]. As known, biofuels including biodiesel, bioethanol, and biohydrogen as potential green alternatives have also been considered for substitute conventional fossil fuels.
The global hydrogen (H2) production accounts for approximately 7.7 EJ/year, which may rise to 10 EJ/year by 2050 [3]. Besides, the H2 market is expected to increase by about 5–10% per year, basically due to its consumption in treating heavy oil fractions. H2 is a clean energy carrier with the highest energy content of 142.0 kJ/g, which is 2.75-fold more than that of the conventional fuels [3]. H2 combustion is pollution-free, as only water is formed as the fuel component. H2 can also be used directly to produce electricity in an internal combustion engine and fuel cell and act as an intermediate for converting liquid fuel such as methanol, ethanol, and gasoline [4].
The developed technologies for the H2 production are thermochemical routes (gasification, liquefaction, and pyrolysis) and biological processes (enzymatic, anaerobic dark fermentation, microbial/electrolysis and photo biological such as light fermentation, direct photolysis, and indirect photolysis) [5]. Thermochemical routes are faster than biological ones, which provide higher stoichiometric H2 yield using pyrolysis and gasification method [6]. However, biological routes are more environmentally friendly and less energy-intensive since they are proficient under mild conditions [7]. In terms of H2 production cost, it has been reported that the cost of both processes is three times higher than the cost of the existing method, methane steam reforming. However, both processes have recently gained attention, with several studies published in the literature over the last years. In addition, a quick search of the available literature also reveals that there are very many different types of original research works and reviews over the use of biomass for the production of H2 by using both processes.
As mentioned previously, LB is the most suitable substrate for H2 production due to the high glucose content in monomer form [2]. LB consists of three components which are cellulose (a linear glucose polymer), hemicellulose (a heteropolymer consisting of C5 and C6 sugars), and lignin (an aromatic macromolecule) [1, 2]. Cellulose in LB can be saccharified to form glucose, which can be fermented to several of biofuels, while hemicellulose is mainly composed of pentose, which rarely ferments to alcohols. Thus, the acid pretreatment must be done for hemicellulose solubilization in order to produce H2 since the pretreatment gave the higher xylose content and a small amount of glucose. This pretreatment gave the maximum production of 1.75 mmol H2. However, xylose contains toxic compounds that inhibit cell growth and glycolytic enzymes and interfere with fermentative H2 production, thus hindering the hemicellulose as a suitable feedstock for H2 production. For lignin, its steric hindrance limits the enzymes’ access, which reduces the H2 productivity yields. Among them, the cellulose-rich organic substrate has intensively attracted as suitable feedstock for H2 production. In order to access cellulose, pretreatment of LB was needed to degrade hemicellulose and lignin, thereby facilitating cellulose hydrolysis [8]. Various pretreatment techniques, physical, chemical, and biological, have been developed to improve enzyme pathways for cellulosic hydrolysis [6].
In order to enhance H2 production, most researchers focused on the use of pure or single cellulose instead of cellulose derived from pretreatment of biomass. It was the additional pretreatment of cellulose which lowered the yield of H2 [9]. Thus, in this review, we briefly explain the reason for the usage of a single component of cellulose. To date, most previous reviews centered solely on biomass as a feedstock for H2 production, but not specifically on the most abundant source of cellulose. To the best of our knowledge, the review of cellulose used for H2 production is still scanty. In this review, recent results and some research trends on H2 production from cellulose by pyrolysis and dark fermentation are discussed. The effect of operating conditions and reactor configuration for both technologies toward H2 production was also discussed.
2 Cellulose resources for biohydrogen production
LB was considered a potential feedstock for biofuel production including biohydrogen, methane and ethanol because it is plentiful, renewable, and readily available [10]. This biomass is the fibrous material that forms the cell walls and consists of three basic polymers, which are 40% wt cellulose [C6H10O5]x, 25% wt hemicelluloses such as xylan [C5H8O4]x, and 20% wt lignin (20% wt [C9H10O3 (OCH3)0.9–1.7]x (Fig. 1). The remaining 15% are minor components with a small amount of inorganic compounds, commonly known as ash and extractives [11].
As shown in Table 1, Wu et al. reported the LB components’ effect on H2 production from the pyrolysis and gasification process [9]. They found that in the absence of steam and catalyst, cellulose produces the largest amount of H2 (5.8 mmol H2 g−1 sample), of which only 1.8 mmol H2 g−1 sample was attained for lignin. The addition of the Ni-based catalyst significantly increased the gas yield, particularly for H2 production from cellulose pyrolysis and gasification (22.2 mmol H2 g−1 sample). In addition, the highest carbon monoxide (CO) concentration was found for cellulose pyrolysis and gasification. It is maybe due to the abundance of C–O compounds in the cellulose.
Compared to the pyrolysis and gasification process, numerous studies have been carried out on the fermentation process of LB for the production of H2 [17]. Since LB has a complex structure, its utilization lowered the H2 production due to its recalcitrance [18]. Therefore, the pretreatment step was needed to enhance the H2 production by extracting a single component from LB. Table 1 shows the H2 production from the different feedstock or single component from LB under batch reactor type.
Prakasham et al. reported all bmr derivatives with lower lignin content produced higher levels of H2 (0.25 mmol) compared to source bmr 3, which has more lignin content [12]. These results demonstrated that the higher recalcitrant lignin is a significant barrier in biofuel, particularly H2 production. The presence of lignin reduces productivity H2 yields in further fermentation due to the lignin degradation and interaction of produced monomeric carbohydrates, which are toxic to microbial growth [19]. Therefore, the removal of lignin from LB is imperative before conversion into biofuels by fermentation.
For hemicellulose, De Sa et al. derived the hemicellulose fractions by acid pretreatment for H2 production via anaerobic fermentation [13]. In fact, acid treatment can extract hemicelluloses, remove lignin, reduce cellulose crystallinity, and increase its surface area. The acid-pretreated samples presented a higher xylose content, which resulted in a maximum production of 1.75 mmol H2. Similarly, Baeta et al. reported that anaerobic digestion of the hemicellulose hydrolysate, which was derived from autohydrolysis (AH) pretreatment, gave the maximum production of 1.76 mmol H2 [14]. However, the AH pretreatment requires high energy and forms hemicellulose with rich fermentable C5 sugars such as xylose. These sugars contain toxic compounds, which inhibit the growth of microorganisms, limiting the use of the hemicellulose [20].
Besides lignin and hemicellulose, cellulose in LB can be saccharified to obtain glucose, which can be fermented to many kinds of biofuels including bio-ethanol and bio-H2. Saratale et al. reported that the maximum cumulative H2 production was 2.63 mmol H2 using the cellulose under solid-state fermentation [15]. This process directly uses the crude fermented product as an enzyme source, reducing the production cost in terms of low energy requirement and high product yield [21]. Ratti et al. also investigated the H2 production from cellulose using rumen fluid as the inoculum and gave the maximum production of 18.5 mmol H2 [16]. They found that H2 was produced from cellulose primarily through the fermentation of butyric acid, a route typical of Clostridium species. In addition, increasing the cellulose concentration favored solvetogenesis and gave the lower H2 yield.
Considering the maximum of bio-H2 production among the LB components, cellulose is the promising feedstock for bio-H2 production. Therefore, this review is more focused on the utilization of cellulose for bio-H2 production. Cellulose is a six-carbon ring or pyranose with a higher molecular polymer composed of glucose units bonded by -1, 4-glycosidic linkages (Fig. 2) [1, 22]. The three hydroxyl groups in each pyranose ring can interact with one another forming intra- and intermolecular hydrogen bonds that give cellulose a crystalline structure and its unique properties of mechanical strength and chemical stability. In addition, the majority of cellulose is well-formed and crystalline of nature and only has small amount of amorphous structure [23].
For degradation, amorphous cellulose is preferred over crystalline cellulose because crystallinity makes degradation difficult. The bonds in cellulose molecules are H2 and covalent with van der Waals force [24]. The H2 bonding present in cellulose involves inter- and intramolecular H2 bonds, which makes cellulose rigid in nature. This rigid form of cellulose opposes and demonstrates resistance to any solubilization in an organic solvent, chemical attack, and deconstruction [25].
3 Technologies for biohydrogen production from cellulose
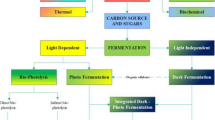
Cellulose as a renewable energy source represents an alternative to conventional methods for H2 production. The methods used for H2 production from cellulose can be divided into two main groups as shown in Fig. 3, which are thermochemical (gasification, liquefaction, and pyrolysis) and biological routes (enzymatic, anaerobic dark fermentation, microbial/electrolysis, and photo biological such as light fermentation, direct photolysis, and indirect photolysis) [11]. The existing methods of H2 production, like combustion and catalytic hydrotreatment, have also been known as potential methods; however, these processes have disadvantages, including contaminated feedstock, higher energy, catalyst deactivation, and formation of tar and char [26].
3.1 Biological process
Biological H2 production processes are considered to be more environmentally friendly and less energy-intensive compared with thermochemical processes [27]. This process is an exciting new field of technological development involving the metabolism of microorganisms, which offers potential for H2 production from different renewable resources, especially cellulose. This process involves several reactions such as direct biophotolysis, indirect biophotolysis, and photofermentation, which involve the presence of light, while dark fermentation is light-independent (Fig. 4). Other than that, bioelectrolysis of water and CO bioconversion are also the other biological processes for H2 production [28].
For direct biophotolysis, water is converted to H2 and O2 by microalgae such as green algae and cyanobacteria in the presence of light under anaerobic conditions [29]. The H2 and oxygen activities are spatially segregated when using cyanobacteria for H2 production under anaerobic conditions [30]. Differently, under aerobic conditions, the cyanobacteria will release the electron during water molecules splitting just only for the reduction of carbon dioxide (CO2) but not for the production of H2. Thus, this phenomenon will cause the activity of the main enzyme (hydrogenase) decreases [31].
For indirect biophotolysis, there are two stages that involve the reactions of H2 and O2 formation (2H2O → 2H2 + O2). Thus, for the second step, hydrogenesis reaction occurred for H2 production and maintained the anaerobic conditions [32]. However, this method has drawbacks including a low process rate and high photobioreactor costs. In addition, it was found that the yield of H2 is less than 10% when using indirect biophotolysis. Similarly, the production of H2 by direct biophotolysis also gave a lower H2 yield [33].
Another type of biological process is photofermentation, in which organic acids are the substrate and converted into H2 and CO2 under anaerobic conditions (Fig. 5). There are many anaerobic bacteria strains used in this reaction such as Rhodospirillum, Rhodopseudomonas, Rhodobacter, and Rhodobium [34]. Generally, the enzyme used in this process is nitrogenase, in which molecular nitrogen reduces the protons to H2 in the presence of O2. In fact, O2 has inhibited H2 production. Referring to the H2 production obtained from previous research, the yield of H2 from fermentation is almost similar to that of biophotolysis [35]. Recently, most researchers often focused on the dark fermentation, since H2 and CH4 are produced with alcohol and fatty acids. Both compounds can be the source of electrons and H2 for sulfur-free photosynthetic bacteria to minimize carbon dioxide and energy supply. In order to increase the H2 yield, fermentation and photo fermentation were combined into a two-step process and is a promising technique [36].
The enzymatic biological route is a common route for bio-H2 production. Majorly, two enzymes, hydrogenase and nitrogenase, are predominantly involved in the production of H2 (Fig. 6) [37]. The former enzymes known as a metalloenzymes are involved in the oxidation of H2 into protons and electrons in the presence of a bimetallic catalytic site, while nitrogenase evolves H2 during nitrogen fixation under anoxic conditions. It is primarily present in archaea and bacteria; however, it is less efficient in hydrogen production than hydrogenase. In fact, the enzymes improved the electron transfer efficiency, thus enhancing the bio-H2 production. Besides those enzymes, pyruvate ferredoxin oxidoreductase, a key enzyme involved in the degradation of pyruvate to reduced ferredoxin and acetyl-CoA, was also found to possess a significant influence on bio-H2 production by transferring an electron to the enzyme hydrogenase [32]. Initially, the metal site was accelerating the electron transfer between ferredoxin and hydrogenase, and then the generated electrons were directed toward hydrogenase for the production of H2.
For bioelectrolysis of water, electric energy was used to convert organic compounds into H2. Meanwhile, microorganisms act as a substrate that will oxidize at the anode and proton movement to the cathode will produce the H2 (Fig. 7a) [37]. Similarly, most research regarding the microbial electrolysis cells also performs a similar procedure (Fig. 7b) [38]. Last but not least is the conversion of CO to CO2 and H2 produced in the presence of Rubrivivax gelatinosus bacteria [39]. In addition, CO also can act as a substrate for H2 production, especially CO, which is derived from the gasification of biomass.
Schematic of a bioelectrolysis with water and b two-chamber microbial electrolysis cell (adopted from [38])
3.1.1 Dark fermentation
Researches toward a new potential method for the regeneration of energy and environment remediation have been attracted nowadays. Dark fermentation is a sustainable and cost-effective way to convert organic waste into biogas like H2 and CH4. This process is a great significance to produce H2 from readily available organic wastes. This process also is light-independent, and many availabilities of carbon source can be used compared to other biological processes. Both advantages can enhance the rate of bio-H2 production as well as the growth rate [40]. Additionally, there are two types of anaerobic microorganisms, which are pure and mixed cultures. The pure cultures are used like Clostridium, Escherichia coli, and Enterobacter; meanwhile, the mixed cultures include bovine manure, anaerobic sludge, and organic compost [41]. Among them, the former cultures have been preferred in the industry since the latter cultures need sterilized surroundings to prevent contamination, which is very costly [42].
The dark fermentation basically occurs via acetate-mediated fermentative pathway for H2 production as shown in the following equation [43]:
In this fermentation, anaerobic bacteria have converted the substrate in the dark. The energy-rich H2 molecules will consume the electrons from the oxidation of H2 to produce energy. Meanwhile, the organism that has excess electrons will reduce the protons to form H2 molecules. There are two types of enzymes that involved in hydrogen metabolism including [NiFe] and [FeFe]-hydrogenase. The [Fe-Fe]-hydrogenase is more active in H2 production; however, it is usually sensitive to oxygen. Meanwhile, the [NiFe]- hydrogenase is primarily catalyzed by oxidation of H2 [44]. Both enzymes catalyze the reversible reaction:
The pretreatment of cellulose is a decisive aspect in the H2 production via dark fermentation as the structure of cellulose is so complex [45]. The requirement of this aspect is to break the heteropolymeric structure of cellulose and, therefore, induce the H2-producing microorganisms by increasing the monomeric sugars [46]. As shown in Fig. 8, there are many products formed as a consequence of the breaking of the cellulose structure. The breakdown of cellulose gave glucose, 5-hydroxymethylfurfural (5-HMF), and cellobiose. According to a previous study [47], the former product is more consumed merely by microorganisms and then metabolized compared to xylose. Other than that, 5-HMF inhibited H2-producing microorganisms in the dark fermentation, thus lowering the H2 production [48].
Recently, more researchers focused on the low operational cost and low capital for the pretreatment process of cellulose and gave the best recovery of cellulose [49]. There are many pretreatment methods that have been established, including physical (hydrothermolysis and steam explosion) and chemical (acid and base) [50]. The most commonly used chemicals are sulfuric acid (H2SO4) and sodium hydroxide (NaOH), respectively. Besides acid and base, solvents are also used for pretreatment of cellulose because solvents can dissolve cellulose by disrupting its structure and thus facilitating its hydrolysis [51].
Among solvents used, ionic liquids (IL) have attracted much interest as new chemical agents for cellulose pretreatment due to their high cellulose dissolubility of up to 39% with no derivatization [52]. In fact, IL have halide anions, which can dissolve the cellulose by strongly disrupting the H2 bonding of the polysaccharide network and facilitating its dissolution [53]. Furthermore, this IL process is economical as it prevents pollution and waste production and has higher reusability.
Nguyen et al. enhanced the H2 production of Thermotoga neapolitana from cellulose by non-derivating ionic liquid like 1-butyl-3-methylimidazolium chloride [C4mim]CI with N2 sparging, which gave a maximum cumulative H2 yield of 2.20 mol H2/mol glucose [54]. They found that N2 sparging maintained the continuous H2 production, thus increasing the H2 yield of T. neapolitana. They also pretreated the cellulose with common acid (H2SO4) and alkali (NaOH), which gave an H2 yield of 0.95 and 1.22 mol H2, respectively. When compared with untreated cellulose, only 0.59 mol H2 was produced. Similarly, Cui also reported that H2 production from cellulose pretreated by acid and alkali was higher than those from untreated cellulose [55]. In addition, Dadi et al. also reported that H2 production was approximately 50-fold higher for the regenerated cellulose as compared to untreated cellulose by using [C4mim]Cl and Trichoderma reesei (ATCC# 26799) [56]. Thus, it can be concluded that pretreated cellulose enhanced H2 production. It was found that pretreatment using [C4mim]Cl is more preferred since the cellulose is more easily enzymatically hydrolyzed than using the NaOH and H2SO4. Also, IL pretreatment offers environmental toxication and a noncorrosive system [54]. However, the high boiling points of IL face difficulties in product separation and solvent recycling. Notably, the retail price of IL is expensive compared to HCI and NaOH since it is a very new chemical. Hence, the cost of the chemical also should be considered in order to enhance H2 production.
Besides the pretreatment of cellulose to enhance H2 production, suitable additives or catalysts also can lower the concentration of the inhibitor and boost the microbial activity [57]. For example, activated zeolite and carbon particles were used as the main transporters in the microbial activity of fermentation. Among them, carbon materials are more favored to provide a good residence of their growth for anaerobic microbes to immobilize [58]. In order to absorb nutrients, the interaction between bacteria and carbon materials may encourage syntrophic microbes and then convert them into more biogas, particularly H2. Zhang et al. claimed that the addition of Fe and Mn into activated carbon enhanced the H2 production by promoting direct interspecies electron transfer [59]. The highest yield of H2 was 55.8% at 37 °C. Because of its magnetism, this catalyst could be recycled by magnetic separation after the bio-H2 process. However, it should be mentioned that over-dosage of the catalyst caused toxicity to H2-producing bacteria and lowered the activities of the bacteria, thus restraining H2 production.
Recently, more researchers more focused on a combination of heterogeneous catalysis with dark fermentation systems for the production of bio-H2 [60]. In fact, a heterogeneous catalyst can catalyze hydrolysis reactions of cellulose [61]. Table 2 shows the heterogeneous catalyst used in H2 production. Guell et al. have shown a two-step combined system involving the hydrolyzing process of cellulose by the ZrO2 catalyst. This catalyst was used to disrupt the structure of cellulose and easily produce fermentable sugar, while this sugar was fermented by using Enterobacter spH1, Citrobacter freundii H3, and Ruminococcus albus DMS2045 to produce H2 [62]. Among the three microorganisms, the yield and production rate of H2 was highest for Enterobacter spH1 (8.71 mol) and C. freundii H3 (7.42 mol). It was found that the bacteria R. albus was unable to produce the fermentable sugars due to the formation of inhibitory compounds such as HMF and furfural, which subsequently inhibited the production of H2 and gave the lowest yield of H2 (4.63 mol).
Other than that, lately, Tondro et al. investigated the use of a heterogeneous catalyst such as sulfonated graphene oxide (SGO) for hydrolyzing the cellulose to produce H2 by Enterobactor aerogens [63]. Generally, this SGO was prepared via chemical exfoliation of graphite and then followed by the sulfonation process [64]. Under optimized conditions, the maximum hydrogen efficiency of 72.4 mL/g was achieved, which was 2.2-fold higher than that of the pretreated MCC substrate as a control in the absence of SGO. It has also been observed that the superior behavior of SGO bearing SO3H, COOH, and OH groups can be correlated with the synergy of multilayered structure and functional groups that provide good catalyst access for cellulose leading to successful cellulose hydrolysis into β-1,4-glucan and glucose [65]. It was concluded that solid acid catalysts with strong acid strength are an effective method for the production of H2 as well as having ease of separation and recyclability or reusability.
3.1.2 Operating conditions
Basically, the H2 production process by dark fermentation is incapable of using the initial substrate totally and this method is not yet commercially feasible due to various processing modes and different types of substrate [66]. Furthermore, this method and its yield rely on various parameters such as temperature, pH, inoculum, and hydraulic retention time (HRT) which are deliberated below [67]. Table 3 displays the parameters used (inoculum, temperature, pH, and HRT) for H2 production in dark fermentation from cellulose. In general, the main source of inoculum used in dark fermentation was a mixed culture. Furthermore, recurring operating parameters are mesophilic temperatures (around 37 °C) and pH close to 7. The HRT results tend to be less studied than the temperature and pH. The HRT has been commonly used for about 60 to 160 h.
There are various types of fermentative bacteria used to produce bio-H2 such as obligate anaerobes (Clostridium), facultative anaerobes (Enterobacter, Escherichia coli, Rhodopseudomonas, Citrobacter), and aerobic bacteria (Bacillus) [41, 43]. The former bacteria require anaerobic conditions. Basically, butyric and acetic acid fermentation was involved by using these bacteria. In addition, the butyric acid and acetic acid fermentation gave the different yield of H2 [41]. For example, the equation of the acetic and butyric acid fermentation is as follows,
Facultative anaerobes are species that generate ATP by aerobic respiration when there is oxygen but may turn to fermentation when there is no oxygen [79]. During dark fermentation, its resistance to the use of oxygen enables its handling. However, these microorganisms have been used in the production of H2, and the H2 is produced by formic acid decomposition, which reduces the development of H2 as opposed to Clostridium [33].
Table 3 shows the usage of Clostridium bacteria for hydrolysis of cellulose. Taguchi et al. used Clostridium sp. for continuous H2 production by fermentation [68]. These bacteria consumed 0.92 mmol/h of glucose and produced 4.10 mmol/h. Similarly, Clostridium sp. was used to produce H2 (0.21 mmol H2) [69]. After that, Lo et al. studied the cellulosic hydrolysates with Clostridium butyricum CGS5, giving a maximum hydrogen yield of 4.79 mmol H2/g reducing sugar under continuous bioreactor via dark fermentation [70]. They also investigated the fermentative H2 production from hydrolyzed cellulose under batch reaction using the same bacteria. It was found that the bacteria displayed the highest H2 production with H2 yield of 7.40 mmol/g as compared with different feedstock (grass hydrolysate) [71]. It can be concluded that H2 production was also dependent on the type of feedstock used.
Clostridium thermocelum is a gram-positive, thermophilic and anaerobic bacterium that degrades cellulose and carries out product fermentation [80]. In fact, C. thermocelum exhibits the highest rate of cellulose degradation. It was due to the interaction of bacteria with cellulose particles via the cellulosome, which is transported into the cell for metabolism after degrading the cellulose into glucose and cellulodextrans [81]. Levin et al. observed H2 production by C. thermocellum 27405 on the cellulose gave an average yield of H2 of 1.60 mol H2/mol glucose [72]. Islam et al. also claimed that H2 yield of 1.30 mol H2/mol glucose was obtained during the direct conversion of cellulose by Clostridium thermocellum DSM 1237 [73].
Similarly, Magnusson et al. also investigated the H2 production by C. thermocellum 27405 on both cellobiose and cellulose, which gave the 1.27 mmol H2/glucose and 1.24 mmol H2/glucose [74]. It was found that there are no changes when using cellobiose and cellulose as a substrate. This result was also confirmed by the Lalaurette et al. study, in which H2 production from cellobiose and cellulose by Clostridium thermocellum was 1.67 mol H2/mol glucose and 1.64 mol H2/mol glucose, respectively [75]. Interestingly, by combining fermentation and electrohydrogenesis, the H2 production was enhanced to 9.95 mol H2/mol glucose using the cellulose, and 8.31 mol H2/mol glucose was produced in the second stage of the electrohydrogenesis. Thus, the two-stage process is a promising approach for H2 production from the more abundant and renewable cellulose. However, in terms of cost, this process is more expensive compared to the single-step dark fermentation process.
Besides Clostridium thermocellum, many researchers also used another Clostridium-type bacteria, including Clostridium acetobutylicum for hydrolyzed cellulose via dark fermentation [82]. Ren et al. reported that C. acetobutylicum underwent typical butyrate-type fermentation metabolism, which gave the H2 yield of 3.5 mmol/g [76]. Meanwhile, Ratti et al. reported the same bacteria, which also produces acetic and butyric acid and gave 19.9 mmol H2. In order to promote the growth of those bacteria, they have added biotin and p-aminobenzoic acid and gave the H2 yield of 2.30 mol H2/mol glucose [16]. This phenomenon shows C. acetobutylicum required the assisted chemical for growth development and formation of cellobiase and cellulase and, thus, needed a longer time for those processes.
Other than that, Chang et al. used many bacteria strains such as Clostridium xylanolyticum, Clostridium papyrosolvens, Clostridium beijerinckii, Ruminococcus sp., Ethanoligenens harbinense, and Desulfovibrio desulfuricans for H2 production [77]. Among them, the Clostridium genus was shown as the dominant population in the system and contributed to the bio-H2 production (50 mL/g). For enhanced H2 production, Nissila et al. used C. stercorarium subsp. Leptospartum, which is the main cellulose, degrades H2 in the 60 °C cultivations, and gives 0.44 mol H2 [78]. This result was obtained by heat treatment and with acetate and ethanol as the main fermentation products.
Recently, mixed cultures are more preferred than pure cultures since they have a low operational cost and are easy to control [42]. In addition, they have the ability to degrade furfural and 5-HMF since these compounds inhibited or lowered the H2 production [83]. In the literature, there are many studies using mixed cultures or co-culture with cellulose for H2 production. Table 4 shows the mixed culture of bacteria used in dark fermentation from cellulose for H2 production. Ueno et al. reported that two bacteria, Clostridium butyricum and Ruminococcus albus, were used for H2 production from cellulose and produce high H2 (2.40 mol/mol-hexose) [84]. Wang et al. also used mixed bacteria such as Clostridium acetobutylicum X9 and Ethanoigenens harbinense B49 for bioaugmented H2 production from microcrystalline cellulose [85]. The maximum H2 yield was 8.10 mmol H2 at pH 5 and 37 °C. The metabolites observed were acetate, ethanol, and butyrate. In addition, the strain B49 rapidly removed reduced sugar; meanwhile, X9 hydrolyzed the cellulose, hence synergistically improving cellulose hydrolysis and subsequent H2 production rate. However, some anaerobic mixed cultures cannot produce H2 as it is rapidly consumed.
Nowadays, robust microorganisms or thermophiles are widely used for H2 production. Thermoanaerobacterium thermosaccharolyticum is one of the thermorphile anaerobes [93]. Liu et al. isolated Clostridium thermocellum JN4 and T. thermosaccharolyticum GD17 from cellulosic [86]. They found that C. thermocellum JN4 can produce H2 by degrading the cellulose; however, cellobiose and glucose produced cannot utilize completely. The H2 yield was approximately 0.80 mol H2/(mol glucose). Interestingly, the H2 yield was increased by twofold (1.80 mol H2/mol glucose) when C. thermocellum JN4 was co-cultured with T. thermosaccharolyticum GD17. Similarly, Nguyen et al. also used Hyperthermophilic eubacterium and Thermotoga neapolitana for H2 production from cellulose and gave 2.20 mol H2 [54].
Other co-cultures for H2 production from cellulose are Clostridium thermocellum and Clostridium thermopalmarium, Thermoanaerobacter and Clostridium spp., and Clostridium cellulovorans 743B and Rhodopseudomonas palustris CGA009, which gave 1387 mL/g, 2.14 mmol, and 0.40 mol H2, respectively [87,88,89]. Lately, the higher H2 yield of about 1.92 mol H2/mol hexose was attained from co-culture of Clostridium termitidis and Clostridium beijerinckii [90]. Firstly, cellulose was hydrolyzed by C. termitidis, and then C. beijerinckii was responsible for increasing the H2 production. In another study, Cellulomonas fimi and Rhodopseudomonas palustris attained 44.0 mmol H2, while Clostridium acetobutylicum X9 and Ethanoigenens harbinense B2 gave the 10.4 mmol H2 from cellulose [91, 92]. Thus, it can be concluded that co-culture hydrolyzed cellulose and produced higher H2.
Table 5 shows other inoculum sources than Clostridium bacteria or pure cultures for H2 production from cellulose. Some studies were conducted on a batch mode reactor and achieved promising yields ranging from 4.20 to 521.4 mL/g and 0.097 to 1.7 mmol H2 [71, 94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109]. Only a few studies described continuous bioreactor usage for H2 production [110,111,112,113] (Table 6). The most significant influences in the anaerobic fermentation process are temperature and pH [18]. Such parameters affect the development of H2-producing bacteria and bio-H2. Bacteria can be categorized into several temperature classes such as psychrophiles (0–25 °C), mesophiles (25–45 °C), thermophiles (45–65 °C), extreme thermophiles (65–80 °C), and hyperthermophiles (above 80 °C) [114].
In general, thermophilic or mesophilic conditions may be performed during the dark fermentation process. In terms of H2 production, the performance of thermophilic fermentation using simple substrates is often seen as good [115]. However, in the case of using cellulose, H2 yields are usually below 1 mol H2/mol hexose. Gadow et al. conducted the dark fermentation under three different temperatures, which are mesophilic (37 °C), thermophilic (55 °C), and hyperthermophilic (80 °C). It was found that 55 and 80 °C resented stable hydrogen yields of 12.3 and 9.72 mmol/g cellulose, respectively [110]. However, mesophilic only produced 3.56 mmol/g cellulose.
Carver et al. reported the usage of mixed microbial consortium in thermophilic fermentation of cellulose gave 0.35 mol H2/mol hexose [88]. They also reported 2.98 mmol H2 was produced at 50 °C by using compost pile (TC60) [108]. In addition, the continuous process with heat-treated anaerobic sludge was maintained for 190 days and gave H2 yield of 2.52 mol H2/mol hexose [107]. Mesophilic processes require less energy input because the process was carried out at lower temperatures. From Table 5, only a few studies described long-term mesophilic hydrogen production from cellulose [110]. In conclusion, the thermophilic temperature is expected to have a better economic performance for cellulosic-hydrogen fermentation.
For the effect of pH, as shown in Table 5, the pH range from 5 to 7 was the optimum pH for fermentation for H2 production. In fact, this pH range is promoting the growth of bacteria. Saripan et al. presented that the cumulative H2 production increased with an initial pH increase from 6 to 7 and decreased sharply with an initial pH increase from 7 to 8 [105]. At initial pH 7, a maximum H2 production was achieved at 761 mL H2/L. At pH 5, H2 could not be generated at low pH, which may probably be due to the formation of acidic metabolites that could destabilize the ability of the microbial cells to retain internal pH, resulting in a decrease in the internal ATP and inhibit substrate uptake. Similarly, the low H2 production was observed at higher pH (pH 8) due to the inhibition of hydrogenase activity. Similarly, Gonzales et al. reported that pH 6 was the optimum pH for maximum cumulative H2 production (1.27 mol H2/mol sugar [116]. Bao et al. also observed the initial pH at 6 shows the maximum H2 yield of Clostridium acetobutylicum X9 + Ethanoigenens harbinense B2 reached 9.63 mmol g-MCC-1, and cellulose degradation was 81% [92].
Another operating condition of the anaerobic fermentation process is HRT, which affects microbial metabolism and, ultimately, the bio-H2 production and final products of fermentation. Based on the Table 6, it was shown that 32 to 240 h is the range used for the HRT [101, 107, 110, 111, 113]. However, the most frequently used for HRT was 240 h. In a previous study for HRT for H2 production from cellulose, Bao et al. revealed that the maximum H2 yield of Clostridium acetobutylicum X9 + Ethanoigenens harbinense B2 reached 10.2 mmol g-MCC-1, cellulose degradation of 85% at HRT 40 h [92]. They claimed multiple microorganisms came to a stable phase.
3.1.3 Bioreactor configurations for dark fermentation
The configuration of the reactor is considered to be critical for the overall output efficiency of fermentative hydrogen. It affects the reactor’s environment, the prevailing microbial population, the hydrodynamic behavior that has been formed, the interaction between substrates and consortia, etc. [18]. The fundamental classification of reactors used for fermentation is based on the mode of implementation of the batch, semi-continuous and continuous. Commonly, batch or continuous bioreactors are used for bio-H2 production in which former reactors are mainly employed for research purposes, whereas latter bioreactors are employed at an industrial scale [43]. In addition, batch reactors have a simple and inexpensive design; fermentation parameters, especially temperature and pH, can be controlled conveniently. Due to these reasons, many researchers most focused on the batch mode of bioreactors [94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109]. Generally, all fermentation batches were incubated at 120 rpm and 60 °C, while anaerobic conditions were generated by purging the bottles with nitrogen gas at 37 °C and 120 rpm for 96 h.
There are various types of bioreactors, including continuous stirred-tank reactors (CSTR), upflow anaerobic sludge blanket reactor (UASB), and anaerobic fluidized bed reactor (AFBR) [18]. The reactors most widely used for continuous fermentation are CSTR. The reactors are cylindrical in shape and are equipped with a mechanical stirring system (Fig. 9a). This reactor is categorized as simple design, which easily adjusts operating conditions such as pH and temperature. In addition, continuous stirring facilitates the medium’s homogeneous conditions and provides microorganisms with a good contact with substrates, and enhances the efficacy of hydrogen removal from the reaction mixture, which decreases its partial pressure and increases the yield of H2 [117]. In general, the microbial population producing bio-H2 is thoroughly circulated and is suspended with the mixed liquor in the reactor with the aid of a stirrer. As a result, in reactor liquor, the microbes are suspended and contain an equal biomass load in the effluent. In such conditions, the best interaction of the inoculum substrate and mass transfer could be obtained.
Schematic of a continuous stirred tank reactors, b upflow anaerobic sludge blanket reactor, and c fluidized-bed reactor for fermentation biohydrogen production (adopted from [18])
The main parameters during the CSTR operation are HRT and organic loading rate. The higher concentration of biomass lowered the H2 production rate. Meanwhile, the H2 production rates in CSTR are also not high when using short HRTs. However, HRT must be less than the maximum growth rate of microorganisms used. Referring to Table 6, it is clearly seen that the longer HRTs used in CSTR gave the higher bio-H2 yield as compared to short HRTs. It might be because the multiple microorganisms came to a stable phase with longer HRT [92]. The literature survey found a range of disadvantages in CSTR, such as highly susceptible to different environmental influences, such as pH and HRT fluctuations, and processing results in biomass washout at an increased dilution rate leading to a lower production rate of biohydrogen [118].
Different processes have been developed, such as microbe immobilization and upflow reactors (UASB) in order to maintain the concentration of biomass in bioreactors for higher bio-H2 production. The UASB reactor consists of an elongated shape with a three-phase separator in the upper part of the reactor (Fig. 9b). The granules are formed and grow during the fermentation process through the aggregation of activated sludge and deposited at the bottom of the reactor. The closeness of microorganisms in granules results in greater conversion of substrates into H2. However, these reactors are sensitive toward channeling effects, which give rise to poor substrate-biomass contact [119, 120].
Another bioreactor used in dark fermentation is AFBR (Fig. 9c). This reactor combines the features of two reactors, which are CSTR and immobilized-bed reactors. In AFBR, biocatalyst substances are moved through the continuous flow of substrate liquor [121]. As the fluid pressure decreases, the weight of the bed will be retained. Meanwhile, as granules, microorganisms are deposited on a solid surface, and then gas is fed to create a fluidized bed from the bottom of the reactor. As microorganisms are accumulated on a solid surface, compared to a UASB reactor, there is a lower risk of biomass being washed off. The literature survey finds that, relative to CSTR, improved biohydrogen development is observed in FBR. A downside of an AFBR is the increased energy necessary to hold the bed in a fluidized state.
3.2 Thermochemical process
Cellulose can be treated by various thermochemical processes including gasification, liquefaction, pyrolysis, steam gasification, and supercritical water gasification to produce biofuels, biochemicals, and energy [122]. The advantages of this process such as low-cost and higher efficiency in thermal treatment [123]. The H2 yield is relatively low when using dry biomass, about 16–18%. In comparison to other thermochemical gasification processes such as steam gasification or air gasification, supercritical water gasification gave a better performance at lower temperatures and can directly use wet biomass without drying [124]. The main drawback of these methods is the feedstock decomposition led to the production of char and tar [125]. Researchers have made a range of efforts to study biomass gasification for H2 production with different types of biomass as well as under different operating conditions in order to improve the thermochemical process for H2 production.
3.2.1 Pyrolysis and gasification
Gasification generally refers to the thermal treatment, which is carried out at high temperature by using a gasifying agent such as steam, air (partial oxidation), or CO for gaseous product formation, especially H2 with lower amounts of char and ash [126]. In particular, the syngas was produced with an H2/CO ratio of 2/1 when steam or O2 was added in the gasification process, and then the latter being used for the formation of higher hydrocarbon from the Fischer-Tropsch reaction [127].
Thermal decomposition of solid mass produced gas products such as H2, CO, CO2, H2O, and CH4 at 600–1000 °C [128]. Meanwhile, superheated steam was used at 900 °C to attain high H2 yields by reforming the dry biomass. However, the tars were formed in the gas product even conducted at a higher temperature in the range of 800–1000 °C [126]. Biomass gasification is typically observed according to the equation:
The gasification method is complex and involves a two-step process of pyrolysis followed by gasification. In fact, pyrolysis provides heat for the endothermic reactions and produces 75 to 90% volatile materials in the form of gaseous and liquid hydrocarbons. Meanwhile, the remaining nonvolatile material, containing a high carbon content, is referred to as char [129]. Both volatile hydrocarbons and char are subsequently converted to syngas in the second step (gasification).
Gasification technology also is known in terms of an energy balance as a self-sufficient autothermic operation. Biomass gasification’s energy recovery and heat efficiency are more than the combustion and pyrolysis due to the optimal use of usable biomass feedstock for heat and power generation as both carbon and hydrogen contribute significantly to the calorific value. At the same time, pyrolysis and liquefaction are complex and highly dependent on operating conditions and the occurrence of a secondary reaction between the hot solid and volatile particles [130]. Therefore CO and H2 conversion are weak in the processes of pyrolysis and liquefaction. However, the syngas produced from gasification is easily convertible by catalytic methanation of CO and CO2 into synthetic natural gas.
Generally, the gasification methods lead to low thermal efficiency because the biomass’s moisture still needs to be vaporized. This method can be conducted in a fluidized-bed reactor compared to a fixed-bed as it performs better [126]. The reactors are usually constructed on a large scale and involve constant fuelling of vast quantities of energy. Hence, it only achieves 35–50% efficiencies since the heating value is lower [131]. One of the problems with this approach is the importance of using large quantities of resources to move the massive volumes of biomass to the central processing plant. The high costs of logistics of gasification plants and the removal of the tar to suitable levels for the processing of pure H2 currently hinder the commercialization of H2 based biomass production. For H2 cost-effective output, this technology will require the future development of smaller and more productive distributed gasification plants.
Another currently promising method for H2 production is pyrolysis (Fig. 10). Raw organic material is heated and gasified within the 500–900 °C range at a pressure of 0.1–0.5 MPa [132]. The cycle is performed without oxygen and air, so the production of dioxins is almost ruled out. Since no water or air is present, no CO or CO2 is produced, which eliminates the need for secondary reaction. This method ultimately provides a substantial reduction in emissions. However, significant COx emissions will be generated when air or water is present. Fuel efficiency, relative simplicity and compactness, clean carbon byproduct, and reduced COx emissions are among the advantages of this process [6]. Generally, the reaction can be represented by the following equation:
General scheme for producing hydrogen through pyrolysis (adopted from [126])
Based on the temperature scale, the pyrolysis process is divided into low (up to 500 °C), medium (500–800 °C), and high (over 800 °C) temperatures [1, 6]. Fast pyrolysis is one of the latest processes in which organic material is converted into higher energy content products. Fast pyrolysis products occur in all the formed phases, including solid, liquid, and gaseous products. One of the issues with this approach is the possibility of carbon-formed fouling, but proponents claim that proper design will mitigate this. Pyrolysis can play an essential role in the future as it has the potential for lower CO and CO2 emissions and can be done so that a significant volume of solid carbon can be extracted, which is quickly sequestered.
Water-gas shift is used in the pyrolysis and gasification processes to transform the revamped gas into H2, and pressure swing adsorption is used to purify the product. Figure 11 shows a general schematic for producing hydrogen from cellulose using gasification or pyrolysis/reforming. The other processes involved in a cellulose thermochemical biorefinery for the production of valuable products such as automotive fuels, light olefins, and H2.
Generally, the pyrolysis of biomass is the main sub-process in thermochemical hydrogen production. The gaseous products can be obtained from the pyrolysis of biomass at high temperatures [133]. However, the H2 concentration in gaseous products from pyrolysis is still too low to be commercially attractive. One of the methods to increase the hydrogen yield is to apply catalytic pyrolysis. Pure cellulose pyrolysis transforms primarily to a monomer, levoglucosan, without a catalyst. Table 7 shows the catalyst used in pyrolysis for H2 production. Su et al. reported that sodium aluminum oxide, Al2O3.Na2O catalyst exhibits good activity for a higher yield of H2 (1.8 mmol) from cellulose at 673 K [134]. At a higher temperature above 673 K, the tar was produced and reduced the utilization efficiency of cellulose [135]. In fact, the catalyst plays a vital role in converting tar and in the production of H2. Thus, controlling the reaction temperature is also a crucial factor for enhancement of H2 production and inhibiting tar formation.
More researchers recently focused on the metallic catalyst, such as nickel (Ni), for the thermochemical conversion process. Zou et al. used the Ni/Al2O3 for H2 production from pyrolysis catalytic reforming of cellulose using a two-stage fixed-bed reaction system [136]. This system has benefitted from the catalytic reforming stage, where direct contacts between catalyst and pollutants from biomass char and ashes are avoided. The used catalyst is easier to be separated and regenerated than single-stage gasification mixing catalyst and raw biomass. It was found that H2 production was around 28 mmol even though the catalyst was reused five times. Wu et al. reported that Ni-based catalysts improve the production of gas and H2, particularly the addition of Zn and Ca to the Ni-Al catalyst [9]. Among both catalysts, Ni-Ca-Al was the most effective for hydrogen production (22.2 mmol H2) from cellulose pyrolysis/gasification compared to the absence of catalyst (5.8 mmol H2). However, the more massive consumption of chemicals to prepare the catalyst can contribute to the higher cost. Although it is a promising performance, a significant effort should be made to reach an economically feasible process.
Rupert et al. also studied Ni supported on ZrO2 for enhanced H2 production (13 mmol) from the thermochemical of cellulose [137]. They found that Ni can catalyze the C–C cleavage and crack products more easily dehydrogenated to form H2. Also, increased contact between the ZrO2 and NiO phases was beneficial for the enhanced H2 yield. Other than that, Ce also was used as a promoter in Ni/Al2O3 for H2 production from catalytic pyrolysis of cellulose [138]. This catalyst exhibited higher H2 production (1.90 mmol) at a higher temperature (650 °C). This result is attributed to the strong acidity of the catalyst favoring the C–O and C–C bond cleavage and subsequently promoting cellulose decomposition.
Besides Ni-based catalyst, Fe-based catalyst also offers relatively high H2 production. In fact, the efficient catalytic metal for water gas shift reaction is Fe2O3. However, the Fe2O3 catalyst was deactivated due to the thermal sintering at higher temperatures [140]. To overcome this problem, Zhou et al. used the CeO2 as the metal oxide for lowering the sintering and enhance the stability of material [139]. It was found that the catalytic performance of the CeO2/Fe2O3 catalyst in relation to H2 production was much better than that of pure CeO2 or Fe2O3. At 800 °C, the H2 yield was 28.6 mmol. After redox reactions, CeFeO3 could be produced at 800 °C without forming the CeO2/Fe2O3 clathrate. However, the decreasing number of CeFeO3 could be attributed to the deactivation catalyst in the lifetime test.
3.2.2 Operating conditions
A variety of parameters influence the cellulose pyrolysis/gasification process, yields, and properties of products. That involves the heating rate, temperature, and vapor residence time [10]. The section below will analyze the effect of those parameters that govern process mechanisms and provide compositions for the desired product.
Pyrolysis/gasification temperature significantly influences the distribution and properties of products. The thermochemical process involves the endothermic reaction and has a significant impact on gas and H2 yields. Furness et al. briefly identified the gas decomposition and defined a three-stage pyrolysis process [141]. CH4, CO2, and H2O were the key pyrolytic products in the first stage at 250 °C. Hydrocarbon and alcohols were the main pyrolytic products in the second stage at 350 °C. Lastly, in the third stage, H2, CH4, CO, CO2, hydrocarbon, and alcohols were the main dominating products at 550 °C.
Based on the previous studies, the gasification temperature should be controlled at < 673 K in order to avoid tar production. However, the formation rates of hydrogen increased sharply at about 1023–1073 K. In addition, at this temperature, the tar was formed, like 1,3-bis(1-methylethyl)-benzene, 1,4-bis(1-methylethyl)-benzene, 1,2,3,4-tetrahydronaphthalene [134]. Similarly, Arregi et al. have summarized the impact of gasification temperature on tar content in the gaseous product stream and found that a more extreme operation resulted in an improved gasification efficiency by reducing the tar content [122].
Zhou et al. also studied the influence of catalytic temperature on the hydrogen production from cellulose gasification and the hydrogen production when cellulose pyrolysis temperature was kept at 800 °C [139]. As shown, the yield of gas increased directly from 62.92 to 85.84 wt%, suggesting that the temperature of gasification had a major impact on the thermal conversion of liquid oil compounds to light gases. The yield of H2 and CO2 increased marginally for that gas concentration as the temperature rose from 500 to 700 °C, while the H2 yield reached the maximum at 28.58 mmol at 800 °C and the H2 yield decreased at 900 °C. The latter phenomenon that occurred might be due to the reverse WGS reaction by the role of catalyst.
Heating rate is a fundamental parameter that defines the type of biomass pyrolysis, i.e., flash, fast, and slow pyrolysis [1]. Fast heating rates favor the rapid fragmentation of the biomass and yield more gases and produce less char. In fact, the heating rate can be considered a function of the temperature and residence time. Higher residence times begin to decompose secondary hydrocarbon reactions to accumulate in the pyrolysis-volatiles as light hydrocarbon gases. Hernandez et al. showed that higher gas residence times increase the H2 yield with decreases in tar formation [142]. Su et al. reported that when the heating rate was increased, the formation rates of H2 also increased [135]. Similarly, Fushimi et al. investigated the H2 gas products of the pyrolysis of cellulose and found that they are increased with heating rate changed from 1 to 100 K s-1 [143]. More specifically, gaseous products, such as H2 and light hydrocarbon gas, are growing with higher heating rates and gas residence times.
3.2.3 Bioreactor configurations for pyrolysis/gasification
A further level of complexity to the pyrolysis operation is added by the type of contact between particles in the reactor and depends on the pyrolysis reactor [144]. There are several reactor configurations that have been used for pyrolysis, including fluidized bed reactors (FBR), ablative reactors, rotating cone, and auger/screw reactor [145].
FBR is one of the most widely used for pyrolysis reaction (Fig. 12a). This reactor involves the rapid heating of biomass by mixing it with the sand particles at high temperatures. There are two basic types of FBR including bubbling and circulating. Among them, bubbling fluidized bed reactors are the most advanced technology, using sand as fluidizing solid because it allows an excellent gas-solid contact and improves heat transfer [146]. Ni et al. [147] reported that hydrogen production from biomass pyrolysis using fluidized bed reactors would be attained at a higher production rate due to its exhibiting higher heating rates. However, large reactor size and the high cost of construction and operation are the disadvantages.
Scheme of a fluidized bed reactor, b ablative reactor, c rotating cone, and d auger/screw reactor of pyrolysis for producing hydrogen (adopted from [145])
Besides FBR, ablative reactors also one of the bioreactors for pyrolysis reaction. It involves the thermal “erosion” of the biomass by pressing against the hot reactor wall (Fig. 12b). This type of reactor accepts a feedstock of large sizes and allows good mechanical abrasion of char. Then, most researches focused more on the rotating cone reactors, which do not involve the carrier gas to transport the vapors, thus reducing the operating cost. The biomass is fed near the bottom of the rotating cone and is carried up the wall of the rotating cone in a spiral motion due to the centrifugal force (Fig. 12c). For the auger/screw reactor, this allows the reactor to be used on the site of biomass generation or where the biomass is abundantly available [148]. This reactor is a tubular, continuous reactor in which solid biomass is transported via a rotating screw, while the heat required for pyrolysis is transported along the tubular wall of the reactor (Fig. 12d).
Refer to Table 7; most researchers use a two-stage pyrolysis catalytic reforming fixed-bed system, as shown in Fig. 13 for H2 production from cellulose [9, 134,135,136, 139]. This system consists of a two-stage fixed-bed furnace with two temperature ranges: pyrolysis zone and gasification zone for the first and second stages. In the first stage, cellulose was pyrolyzed in a stainless tube reactor, while the derived pyrolysis vapors were catalytic steam reformed under a second reactor tube in the presence of the catalyst. Fast pyrolysis of cellulose samples happened in the first stage, and the volatiles are entering the second reactor. The outlet products passed through two condensers, where the liquid products were collected. Finally, the noncondensable gases were cleaned, dried, and collected with a gas bag.
Scheme diagram of a two-stage pyrolysis catalytic reforming system (adopted from [136])
4 Economic aspects of biohydrogen production
World demand for H2 is about 70 million tonnes/year since its potential utilization in fuel, vehicle, and industrial feedstock. However, the major problem is its unavailability in nature. Therefore, the supply of H2 by inexpensive production methods is necessary in order to meet those needs. The most widely used and cheapest method for H2 production is the steam reforming of methane (SRM) from natural gas, with a cost of 1.5–2.2 USD/kg [37]. However, nowadays, biological processes such as dark fermentation and thermochemical processes, including pyrolysis, have been extensively studied in a laboratory scale using different biomass sources, particularly cellulose feedstock. Generally, the molar yield of H2 and the feedstock’s cost are the two main barriers for both fermentative and pyrolysis hydrogen production.
H2 production by gasification and pyrolysis of biomass is not generally considered economically competitive with SRM processes. The price of H2 obtained by direct gasification of lignocellulosic biomass, however, is about three times higher than that for H2 produced by SRM [149]. According to Hamelinck and Faaij [150], the cost of producing H2 from biomass ranges from 10 to 14 USD, with a net higher heating value (HHV) energy efficiency of 56–64%. For fermentation, Ochs et al. [151] demonstrated a study that H2 from a combined dark and photo fermentation impacts 5.7 higher than SMR, most from the use of phosphate in the fermentation process, which used the higher cost. It can be concluded that biological hydrogen was comparatively higher than that of H2 from pyrolysis.
5 Challenges and future perspective for enhancing biohydrogen production from cellulose
Presently, H2 is the most expensive product among the biofuels and H2 generation from renewable sources, especially cellulose feedstock. This feedstock can be transformed into H2 by thermochemical processes (pyrolysis and gasification) and biological process (dark fermentation). Both processes have weaknesses in a low degree of conversion of the substrate to product, thus limiting the production of H2. Due to the pretreatment of cellulose and enzymatic hydrolysis, it is the most cost-intensive step, including the major cost for H2 production [45]. The pretreatment of cellulose by acid or alkali inhibited its utilization in industrialization, which is mainly due to the system corrosion and environmental toxication [54]. In response to that matter, the catalyst or nanomaterials for pretreatment of cellulose might reduce the chemical consumption by green synthesis via plant extracts and microorganisms [58]. This process offers many advantages, such as high stability, utilizing fewer chemical agents, and low cost. Thus, the green method for synthesis of nanomaterials led to lower pretreatment of cellulose for H2 production.
As previously discussed the reactor configuration of dark fermentation, many researchers most focused on the batch mode of bioreactors. However, this reactors normally brings about lower H2 production rates. Thus, a suitable design of a fermenter like a bioreactor configuration and operation mode has to be developed to make the production of H2 more efficient [40]. The fermentation should be carried out in continuous mode rather than in batch method, with particular attention given to the starting conditions—up strategy [41]. Such fermentation requires more rigorous large-scale studies to address technical and economic obstacles to be a viable and competitive technology. Besides the high cost for the pretreatment step, enzymatic hydrolysis also involves the major cost of incomplete fermentation when its deactivation at higher temperatures and incomplete conversion of the cellulose into the H2 product [45]. Thus, combining dark fermentation with photo fermentation is one of the approaches for economic technology since the latter process can consume unconverted metabolites from former fermentation such as acetic acid and then increase the process efficiency.
In recent years, there are still few studies using the additives to improve dark fermentative bio-H2 production. In general, the main additives used were metal zero-valent (Fe0, Ag0, Ni0, Pd0, etc.), metal ions (Fe2+, Ni2+, Na2+, Mg2+, etc.), and metal oxides (Fe2O3, Fe3O4, CoO, and ZrO2). In fact, the addition of metal into dark fermentation media facilitated intracellular electron transportation and provided the essential nutrition for microbial growth [59]. Among those metal additives, Fe is the most used for the dark fermentation process. Zhang et al., for example, evaluated the concentration of Fe and Mn on bio-H2 production. The addition of Fe and Mn into activated carbon enhanced the H2 production by 55.8%. These additives provided favorable sites for microbial colonization and promoted the direct interspecies electron transfer [59]. In addition, this catalyst could be recycled by magnetic separation after the bio-H2 process.
Electrofermentation is another technology that is being investigated to improve hydrogen production by dark fermentation. It consists of electrochemically controlling the metabolism of microbial fermentation using electrodes [152]. The electrofermentation inoculum must consist of electroactive and fermenting bacteria that are specialized in the desired product. Using co-cultures of microorganisms has shown cooperation between bacteria of the genus Geobacter (electroactive) and bacteria of the genus Clostridium (fermenter). Electrofermentation experiences with promising results are reported in the literature [153]. However, there is still much work to do to apply this process to real substrates.
For pyrolysis and gasification, tars from gasification and pyrolysis present a significant issue for low-temperature gasification systems, while contaminants such as heavy metals, chlorine, and sulfur also need to be removed in the syngas conditioning section [10]. To overcome this problem, the catalyst was loaded for enhancing the H2. The previous study suggested that if the catalyst load is lower, then the catalyst displays greater H2 potential. However, the usage of catalysts may cause poisoning and deactivation, coking, and lower H2 yields. Thus, two-step pyrolysis and reforming of cellulose are one of the approaches for H2 production, in which this process produces the free tar. Furthermore, adding the metal phases, support, and promoter can activate the catalyst by inhibited the coke formation. However, it is evident from the literature that H2 generation through pyrolysis still has many obstacles to be overcome, such as volatile composition, separation and purification, cost of development, selection of catalysts, or other materials that ease the process balance, and availability of sustainable biomass.
6 Conclusion
The present review mainly focused on the application of promising substrates or feedstock, cellulose in bio-H2 production under the thermochemical process, and biological process. Notably, the most promising methods of producing H2 from cellulose are pyrolysis/gasification and dark fermentation. Both processes need pretreatment of cellulose in order to enhance bio-H2 production. In fact, the pretreatment aims to break the heteropolymeric structure of cellulose and induce the H2-producing microorganisms by increasing the monomeric sugars. The most promising pretreatment for cellulose is an ionic liquid such as 1-butyl-3-methylimidazolium chloride (2.20 mol H2) instead of alkali and acid treatment (0.95 mol H2). This paper also reviews the parameters that influence cellulose pyrolysis such as temperature, heating rate, and vapor residence time, while for dark fermentation, including the inoculum source, temperature, pH, and HRT. Interestingly, the formation of H2 increases with a higher heating rate and gas residence times, which decreases the tar formation. In addition, the optimum temperature for cellulose pyrolysis is 800 °C, which gave H2 yield reaching a maximum of about ~ 30 mmol H2. For dark fermentation, co-culture or mixed cultures of thermophilic fermentation of cellulose at pH 7 for 240 h is often considered better in H2 yield (~ 50 mmol H2). In terms of the cost aspect, H2 production by both processes is not generally considered economically competitive with the existing process (SRM processes). It is believed that cellulose is thought to become a significant renewable source of H2 in the future. The share of H2 from cellulose in the automotive fuel market will rise rapidly in the next decade due to its environmental benefits.
References
Hassan NS, Jalil AA, Hitam CNC, Vo DVN, Nabgan W (2020) Biofuels and renewable chemicals production by catalytic pyrolysis of cellulose: a review. Environ Chem Lett 18:1625–1648. https://doi.org/10.1007/s10311-020-01040-7
Balat M, Balat M (2009) Political, economic and environmental impacts of biomass based hydrogen. Int J Hydrog Energy 34:3589–3603. https://doi.org/10.1016/j.ijhydene.2009.02.067
Parthasarathy P, Narayanan KS (2014) Hydrogen production from steam gasification of biomass: influence of process parameters on hydrogen yield – a review. Renew Energy 66:570–579. https://doi.org/10.1016/j.renene.2013.12.025
Nagarajan D, Lee DJ, Kondo A, Chang JS (2017) Recent insights into biohydrogen production by microalgae–from biophotolysis to dark fermentation. Bioresour Technol 227:373–387. https://doi.org/10.1016/j.biortech.2016.12.104
Osman AI, Deka TJ, Baruah DC, Rooney DW (2020) Critical challenges in biohydrogen production processes from the organic feedstocks. Biomass Convers Bior 1–19. https://doi.org/10.1007/s13399-020-00965-x
Dou B, Zhang H, Song Y, Zhao L, Jiang B, He M, Xu Y (2019) Hydrogen production from the thermochemical conversion of biomass: issues and challenges. Sustain Energy Fuels 3:314–342. https://doi.org/10.1039/C8SE00535D
Srivastava N, Srivastava M, Malhotra BD, Gupta VK, Ramteke PW, Silva RN, Mishra PK (2019) Nanoengineered cellulosic biohydrogen production via dark fermentation: a novel approach. Biotechnol Adv 37:107384. https://doi.org/10.1016/j.biotechadv.2019.04.006
Zheng Y, Pan Z, Zhang R (2009) Overview of biomass pretreatment for cellulosic ethanol production. Int J Agric Biol 2:51–68. https://doi.org/10.3965/j.issn.1934-6344.2009.03.051-068
Wu C, Wang Z, Huang J, Williams PT (2013) Pyrolysis/gasification of cellulose, hemicellulose and lignin for hydrogen production in the presence of various nickel-based catalysts. Fuel 106:697–706. https://doi.org/10.1016/j.fuel.2012.10.064
Kan T, Strezov V, Evans TJ (2016) Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters. Renew Sust Energ Rev 57:1126–1140. https://doi.org/10.1016/j.rser.2015.12.185
Shahabuddin M, Krishna BB, Bhaskar T, Perkins G (2020) Advances in the thermo-chemical production of hydrogen from biomass and residual wastes: summary of recent techno-economic analyses. Bioresour Technol 299:122557. https://doi.org/10.1016/j.biortech.2019.122557
Prakasham RS, Brahmaiah P, Nagaiah D, Rao PS, Reddy BV, Rao RS, Hobbs PJ (2012) Impact of low lignin containing brown midrib sorghum mutants to harness biohydrogen production using mixed anaerobic consortia. Int J Hydrog Energy 37:3186–3190. https://doi.org/10.1016/j.ijhydene.2011.11.082
de Sá LRV, de Oliveira MR, da Silva Bon EP, Cammarota MC, Ferreira-Leitao VS (2015) Fermentative biohydrogen production using hemicellulose fractions: analytical validation for C5 and C6-sugars, acids and inhibitors by HPLC. Int J Hydrog Energy 40:13888–13900. https://doi.org/10.1016/j.ijhydene.2015.08.014
Baêta BEL, Lima DRS, Balena Filho JG, Adarme OFH, Gurgel LVA, de Aquino SF (2016) Evaluation of hydrogen and methane production from sugarcane bagasse hemicellulose hydrolysates by two-stage anaerobic digestion process. Bioresour Technol 218:436–446. https://doi.org/10.1016/j.biortech.2016.06.113
Saratale GD, Kshirsagar SD, Sampange VT, Saratale RG, Oh SE, Govindwar SP, Oh MK (2014) Cellulolytic enzymes production by utilizing agricultural wastes under solid state fermentation and its application for biohydrogen production. App Biochem Biotechnol 174:2801–2817. https://doi.org/10.1007/s12010-014-1227-1
Ratti RP, Botta LS, Sakamoto IK, Varesche MBA (2013) Microbial diversity of hydrogen-producing bacteria in batch reactors fed with cellulose using leachate as inoculum. Int J Hydrog Energy 38:9707–9717. https://doi.org/10.1016/j.ijhydene.2013.05.089
Soares JF, Confortin TC, Todero I, Mayer FD, Mazutti MA (2020) Dark fermentative biohydrogen production from lignocellulosic biomass: technological challenges and future prospects. Renew Sust Energ Rev 117:109484. https://doi.org/10.1016/j.rser.2019.109484
Łukajtis R, Hołowacz I, Kucharska K, Glinka M, Rybarczyk P, Przyjazny A, Kamiński M (2018) Hydrogen production from biomass using dark fermentation. Renew Sust Energ Rev 91:665–694. https://doi.org/10.1016/j.rser.2018.04.043
Shanmugam S, Krishnaswamy S, Chandrababu R, Veerabagu U, Pugazhendhi A, Mathimani T (2020) Optimal immobilization of Trichoderma asperellum laccase on polymer coated Fe3O4@ SiO2 nanoparticles for enhanced biohydrogen production from delignified lignocellulosic biomass. Fuel 273:117777. https://doi.org/10.1016/j.fuel.2020.117777
Santucci BS, Maziero P, Rabelo SC, Curvelo AAS, Pimenta MTB (2015) Autohydrolysis of hemicelluloses from sugarcane bagasse during hydrothermal pretreatment: a kinetic assessment. Bioenergy Res 8:1778–1787. https://doi.org/10.1007/s12155-015-9632-z
Deswal D, Khasa YP, Kuhad RC (2011) Optimization of cellulase production by a brown rot fungus Fomitopsis sp. RCK2010 under solid state fermentation. Bioresour Technol 102:6065–6072. https://doi.org/10.1016/j.biortech.2011.03.032
Campos L, Moura H O, Cruz A J, Assumpcao S, de Carvalho LS, Pontes LA (2020) Response surface methodology (RSM) for assessing the effects of pretreatment, feedstock, and enzyme complex association on cellulose hydrolysis. Biomass Convers Bior https://doi.org/10.1007/s13399-020-00756-4
Xu Y, Liu G, Fu J, Kang S, Xiao Y, Yang P, Liao W (2019) Catalytic hydrolysis of cellulose to levulinic acid by partly replacing sulfuric acid with Nafion® NR50 catalyst. Biomass Convers Bior 9:609–616. https://doi.org/10.1007/s13399-019-00373-w
de Amorim JDP, de Souza KC, Duarte CR, da Silva Duarte I, Ribeiro FDAS, Silva GS, Sarubbo LA (2020) Plant and bacterial nanocellulose: production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ Chem Lett 18:851–869. https://doi.org/10.1007/s10311-020-00989-9
Li Y, Liao Y, Cao X, Wang T, Ma L, Long J, Xua Y (2015) Advances in hexitol and ethylene glycol production by one-pot hydrolytic hydrogenation and hydrogenolysis of cellulose. Biomass Bioenergy 74:148–161. https://doi.org/10.1016/j.biombioe.2014.12.025
Lam SS, Russell AD, Lee CL, Chase HA (2012) Microwave-heated pyrolysis of waste automotive engine oil: influence of operation parameters on the yield, composition, and fuel properties of pyrolysis oil. Fuel 92:327–339. https://doi.org/10.1016/j.fuel.2011.07.027
Trchounian K, Sawers RG, Trchounian A (2017) Improving biohydrogen productivity by microbial dark- and photo-fermentations: novel data and future approaches. Renew Sust Energ Rev 80:1201–1216. https://doi.org/10.1016/j.rser.2017.05.149
Wong YM, Wu TY, Juan JC (2014) A review of sustainable hydrogen production using seed sludge via dark fermentation. Renew Sust Energ Rev 34:471–482. https://doi.org/10.1016/j.rser.2014.03.008
Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M (2000) Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga chlamydomonas reinhardtii 1. Plant Physiol 122:127–135. https://doi.org/10.1104/pp.122.1.127
Winkler M, Hemschemeier A, Gotor C, Melis A, Happe T (2002) [Fe]-hydrogenases invgreen algae: photo-fermentation and hydrogen evolution under sulfur deprivation. Int J Hydrog Energy 27:1431–1439. https://doi.org/10.1016/S0360-3199(02)00095-2
Hallenbeck PC, Benemann JR (2002) Biological hydrogen production; fundamentals and limiting processes. Int J Hydrog Energy 27:1185–1193. https://doi.org/10.1016/S0360-3199(02)00131-3
Tamagnini P, Axelsson R, Lindberg P, Oxelfelt F, Wünschiers R, Lindblad P (2002) Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiol Mol Biol Rev 66:1–20. https://doi.org/10.1128/MMBR.66.1.1-20.2002
Kapdan IK, Kargi F (2006) Bio-hydrogen production from waste materials. Enzym Microb Technol 38:569–582. https://doi.org/10.1016/j.enzmictec.2005.09.015
He D, Bultel Y, Magnin JP, Roux C, Willison JC (2005) Hydrogen photosynthesis by Rhodobacter capsulatus and its coupling to a PEM fuel cell. J Power Sources 141:19–23. https://doi.org/10.1016/j.jpowsour.2004.09.002
Chen JS, Toth J, Kasap M (2001) Nitrogen-fixation genes and nitrogenase activity in Clostridium acetobutylicum and Clostridium beijerinckii. J Ind Microbiol Biotechnol 27:281–286. https://doi.org/10.1038/sj.jim.7000083
Zagrodnik R, Łaniecki M (2017) Hydrogen production from starch by co-culture of Clostridium acetobutylicum and Rhodobacter sphaeroides in one step hybrid dark and photofermentation in repeated fed-batch reactor. Bioresour Technol 224:298–306. https://doi.org/10.1016/j.biortech.2016.10.060
Manish S, Banerjee R (2008) Comparison of biohydrogen production processes. Int J Hydrog Energy 33:279–286. https://doi.org/10.1016/j.ijhydene.2007.07.026
Logan BE, Call D, Cheng S, Hamelers HVM, Sleutels THJA, Jeremiasse AW (2008) Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ Sci Technol 42:8630–8640. https://doi.org/10.1021/es801553z
Cha M, Chung D, Elkins JG, Guss AM, Westpheling J (2013) Metabolic engineering of Caldicellulosiruptor bescii yields increased hydrogen production from lignocellulosic biomass. Biotechnol Biofuels 6:85. https://doi.org/10.1186/1754-6834-6-85
Azman NF, Abdeshahian P, Kadier A, Nasser Al-Shorgani NK, Salih NKM, Lananan I, Kalil MS (2016) Biohydrogen production from de-oiled rice bran as sustainable feedstock in fermentative process. Int J Hydro Energy 41:145–156. https://doi.org/10.1016/j.ijhydene.2015.10.018
Nandi R, Sengupta S (1998) Microbial production of hydrogen: an overview. Crit Rev Microbiol 24:61–84. https://doi.org/10.1080/10408419891294181
Li C, Fang HHP (2007) Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Crit Rev Environ Sci Technol 37:1–39. https://doi.org/10.1080/10643380600729071
Hallenbeck PC (2012) Microbial technologies in advanced biofuels production. Springer Science+Business Media, LLC, New York. https://doi.org/10.1007/978-1-4614-1208-3
Mishra J, Khurana S, Kumar N, Ghosh AK, Das D (2004) Molecular cloning, characterization, and overexpression of a novel [Fe]-hydrogenase isolated from a high rate of hydrogen producing Enterobacter cloacae IIT-BT 08. Biochem Biophys Res Commun 324:679–685. https://doi.org/10.1016/j.bbrc.2004.09.108
Srivastava N, Srivastava M, Kushwaha D, Gupta VK, Manikanta A, Ramteke PW, Mishra PK (2017) Effcient dark fermentative hydrogen production from enzyme hydrolyzed rice straw by clostridium pasteurianum (MTCC116). Bioresour Technol 238:552–558. https://doi.org/10.1016/j.biortech.2017.04.077
Pandey A, Nigam P, Soccol CR, Soccol VT, Singh D, Mohan R (2000) Advances in microbial amylases. Biotechnol Appl Biochem 31:135–152. https://doi.org/10.1042/ba19990073
Xu J, Deshusses MA (2015) Fermentation of swine wastewater-derived duckweed 70for biohydrogen production. Int J Hydrog Energy 40:7028–7036. https://doi.org/10.1016/j.ijhydene.2015.03.166
Lin R, Cheng J, Ding L, Song W, Zhou J, Cen K (2015) Inhibitory effects of furan derivatives and phenolic compounds on dark hydrogen fermentation. Bioresour Technol 196:250–255. https://doi.org/10.1016/j.biortech.2015.07.097
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4:7. https://doi.org/10.1186/s40643-017-0137-9
Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efcient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 10:4851–4861. https://doi.org/10.1016/j.biortech.2009.11.093
Zhang J, Wu J, Yu J, Zhang X, He J, Zhang J (2017) Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: state of the art and future trends. Mater Chem Frontiers 1:1273–1290. https://doi.org/10.1039/C6QM00348F
Low FW, Samsudin NA, Yusoff Y, Tan XY, Lai CW, Amin N, Tiong SK (2020) Hydrolytic cleavage of glycosidic bonds for cellulose nanoparticles (CNPs) production by BmimHSO4 ionic liquid catalyst. Thermochim Acta 684:178484
Han J, Zhou C, French AD, Han G, Wu Q (2013) Characterization of cellulose II nanoparticles regenerated from 1-butyl-3-methylimidazolium chloride. Carbohydr Polym 94:773–781. https://doi.org/10.1016/j.tca.2019.178484
Nguyen TAD, Han SJ, Kim JP, Kim MS, Oh YK, Sim SJ (2008) Hydrogen production by the hyperthermophilic eubacterium, Thermotoga neapolitana, using cellulose pretreated by ionic liquid. Int J Hydrog Energy 33:5161–5168. https://doi.org/10.1016/j.ijhydene.2008.05.019
Cui M, Shen J (2012) Effects of acid and alkaline pretreatments on the biohydrogen production from grass by anaerobic dark fermentation. Int J Hydrog Energy 37:1120–1124. https://doi.org/10.1016/j.ijhydene.2011.02.078
Dadi AP, Varanasi S, Schall CA (2006) Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step. Biotechnol Bioeng 95:904–910. https://doi.org/10.1002/bit.21047
Zhang J, Zhao W, Zhang H, Wang Z, Fan C, Zang L (2018) Recent achievements in enhancing anaerobic digestion with carbon-based functional materials. Bioresour Technol 266:555–567. https://doi.org/10.1016/j.biortech.2018.07.076
Lee J, Lee S, Park H (2016) Enrichment of specific electro-active microorganisms and enhancement of methane production by adding granular activated carbon in anaerobic reactors. Bioresour Technol 205:205–212. https://doi.org/10.1016/j.biortech.2016.01.054
Zhang J, Fan C, Zhao W, Zang L (2019) Improving bio-H2 production by manganese doped magnetic carbon. Int J Hydrog Energy 44:26920–26932. https://doi.org/10.1016/j.ijhydene.2019.08.148
Zhang X, Zhang Z, Wang F, Wang Y, Song Q, Xu J (2013) Lignosulfonate-based heterogeneous sulfonic acid catalyst for hydrolyzing glycosidic bonds of polysaccharides. J Mol Catal A Chem 377:102–107. https://doi.org/10.1016/j.molcata.2013.05.001
Wang H, Zhang C, He H, Wang L (2012) Glucose production from hydrolysis of cellulose over a novel silica catalyst under hydrothermal conditions. J Environ Sci 24:473–478. https://doi.org/10.1016/S1001-0742(11)60795-X
Güell EJ, Maru BT, Chimentão RJ, Gispert-Guirado F, Constantí M, Medina F (2015) Combined heterogeneous catalysis and dark fermentation systems for the conversion of cellulose into biohydrogen. Biochem Eng J 101:209–219. https://doi.org/10.1016/j.bej.2015.06.004
Tondro H, Zilouei H, Zargoosh K, Bazarganipour M (2020) Investigation of heterogeneous sulfonated graphene oxide to hydrolyze cellulose and produce dark fermentative biohydrogen using Enterobacter aerogenes. Bioresour Technol 306:123124. https://doi.org/10.1016/j.biortech.2020.123124
Wei Z, Yang Y, Hou Y, Liu Y, He X, Deng S (2014) A new approach towards acid catalysts with high reactivity based on graphene nanosheets. ChemCatChem 6:2354–2363. https://doi.org/10.1002/cctc.201402100
Zhang M, Wu M, Liu Q, Wang X, Lv T, Jia L (2017) Graphene oxide mediated cellulose-derived carbon as a highly selective catalyst for the hydrolysis of cellulose to glucose. Appl Catal A Gen 543:218–224. https://doi.org/10.1016/j.apcata.2017.06.033
Goud RK, Raghavulu SV, Mohanakrishna G, Naresh K, Mohan SV (2012) Predominance of bacilli and clostridia in microbial community of biohydrogen producing bioflm sustained under diverse acidogenic operating conditions. Int J Hydrog Energy 37:4068–4076. https://doi.org/10.1016/j.ijhydene.2011.11.134
Wang J, Wan W (2009) Factors influencing fermentative hydrogen production: a review. Int J Hydrog Energy 34:799–811. https://doi.org/10.1016/j.ijhydene.2008.11.015
Taguchi F, Yamada K, Hasegawa K, Taki-Saito T, Hara K (1996) Continuous hydrogen production by Clostridium sp. strain no. 2 from cellulose hydrolysate in an aqueous two-phase system. J Ferment Bioeng 82:80–83. https://doi.org/10.1016/0922-338X(96)89460-8
Ho KL, Lee DJ, Su A, Chang JS (2012) Biohydrogen from cellulosic feedstock: dilution-to-stimulation approach. Int J Hydrog Energy 37:15582–15587. https://doi.org/10.1016/j.ijhydene.2012.01.093
Lo YC, Saratale GD, Chen WM, Bai MD, Chang JS (2009) Isolation of cellulose-hydrolytic bacteria and applications of the cellulolytic enzymes for cellulosic biohydrogen production. Enzym Microb Technol 44:417–425. https://doi.org/10.1016/j.enzmictec.2009.03.002
Lo YC, Huang CY, Fu TN, Chen CY, Chang JS (2009) Fermentative hydrogen production from hydrolyzed cellulosic feedstock prepared with a thermophilic anaerobic bacterial isolate. Int J Hydrog Energy 34:6189–6200. https://doi.org/10.1016/j.ijhydene.2009.05.104
Levin DB, Islam R, Cicek N, Sparling R (2006) Hydrogen production by Clostridium thermocellum 27405 from cellulosic biomass substrates. Int J Hydrog Energy 31:1496–1503. https://doi.org/10.1016/j.ijhydene.2006.06.015
Islam R, Sparling R, Cicek N, Levin DB (2015) Optimization of influential nutrients during direct cellulose fermentation into hydrogen by Clostridium thermocellum. Int J Mol Sci 16:3116–3132. https://doi.org/10.3390/ijms16023116
Magnusson L, Islam R, Sparling R, Levin D, Cicek N (2008) Direct hydrogen production from cellulosic waste materials with a single-step dark fermentation process. Int J Hydrog Energy 33:5398–5403. https://doi.org/10.1016/j.ijhydene.2008.06.018
Lalaurette E, Thammannagowda S, Mohagheghi A, Maness PC, Logann BE (2009) Hydrogen production from cellulose in a two-stage process combining fermentation and electrohydrogenesis. Int J Hydrog Energy 34:6201–6210. https://doi.org/10.1016/j.ijhydene.2009.05.112
Ren N, Wang A, Gao L, Xin L, Lee DJ, Su A (2008) Bioaugmented hydrogen production from carboxymethyl cellulose and partially delignified corn stalks using isolated cultures. Int J Hydrog Energy 33:5250–5255. https://doi.org/10.1016/j.ijhydene.2008.05.020
Chang JJ, Lin JJ, Ho CY, Chin WC, Huang CC (2010) Establishment of rumen-mimic bacterial consortia: a functional union for bio-hydrogen production from cellulosic bioresource. Int J Hydrog Energy 35:13399–13406. https://doi.org/10.1016/j.ijhydene.2009.11.119
Nissilä ME, Tähti HP, Rintala JA, Puhakka JA (2011) Thermophilic hydrogen production from cellulose with rumen fluid enrichment cultures: effects of different heat treatments. Int J Hydrog Energy 36:1482–1490. https://doi.org/10.1016/j.ijhydene.2010.11.010
Rosseau JD, Zajic JE (2007) Hydrogen-gas production with Citrobacter intermedim and Clostridium pasteurianum. J Chem Technol Biotechnol 32:496–502. https://doi.org/10.1002/jctb.5030320310
Chen WM, Tseng ZJ, Lee KS, Chang JS (2005) Fermentative hydrogen production with Clostridium butyricum CGS5 isolated from anaerobic sewage sludge. Int J Hydrog Energy 30:1063–1070. https://doi.org/10.1016/j.ijhydene.2004.09.008
Islam R, Cicek N, Sparling R, Levin DB (2009) Influence of initial cellulose concentration on the carbon flow distribution during batch fermentation of cellulose by Clostridium thermocellum. Appl Microbiol Biotechnol 82:141–148. https://doi.org/10.1007/s00253-008-1763-0
Ratti RP, Botta LS, Sakamoto IK (2014) Production of H2 from cellulose by rumen microorganisms: effects of inocula pre-treatment and enzymatic hydrolysis. Biotechnol Lett 36:537–546. https://doi.org/10.1007/s10529-013-1395-z
Valdez-Vazquez I, Ríos-Leal E, Esparza-García F, Cecchi F, Poggi-Varaldo HM (2005) Semi-continuous solid substrate anaerobic reactors for H2 production from organic waste: mesophilic versus thermophilic regime. Int J Hydrog Energy 30:1383–1391. https://doi.org/10.1016/j.ijhydene.2004.09.016
Ueno Y, Kawai T, Sato S, Otsuka S, Morimoto M (1995) Biological production of hydrogen from cellulose by natural anaerobic microflora. J Ferment Bioeng 79:395–397. https://doi.org/10.1016/0922-338X(95)94005-C
Wang A, Re N, Shi Y, Le DJ (2008) Bioaugmented hydrogen production from microcrystalline cellulose using co-culture-Clostridium acetobutylicum X9 and Ethanoigenens harbinense B49. Int J Hydrog Energy 33:912–917. https://doi.org/10.1016/j.ijhydene.2007.10.017
Liu Y, Yu P, Song X, Qu Y (2008) Hydrogen production from cellulose by co-culture of Clostridium thermocellum JN4 and Thermoanaerobacterium thermosaccharolyticum GD17. Int J Hydrog Energy 33:2927–2933. https://doi.org/10.1016/j.ijhydene.2008.04.004
Geng A, He Y, Qian C, Yan X, Zhou Z (2010) Effect of key factors on hydrogen production from cellulose in a co-culture of Clostridium thermocellum and Clostridium thermopalmarium. Bioresour Technol 101:4029–4033. https://doi.org/10.1016/j.biortech.2010.01.042
Carver SM, Nelson MC, Lepistö R, Yu Z, Tuovinen OH (2012) Hydrogen and volatile fatty acid production during fermentation of cellulosic substrates by a thermophilic consortium at 50 and 60°C. Bioresour Technol 104:424–431. https://doi.org/10.1016/j.biortech.2011.11.013
Lu H, Lee PK (2015) Effects of cellulose concentrations on the syntrophic interactions between Clostridium cellulovorans 743B and Rhodopseudomonas palustris CGA009 in coculture fermentation for biohydrogen production. Int J Hydrog Energy 40:11800–11808. https://doi.org/10.1016/j.ijhydene.2015.05.135
Gomez-Flores M, Nakhla G, Hafez H (2017) Hydrogen production and microbial kinetics of Clostridium termitidis in mono-culture and co-culture with Clostridium beijerinckii on cellulose. AMB Express 7:84. https://doi.org/10.1186/s13568-016-0256-2
Hitit ZY, Lazaro CZ, Hallenbeck PC (2017) Single stage hydrogen production from cellulose through photo-fermentation by a co-culture of Cellulomonas fimi and 127Rhodopseudomonas palustris. Int J Hydrog Energy 42:6556–6566. https://doi.org/10.1016/j.ijhydene.2016.12.035
Bao H, Chen C, Jiang L, Liu Y, Shen M, Liu W, Wang A (2016) Optimization of key factors affecting biohydrogen production from microcrystalline cellulose by the co-culture of Clostridium acetobutylicum X9+ Ethanoigenens harbinense B2. RSC Adv 6:3421–3427. https://doi.org/10.1039/C5RA14192C
Ueno Y, Haruta S, Ishii M, Igarashi Y (2001) Characterization of a microorganism isolated from the effluent of hydrogen fermentation by microflora. J Biosci Bioeng 92:397–400. https://doi.org/10.1016/S1389-1723(01)80247-4
Chu Y, Wei Y, Yuan X, Shi X (2011a) Bioconversion of wheat stalk to hydrogen by dark fermentation: effect of different mixed microflora on hydrogen yield and cellulose solubilisation. Bioresour Technol 102:3805–3809. https://doi.org/10.1016/j.biortech.2010.11.092
Lin CY, Hung WC (2008) Enhancement of fermentative hydrogen/ethanol production from cellulose using mixed anaerobic cultures. Int J Hydrog Energy 33:3660–3667. https://doi.org/10.1016/j.ijhydene.2008.04.036
Fan YT, Xing Y, Ma H, Pan CM, Hou HW (2008) Enhanced cellulose-hydrogen production from corn stalk by lesser panda manure. Int J Hydrog Energy 33:6058–6065. https://doi.org/10.1016/j.ijhydene.2008.08.005
Lo YC, Bai MD, Chen WM, Chang JS (2008) Cellulosic hydrogen production with a sequencing bacterial hydrolysis and dark fermentation strategy. Bioresour Technol 99:8299–8303. https://doi.org/10.1016/j.biortech.2008.03.004
Ren NQ, Xu JF, Gao LF, Xin L, Qiu J, Su DX (2010) Fermentative bio-hydrogen production from cellulose by cow dung compost enriched cultures. Int J Hydrog Energy 35:2742–2746. https://doi.org/10.1016/j.ijhydene.2009.04.057
Saratale G, Saratale RG, Lo YC, Chang JS (2010) Multicomponent cellulase production by Cellulomonas biazotea NCIM-2550 and its applications for cellulosic biohydrogen production. Biotechnol Prog 26:406–416. https://doi.org/10.1002/btpr.342
Guo YP, Fan SQ, Fan YT, Pan CM, Hou HW (2010) The preparation and application of crude cellulase for cellulose-hydrogen production by anaerobic fermentation. Int J Hydrog Energy 35:459–468. https://doi.org/10.1016/j.ijhydene.2009.10.021
Wang A, Gao L, Ren N, Xu J, Liu C, Lee DJ (2010) Enrichment strategy to select functional consortium from mixed cultures: consortium from rumen liquor for simultaneous cellulose degradation and hydrogen production. Int J Hydrog Energy 35:13413–13418. https://doi.org/10.1016/j.ijhydene.2009.11.117
Chu CY, Wu S, Tsai CY, Lin CY (2011b) Kinetics of cotton cellulose hydrolysis using concentrated acid and fermentative hydrogen production from hydrolysate. Int J Hydrog Energy 36:8743–8750. https://doi.org/10.1016/j.ijhydene.2010.07.072
Pan CM, Ma HC, Fan YT, Hou HW (2011) Bioaugmented cellulosic hydrogen production from cornstalk by integrating dilute acid-enzyme hydrolysis and dark fermentation. Int J Hydrog Energy 36:4852–4862. https://doi.org/10.1016/j.ijhydene.2011.01.114
Lay CH, Chang FY, Chu CY, Chen CC, Chi YC, Hsieh TT, Lin CY (2011) Enhancement of anaerobic biohydrogen/methane production from cellulose using heat-treated activated sludge. Water Sci Technol 63:1849–1854. https://doi.org/10.2166/wst2011.390
Saripan AF, Reungsang A (2014) Simultaneous saccharification and fermentation of cellulose for bio-hydrogen production by anaerobic mixed cultures in elephant dung. Int J Hydrog Energy 39:9028–9035. https://doi.org/10.1016/j.ijhydene.2014.04.066
Gadow SI, Jiang H, Li YY (2016) Characterization and potential of three temperature ranges for hydrogen fermentation of cellulose by means of activity test and 16s rRNA sequence analysis. Bioresour Technol 209:80–89. https://doi.org/10.1016/j.biortech.2016.02.098
Jiang Y, Lu J, Lv Y, Wu R, Dong W, Zhou J, Xin F (2019) Efficient hydrogen production from lignocellulosic feedstocks by a newly isolated thermophlic Thermoanaerobacterium sp. strain F6. Int J Hydrog Energy 44:14380–14386. https://doi.org/10.1016/j.ijhydene.2019.01.226
Carver SM, Lepistö R, Tuovinen OH (2019) Effect of the type and concentration of cellulose and temperature on metabolite formation by a fermentative thermophilic consortium. Int J Hydrog Energy 44:17248–17259. https://doi.org/10.1016/j.ijhydene.2019.02.177
Zagrodnik R, Seifert K (2020) Direct fermentative hydrogen production from cellulose and starch with mesophilic bacterial consortia. Pol J Microbiol 69:109–120
Gadow SI, Li YY, Liu Y (2012) Effect of temperature on continuous hydrogen production of cellulose. Int J Hydrog Energy 37:15465–15472. https://doi.org/10.1016/j.ijhydene.2012.04.128
Gadow SI, Jiang H, Watanabe R, Li YY (2013) Effect of temperature and temperature shock on the stability of continuous cellulosic-hydrogen fermentation. Bioresour Technol 142:304–311. https://doi.org/10.1016/j.biortech.2013.04.102
Wang A, Sun D, Ca G, Wang H, Ren N, Wu WM, Logan BE (2011) Integrated hydrogen production process from cellulose by combining dark fermentation, microbial fuel cells, and a microbial electrolysis cell. Bioresour Technol 102:4137–4143. https://doi.org/10.1016/j.biortech.2010.10.137
Boonsayompoo O, Reungsang A (2013) Thermophilic biohydrogen production from the enzymatic hydrolysate of cellulose fraction of sweet sorghum bagasse by Thermoanaerobacterium thermosaccharolyticum KKU19: optimization of media composition. Int J Hydrog Energy 38:15777–15786. https://doi.org/10.1016/j.ijhydene.2013.04.129
Levin DB, Pitt L, Love M (2004) Biohydrogen production: prospects and limitations to practical application. Int J Hydrog Energy 29:173–185. https://doi.org/10.1016/S0360-3199(03)00094-6
Kumar G, Bakony P, Sivagurunathan P, Nemestothy N, Belaf-Bako K, Lin C-Y (2015) Improved microbial conversion of de-oiled Jatropha waste into biohydrogen via inoculum pretreatment: process optimization by experimental design approach. Biofuel Res J 5:209–214. https://doi.org/10.18331/brj2015.2.1.7
Gonzales RR, Kim SH (2017) Dark fermentative hydrogen production following the sequential dilute acid pretreatment and enzymatic saccharifcation of rice husk. Int J Hydrog Energy 42:27577–27583. https://doi.org/10.1016/j.ijhydene.2017.08.185
Hawkes FR, Hussy I, Kyazze G, Dinsdale R, Hawkes DL (2007) Continuous dark fermentative hydrogen production by mesophilic microflora: principles and progress. Int J Hydrog Energy 32:172–184. https://doi.org/10.1016/j.ijhydene.2006.08.014
Show KY, Lee DJ, Chang JS (2011) Bioreactor and process design for biohydrogen production. Bioresour Technol 102:8524–8533. https://doi.org/10.1016/j.biortech.2011.04.055
Banu JR, Arulazhagan P, Adish Kumar S, Kaliappan S, Lakshmi AM (2015) Anaerobic co-digestion of chemical and ozone-pretreated sludge in hybrid upflow anaerobic sludge blanket reactor. Desalination Water Treat 54:3269–3278. https://doi.org/10.1080/19443994.2014.912156
Lin C, Chang R-C (2004) Fermentative hydrogen production at ambient temperature. Int J Hydrog Energy 29:715–720. https://doi.org/10.1016/j.ijhydene.2003.09.002
Anjana AAS, Adish Kumar S, Banu RJ, Ginni G (2016) The performance of fluidized bed solar photo Fenton oxidation in the removal of COD from hospital wastewaters. Desalination Water Treat 57:8236–8242. https://doi.org/10.1080/19443994.2015.1021843
Arregi A, Amutio M, Lopez G, Bilbao J, Olazar M (2018) Evaluation of thermochemical routes for hydrogen production from biomass: a review. Energy Convers Manag 165:696–719. https://doi.org/10.1016/j.enconman.2018.03.089
Balat H, Kırtay E (2010) Hydrogen from biomass–present scenario and future prospects. Int J Hydrog Energy 35:7416–7426. https://doi.org/10.1016/j.ijhydene.2010.04.137
Yan Q, Guo L, Lu Y (2006) Thermodynamic analysis of hydrogen production from biomass gasification in supercritical water. Energy Convers Manag 47:1515–1528. https://doi.org/10.1016/j.enconman.2005.08.004
Swami SM, Chaudhari V, Kim DS, Sim SJ, Abraham MA (2008) Production of hydrogen from glucose as a biomass simulant: integrated biological and thermochemical approach. Ind Eng Chem Res 47:3645–3651
Sansaniwal SK, Pal K, Rosen MA, Tyagi SK (2017) Recent advances in the development of biomass gasification technology: a comprehensive review. Renew Sustainable Energy Rev 72:363–384. https://doi.org/10.1016/j.rser.2017.01.038
Zhang L, Champagne P (2010) Overview of recent advances in thermochemical conversion of biomass. Energy Convers Manag 51:969–982. https://doi.org/10.1016/j.enconman.2009.11.038
Mazloomi K, Gomes C (2012) Hydrogen as an energy carrier: prospects and challenges. Renew Sustainable Energy Rev 16:3024–3033. https://doi.org/10.1016/j.rser.2012.02.028
Zhang B, Xiong S, Xiao B, Yu D, Jia X (2011) Mechanism of wet sewage sludgepyrolysis in a tubular furnace. Int J Hydrog Energy 36:355–363. https://doi.org/10.1016/j.ijhydene.2010.05.100
Salam MA, Ahmed K, Akter N, Hossain T, Abdullah B (2018) A review of hydrogen production via biomass gasification and its prospect in Bangladesh. Int J Hydrog Energy 43:14944–14973. https://doi.org/10.1016/j.ijhydene.2018.06.043
Balat H (2010) Prospects of biofuels for a sustainable energy future: a critical assessment. Energy Educ Sci Technol Part A 24:85–111
Al-Rahbi AS, Onwudili JA, Williams PT (2016) Thermal decomposition and gasification of biomass pyrolysis gases using a hot bed of waste derived pyrolysis char. Bioresour Technol 204:71–79. https://doi.org/10.1016/j.biortech.2015.12.016
Velden MVD, Baeyens J, Brems A, Janssens B, Dewil R (2010) Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew Energy 35:232–242. https://doi.org/10.1016/j.renene.2009.04.019
Su S, Li W, Bai Z, Xiang H (2008) A preliminary study of a novel catalyst Al2O3· Na2O· xH2O/NaOH/Al(OH)3 for production of hydrogen and hydrogen-rich gas by steam gasification from cellulose. Int J Hydrog Energy 33:6947–6952. https://doi.org/10.1016/j.ijhydene.2008.09.003
Su S, Li W, Bai Z, Xiang H, Ba J (2010) Effects of ionic catalysis on hydrogen production by the steam gasification of cellulose. Int J Hydrog Energy 35:4459–4465. https://doi.org/10.1016/j.ijhydene.2010.02.066
Zou J, Yang H, Zeng Z, Wu C, Williams PT, Chen H (2016) Hydrogen production from pyrolysis catalytic reforming of cellulose in the presence of K alkali metal. Int J Hydrog Energy 41:10598–10607. https://doi.org/10.1016/j.ijhydene.2016.04.207
Ruppert AM, Niewiadomski M, Grams J, Kwapiński W (2014) Optimization of Ni/ZrO2 catalytic performance in thermochemical cellulose conversion for enhanced hydrogen production. Appl Catal B Environ 145:85–90. https://doi.org/10.1016/j.apcatb.2013.01.010
Kang K, Azargohar R, Dalai AK, Wang H (2016) Hydrogen production from lignin, cellulose and waste biomass via supercritical water gasification: catalyst activity and process optimization study. Energy Convers Manag 117:528–537. https://doi.org/10.1016/j.enconman.2016.03.008
Zou J, Oladipo J, Fu S, Al-Rahbi A, Yang HWC, Chen H (2018) Hydrogen production from cellulose catalytic gasification on CeO2/Fe2O3 catalyst. Energy Convers Manag 171:241–248. https://doi.org/10.1016/j.enconman.2018.05.104
Khan A, Chen P, Boolchand P, Smirniotis PG (2008) Modified nano-crystalline ferrites for high-temperature WGS membrane reactor applications. J Catal 253:91–104. https://doi.org/10.1016/j.jcat.2007.10.018
Furness D, Hoggett L, Judd S (2000) Thermochemical treatment of sewage sludge. Water Environ J 14:57–65. https://doi.org/10.1111/j.1747-6593.2000.tb00227.x
Hernández C, Alamilla-Ortiz ZL, Escalante AE, Navarro-Díaz M, Carrillo-Reyes J, Moreno-Andrade I, Valdez-Vazquez I (2019) Heat-shock treatment applied to inocula for H2 production decreases microbial diversities, interspecific interactions and performance using cellulose as substrate. Int J Hydrog Energy 44:13126–13134. https://doi.org/10.1016/j.ijhydene.2019.03.124
Fushimi C, Araki K, Yamaguchi Y, Tsutsumi A (2003) Effect of heating rate on steam gasification of biomass. 2. Thermogravimetric-mass spectrometric (TG-MS) analysis of gas evolution. Ind Eng Chem Res 42:3929–3936. https://doi.org/10.1021/ie0300575
Dhyani V, Bhaskar T (2018) A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew Energy 129:695–716. https://doi.org/10.1016/j.renene.2017.04.035
Yu J, Paterson N, Blamey J, Millan M (2017) Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 191:140–149. https://doi.org/10.1016/j.fuel.2016.11.057
Bridgwater AV (2012) Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenerg 38:68–94. https://doi.org/10.1016/j.biombioe.2011.01.048
Ni M, Leung DY, Leung MK, Sumathy K (2006) An overview of hydrogen production from biomass. Fuel Process Technol 87:461–472. https://doi.org/10.1016/j.fuproc.2005.11.003
Ingram L, Mohan D, Bricka M, Steele P, Strobel D, Crocker D, Mitchell B, Mohammad J, Cantrell K, Pittman CU Jr (2007) Pyrolysis of wood and bark in an auger reactor: physical properties and chemical analysis of the produced bio-oils. Energy Fuel 22:614–625. https://doi.org/10.1021/ef700335k
Demirbaş A (2005) Thermochemical conversion of hazelnut shell to gaseous products for production of hydrogen. Energy Sources 27:339–347. https://doi.org/10.1080/00908310490441728
Hamelinck CN, Faaij APC (2002) Future prospects for production of methanol and hydrogen from biomass. J Power Sources 111:1–22. https://doi.org/10.1016/S0378-7753(02)00220-3
Ochs D, Wukovits W, Ahrer W (2010) Life cycle inventory analysis of biological hydrogen production by thermophilic and photo fermentation of potato steam peels (PSP). J Clean Prod 18:88–94. https://doi.org/10.1016/j.jclepro.2010.05.018
Moscoviz R, Trably E, Bernet N (2018) Electro-fermentation triggering population selection in mixed-culture glycerol fermentation. Microb Biotechnol 11:74–83. https://doi.org/10.1111/1751-7915.12747
Toledo-Alarcon J, Moscoviz R, Trably E, Bernet N (2019) Glucose electro-fermentation as main driver for efficient H2-producing bacteria selection in mixed cultures. Int J Hydrog Energy 4:2230–2238. https://doi.org/10.1016/j.ijhydene.2018.07.091
Acknowledgments
This research study was sponsored by the Universiti Teknologi Malaysia through the Professional Development Research University Grant (No. 04E73), UTM Transdisciplinary Research Grant (Grant No. 06G53), and Collaborative Research Grant (Grant No. 07G62).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hassan, N.S., Jalil, A.A., Vo, D.V.N. et al. An overview on the efficiency of biohydrogen production from cellulose. Biomass Conv. Bioref. 13, 8485–8507 (2023). https://doi.org/10.1007/s13399-020-01125-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01125-x